Abstract
AIM: To explore whether there was anti-Saccharomyces cerevisiae antibodies (ASCA) positivity in our patients with biopsy-confirmed celiac disease.
METHODS: A cohort of patients with inflammatory bowel diseases (42 patients with Crohn’s disease and 10 patients with ulcerative colitis) and gluten sensitive enteropathy (16 patients) from Debrecen, Hungary were enrolled in the study. The diagnosis was made using the formally accepted criteria. Perinuclear antineutrophil cytoplasmic antibodies (pANCA) and anti-Saccharomyces cerevisiae antibodies (ASCA), antiendomysium antibodies (EMA), antigliadin antibodies (AGA) and anti human tissue transglutaminase antibodies (tTGA) were investigated.
RESULTS: The results showed that ASCA positivity occurred not only in Crohn’s disease but also in Celiac disease and in these cases both the IgG and IgA type antibodies were proved.
CONCLUSION: It is conceivable that ASCA positivity correlates with the (auto-) immune inflammation of small intestines and it is a specific marker of Crohn’s disease.
INTRODUCTION
Gluten-sensitive enteropathy (GSE) or, as it is more commonly called, celiac disease, is an immune-mediated enteropathic condition (specified as an autoimmune inflammatory disease of the small intestine) that is precipitated by the ingestion of gluten, a component of wheat protein, in genetically susceptible persons. Exclusion of dietary gluten results in healing of the mucosa, resolution of the malabsorptive state, and reversal of most, if not all, effects of celiac disease. GSE commonly manifests as “silent” celiac disease (i.e., minimal or no symptoms). Histological examination and serologic tests for antibodies against endomysium, transglutaminase, and gliadin identify most patients with the disease[1].
Crohn’s disease (CD) and ulcerative colitis (UC) are both classified under the medical rubric of inflammatory bowel disease (IBD). It is currently accepted that the term “IBD” does not encompass just two diseases (CD or UC), but rather a group of diseases, triggered and perpetuated by a variety of diverse genetic, environmental, and immunologic factors that share similar clinical manifestations. They cause life-impaired symptoms, necessitate long-term dependence on powerful drugs, and often result in debilitating surgery and even death. Although the etiology of UC and CD remains unclear, in addition to genetic and other environmental factors (food allergens, etc.), microorganisms have been discussed as possibly playing an important role. The gastrointestinal (GI) tract has direct contact with the environment and therefore forms a very important protective barrier within the human’s organism. The gut mucosa has to stop foreign materials, such as bacteria or antigens, from entering the body and also prevent excessive loss of “body material” into the lumen. If the mucosal barrier is broken, an influx of luminal antigens may result in the perpetuation of intestinal inflammation by chronically stimulating resident and recruited immunocompetent cells of the lamina propria. Bacteria present within the intestinal lumen or in the intestinal wall are important in the development of mucosal inflammation. Recent reports in the literature do not suggest that a specific persistent infection causes IBD (i.e. the repeatedly blamed Mycobacterium paratuberculosis avium), but indicate that enteric pathogens could cause initial onset of IBD and are associated with reactivation of quiescent disease. CD patients may have a heterogeneous serological response to specific bacteria and bacterial related antigens. The serologic responses seen in CD patients include antibodies to Saccharomyces cerevisiae, Mycobacteria, Bacteriodes, Listeria, and E. coli. Many of the specific organisms have been proposed to directly or indirectly contribute to the pathogenesis of CD. Despite their self-limited character, these infections initiate a cascade of inflammatory events leading to chronic, relapsing disease in a genetically susceptible host (“hit-and-run” hypothesis). So, epidemiological and microbiologic studies suggest that enteropathogenic microorganisms play a substantial role in the clinical initiation and relapses of IBD. Thus microbiologic screening might be helpful in patients with flares of IBD for optimal medical treatment and as many bacteria cannot be cultured, culture-independent techniques are expected to be of great help in identifying microorganisms in IBD. With more comprehensive knowledge of the intestinal microflora and how the latter interacts with the host’s immune system, it may be possible to define specific microbial substances in specific patients that are either present or absent from the normal flora to cause disease. The diagnoses of CD, UC and GSE are based on clinical features and the results of barium X-rays, endoscopy, mucosal biopsy histology, and in some cases operative findings and resected bowel pathology and histology. Serologic disease markers are accepted in GSE but unambiguously in IBD. Serologic tests that could serve as an adjunct to these invasive and expensive diagnostic studies, or possibly replace them, would have clinical utility[2-5]. Antibodies have attracted much interest for the study of the immune response in inflammatory bowel disease (IBD) and are also used as a tool for phenotyping. Antibodies to baker’s yeast and brewer’s yeast (anti-Saccharomyces cerevisiae antibodies or ASCA) directed against cell wall oligomannoside epitopes have been proposed as a serological marker for CD[6,7]. They have a sensitivity of 60% and a specificity of 80%-95% for differentiating CD from controls[8]. One small study also reported the production of ASCA by a fraction of unaffected relatives of Crohn’s patients. The role of ASCA’s in CD is completely unknown, but one hypothesis links them to increased intestinal permeability. 50%-60% of patients with CD exhibit an activity-related increase in intestinal permeability, and this increase might be predictive for relapse. A variable proportion of healthy first-degree relatives of CD patients also have abnormal permeability of the small intestine, which lead some authors to postulate a primary defect of the tight junctions as etiologic factor in CD. Increased exposure of the epithelium to common food antigens such as yeasts due to a break in the epithelial barrier may then result in an antibody response. The effect of dietary yeast (Saccharomyces cerevisiae) on the activity of stable CD disease was assessed in patients. The patients’ mean CD activity index (CDAI) while taking baker’s yeast was significantly greater than that during yeast exclusion and patients with elevated yeast antibodies tended to develop a higher CDAI while receiving baker’s yeast. These results suggest that dietary yeast may affect the activity of CD[9]. Antinuclear cytoplasmic antibodies (ANCA) with a perinuclear staining pattern have been proposed as a serologic marker for UC. pANCA’s were found in 60%-70% of patients with UC. There is no clear association of pANCA with severity of disease but it is predictive of pouchitis after restorative proctocolectomy with ileoanal pouch anastomosis[10]. pANCA’s are also found in 15%-20% of patients with CD. These patients present diffuse left-sided colitis with symptoms such as rectal bleeding, urgency, and mucus discharge and this phenotype is labeled “UC-like CD”.
Saccharomyces cerevisiae (SC) is “ubiquitous” yeast and occurs in diverse places in naturally on plants and in the ground. Mankind used in the past and use now yeast for making miscellaneous foods (i.e. beer, bread) so we can come into contact with it principally with our consumed food products and beverages. It is generally accepted that SC is not a pathogen, but it has been suggested that in case of fungemia with SC (in patients with damaged/defective immune system) various organs can be affected and injured. The titer of pANCA does not change with the activity of the disease, does not depend on the therapy or the surgical intervention and but persists after resection of the affected part of the bowels. Similarly, the titer of the ASCA is stable irrespective of the activity of the disease and the drug-therapy. ASCA titers correlated with small bowel involvement and occurred particularly in cases of young adults with CD.
The aim of the study was to determine the prevalence of serological markers for GSE and IBD in patients with celiac disease (16 patients), UC (10 patients) and Crohn’s disease (42 patients) and to correlate the presence with the characteristics of these diseases.
MATERIALS AND METHODS
Patients
The entire cohort of patients with IBD (42 patients with CD and 10 patients with UC) and GSE (16 patients) from Debrecen, Hungary were enrolled in the study. Adult patients of both sexes were included. The diagnosis of CD, UC or GSE was made using the formally accepted criteria. The medical records for these patients have previously been reviewed by investigators and abstracted for patient characteristics. The blood samples for the study were collected between January 2000 and March 2000. Their sera were separated and stored at -70 °C. Determination of serum values was performed by individuals blinded to the clinical data for the patients in our Regional Immunology Laboratory.
Specific antibody measurement
Gliadin Glia-test from Diagnosticum Rt. (Budapest, Hungary) was warmed up to room temperature and the dilution of the diluents solution and the buffer solution was dispensed. For the measurement of specific IgG and IgA, the samples were diluted 1:500 (v/v). Micro standardization strips were incubated with one hundred microlitres of washing buffer, negative control, IgA positive control, IgG positive control, and the samples from the patients, for 30 min at 37 °C. After the incubation, the plate was washed three times with a Labsystems ELISA washer (Beverly, MA). Both of the anti-IgG and anti-IgA conjugate were diluted 1:200 in diluents solution (100 μL per well divided). Wells were again incubated for 30 min at 37 °C and washed three times with two hundred microlitres of washing buffer. Next step was incubation with 100 μL of TM B substrate buffer (protected from light) at room temperature for 30 min. Reaction was stopped with 50 μL 4N sulphuric acid per well. Optical density (OD) was determined at 450 nm. All chemical reagents were reagent grade, from Sigma unless otherwise stated.
Transglutaminase Greiner plates (N°655180) were incubated with transglutaminase, dissolved in PBS at 10 μg per milliliters, 50 μL per well, 16 hours at +4 °C. Binding sites were blocked by incubating with two hundred microlitres per well of 1% (w/v) bovine serum albumin (BSA) in PBS for 2 hours at 37 °C. 100 μL per well of each sample as incubated at room temperature for 2 hours. (For the measurement, the serum samples were diluted 1:100) Anti-human IgA/HRPO conjugate, diluted 1:4000 (v/v) in diluents solution (1% BSA-PBS) were incubated (100 μL per well) for 1 hour at room temperature. The color reaction was developed by adding a solution (containing 12.5 mL citrate-phosphate buffer pH5.0, 4.25 mg OPD, 5 μL of 30% H2O2). The enzymatic reaction was stopped after 15 min with 50 μL per well of 4N H2SO4. Optical density (OD) was determined at 492 nm. After each incubation step, wells were washed two times with PBS containing 0.05% (v/v) Tween 20 (PBS-T) during 10 minutes. Each sample was analyzed in duplicate. All chemical reagents were reagent grade, from Sigma unless otherwise stated.
Endomysium Indirect immunofluorescent method was developed in our laboratory. The substrate (umbilical cord) was incubated in PBS with sera (diluted 1:10 in diluent), washed twice with PBS for 10 min, then incubated with anti-human IgA/FITC and IgG/FITC, washed twice with PBS for 10 min. The preparates were coated with a mixture of glycerin/PBS (1:1) and covered with cover plate. Evaluation was interpreted with fluorescent microscopy. All chemical reagents were reagent grade, from Sigma.
ASCA IgG and IgA Medizym® ASCA IgA is an enzyme immunoassay for the quantitative determination of IgA antibodies to Saccharomyces cerevisiae in human serum. Autoantibodies of the diluted patient samples and calibrators reacted with mannan (cell surface component of baker’s yeast) immobilized on the solid phase of a microtiter plate. Following an incubation period of 60 min at 37 °C, unbound serum components are removed by a washing step. The bound antibodies react specifically with anti-human-IgA-antibodies conjugated to horseradish peroxidase (HRPO). Within the incubation period of 30 min at 37 °C, excessive conjugate was separated from the solid-phase immune complexes by the following washing step. Horseradish peroxidase converted the colorless substrate solution of 3, 3’, 5, 5’-tetramethylbenzidine (TMB) added into a blue product. This enzyme reaction was stopped by dispensing an acidic solution (H2SO4) into the wells after 10 min at room temperature turning the solution from blue to yellow. The optical density (OD) of the solution at 450 nm was directly proportional to the amount of specific antibodies bound. The standard curve was established by plotting the concentrations of the antibodies of the standards (x-axis) and their corresponding OD values (y-axis) were measured. The concentration of antibodies of the specimen was directly read off the standard curve.
ANCA The presence of ANCA was first screened by means of a fixed neutrophil enzyme-linked immunosorbent assay (ELISA). Methanol-fixed neutrophils were incubated with control and coded sera at 1:100 dilutions. Neutrophil-bound antibody was labeled by alkaline phosphatase-conjugated goat antihuman IgG. After the addition of p-nitrophenol, specific absorbance was measured at 405 nm. The cutoff for positivity was determined by positive controls from well-defined patients with UC. Indirect immunofluorescent staining was then performed on ANCA ELISA-positive samples to determine whether a predominantly perinuclear (pANCA) or cytoplasmic (cANCA) staining pattern was present. Methanol-fixed neutrophils on glass slides were incubated with the coded sera samples (1:20 dilution). Specific binding was visualized by fluorescence microscopy after the addition of fluorescein-labeled antihuman IgG. The specificity of the perinuclear staining pattern in UC was finally confirmed by its disappearance after DNase treatment of the neutrophils. Results were considered positive when both the ANCA titers were above the cutoff and the indirect immunofluorescence revealed a perinuclear binding of ANCA that disappeared after DNase treatment.
Statistical analysis
The relations were concluded from the evidences with statistical methods and summarized them in tables. Data was presented as percentages or mean values ± standard deviation (SD). The statistical package used in data interpretation (the two-tailed Student’s t-test, Pierce regression coefficient assay) was Statistica for Windows software (StatSoft, Inc., OK, U.S.A.).
RESULTS
Results are summarized in (Table 1, Table 2, Table 3, Figure 1, Figure 2, Figure 3, Figure 4). Twenty-seven patients with CD had only colonic localization (27/42), terminal ileitis was in 7 cases (7/42). Both the small and large bowels were affected in 8 cases (8/42). IgG type ASCA was found in 9 patients (1 colitis, 4 ileitis, 4 duodenitis-ileitis-colitis). IgA type ASCA was positive only in the patients with terminal ileitis (6/10) and in the patients with the duodenitis-ileitis-colitis type CD (10/15). The 27 patients with only colonic localization (27/42) had no IgA type ASCA positivity (Figure 1, Figure 2). All together, we detected that the increased IgG and IgA type ASCA titers, were mainly in patients with small bowel type CD (with the exception of one patient with colitis-type form with only IgG type ASCA) and celiac disease (Figure 3, Figure 4). IgG and IgA ASCA progressed together. IgA type ASCA values were between 0.35-938.19 U/mL (positive ≥ 20 U/mL). The prevalence of ASCA in patients with celiac disease outlined a possible role of anti-Saccharomyces cerevisiae antibodies in GSE. These findings outlined a noticeable startling resemblance, suggesting a possible kind of connection between CD and GSE.
Table 1.
ASCA positive patients in different diseases of bowels
| ASCA IgG+ | ASCA IgA+ | ASCA IgG+IgA+ | IgG vs IgA corr.(r) | |
| All patients (n = 68) | 5 | 4 | 10 | 0.75 |
| Crohn’s disease (n = 42) | 1 | 2 | 8 | 0.81 |
| Ulcerative colitis (n = 10) | 2 | 1 | 0 | -0.01 |
| Celiac disease (n = 16) | 2 | 1 | 2 | 0.50 |
| Controls (n = 20) | 0 | 0 | 0 | -0.18 |
Notes: It is clear that there was no ASCA positivity in the controls. ASCA positivity was common in patients with Crohn’s disease. The ASCA IgG- and IgA-type seropositivity was concomitant.
Table 2.
ASCA positive patients with different forms of Crohn’s disease
| Affected section of the bowels | ASCA IgG+ | ASCA IgA+ | ASCA IgG+IgA+ |
| Only the colon (n = 27) | 1 | 0 | 0 |
| Large and small bowels both (n = 8) | 0 | 0 | 4 |
| Only small bowels (n = 7) | 0 | 2 | 4 |
| Small bowel together (n = 15) | 0 | 2 | 8 |
Notes: Both the IgA and IgG type ASCA associated to the small bowels. P < 0.0004, ASCA IgG+ vs small bowels; P < 0.0001 ASCA IgA+ vs small bowels.
Table 3.
Summary of ASCA positive cases with Crohn’s disease
| Case: | ASCA IgG | ASCA IgA | ASCA IgG+IgA | Age (years) | Sex | Affected sections of gastrointestinal tract |
| 1. | + | 19 | Female | Terminal ileum | ||
| 2. | + | 46 | Male | Terminal ileum | ||
| 3. | + | 37 | Male | Terminal ileum | ||
| 4. | + | 38 | Female | Terminal ileum | ||
| 5. | + | 50 | Female | Colon | ||
| 6. | + | 29 | Male | From the duodenum to the rectum | ||
| 7. | + | 40 | Female | Terminal ileum | ||
| 8. | + | 24 | Male | Terminal ileum + colon | ||
| 9. | + | 49 | Female | From the duodenum to the rectum | ||
| 10. | + | 41 | Female | Terminal ileum | ||
| 11. | + | 27 | Male | Terminal ileum + colon |
Figure 1.
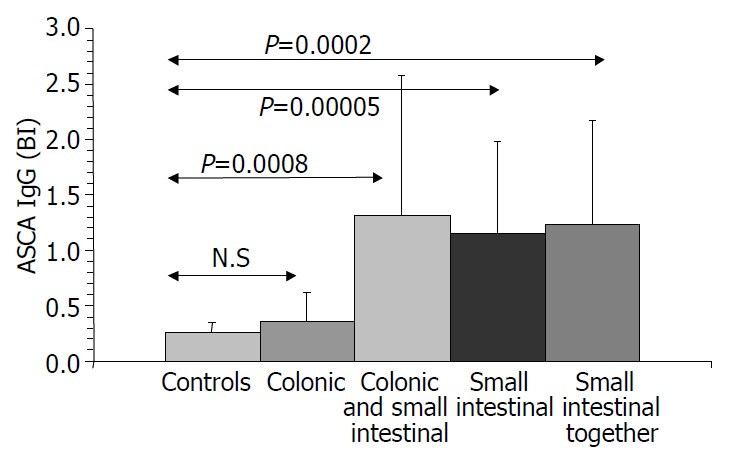
ASCA IgG values in Crohn’s disease.
Figure 2.
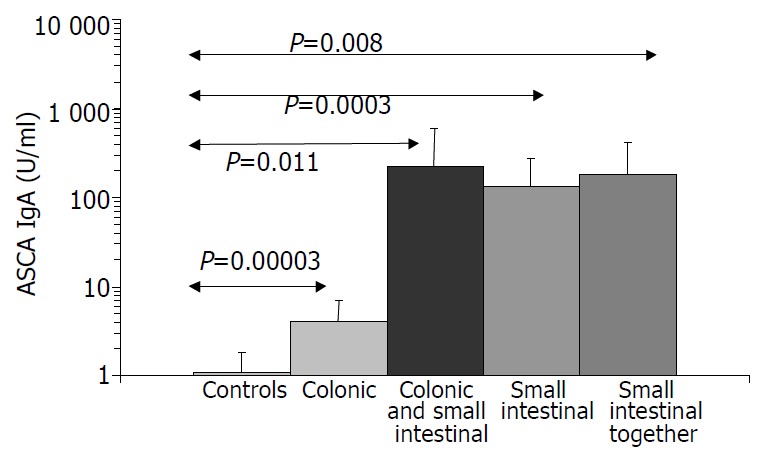
ASCA IgA values in Crohn’s disease.
Figure 3.
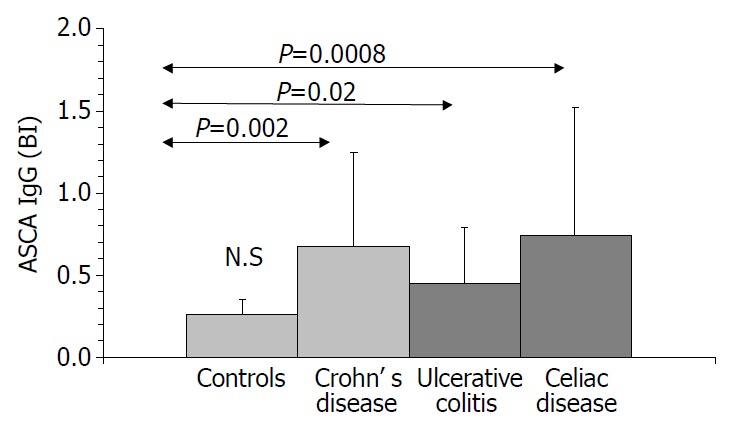
ASCA IgG values in inflammatory diseases of intestines.
Figure 4.
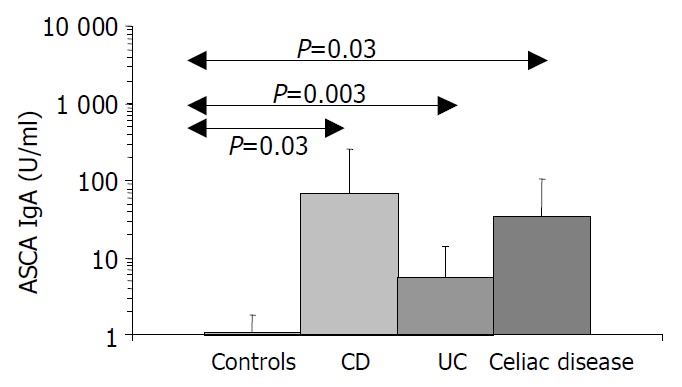
ASCA IgA values in inflammatory diseases of intestines. [The IgA type ASCA values are between 0.35-938.19 U/mL (positive: IgA ≥ 20 U/mL).]
DISCUSSION
Both celiac disease and Crohn’s disease are characterized by the presence of distinct (auto) antibodies. Based upon our results, theoretically and practically it is thinkable that ASCA positivity is not only a specific marker of Crohn’s disease but correlates with the (auto-) immune inflammation of the small intestines How does it happen?
Macrophages can produce proinflammatory cytokines (i.e. TNF-α) with direct and indirect microbicide activity. Several cell-wall components of Candida albicans were investigated in relation with the TNF-alfa secretion of macrophages and confirmed that they all had beta-1,2-oligomannoside[11,12]. The incubation with purified oligomannoside activated macrophages and they secreted TNF-alfa (in dose and molecule-size dependently) in vitro and in vivo[13,14]. Similar observations were demonstrated with Saccharomyces cerevisiae: the oligomannose structures influenced per interaction the cytokine network[15-17]. It is conceivable that miscellaneous fungus-oligosaccharides (their signal function in phytopathology is well-known) could play a key role in the regulation of human infections[18]. Oligomannosids of Saccharomyces cerevisiae with modification by ASCA can change their immunopathogenicity and trigger a process that results in specific inflammation such as CD.
The human gastrointestinal tract possesses a complex ecosystem, the components of which are multifaceted and metabolically diverse. Although the presence of intestinal microflora certainly contributes to the maintenance of human health, intestinal mucosa has the task, among others, of preventing the passage of commensal microflora and occasional pathogens to other compartments. To carry out such a function, the mucosa has to behave as a physical barrier but it also has to play an active role. Oral tolerance (OT) consists of the oral administration of antigens that could alter the response of the immune system[19]. This is a form of peripheral immune tolerance in which mature lymphocytes in the peripheral lymphoid tissues are rendered non functional or hyporesponsive by prior oral administration of antigens. The mechanisms by which OT is mediated include deletion or anergy and active cellular suppression. The primary factor determining which form of tolerance will be developed after oral administration of antigen is its dosage. Thus, it is thought that low doses of antigen can induce the generation of active suppression, via regulatory T cells in the gut-associated lymphoid tissue (GALT), which then migrate to the systemic immune system. These regulatory T cells produce down-regulatory cytokines such as IL-4, IL-10 and TGF-β in a Th2/Th3 cytokine pattern. Conversely, high dose of antigen favors anergy or clonal deletion. The phenomenon in which regulatory cells, as generated by oral tolerance, are primed in an antigen specific manner, but act in the respective microenvironment in a non-antigen specific manner is called bystander suppression. This phenomenon is of particular interest and can explain the use of oral/mucosal tolerance in T cell mediated autoimmune diseases such as rheumatoid arthritis and some diseases in which the autoantigen remains unknown or where there are reactivities to multiple autoantigens. T helper type 1 (Th1) lymphocytes secrete interleukin (IL)-2, interferon-γ and lymphotoxin-α, and stimulate type 1 immunity, which is characterized by intense phagocytic activity. Conversely, Th2 cells secrete IL-4, IL-5, IL-9, IL-10 and IL-13, and stimulate type 2 immunity, which is characterized by high antibody titers. Type 1 and type 2 immunity are not strictly synonymous with cell-mediated and humoral immunity, because Th1 cells also stimulate moderate levels of antibody production, whereas Th2 cells actively suppress phagocytosis. For most infections caused by large eukaryotic pathogens, type 1 immunity is protective, whereas type 2 responses assist with the resolution of cell-mediated inflammation. Severe systemic stress, immunosuppression, or overwhelming microbial inoculation causes the immune system to mount a type 2 response to an infection normally controlled by type 1 immunity. Macrophages also play a crucial role in the mucosal network as they must perform a number of diverse cellular functions that allow them to kill invading micro-organisms and neoplastic cells as well as produce growth factors involved in wound healing. Macrophages that develop these diverse functions arise from a common precursor. By a process of selective adaptation, the common precursor monocyte/macrophage differentiates into a distinctive macrophage with a different and specific phenotype, characterized by the expression of a specific set of gene products. The local environment plays a critical role in shaping or directing the pattern or pathway of macrophage differentiation: one pathway was believed to play a role in wound repair and characterized by the induction of insulin-like growth factor-1 (IGF-I) and a second pathway was involved in macrophage cytocidal activation and characterized by the induction of the inducible form of nitric oxide synthase (iNOS)[20,21].
IBDs are a group of diseases due to chronic inflammation of the gastrointestinal tract, but without proved etiology. IBD appears to be resulted from a dysregulated immune response with contributions from environmental, genetic, and bacterial factors. In the last decades, our understanding of the pathogenesis of IBD has greatly expanded but a better insight is needed into the environmental agents responsible for either initiation or perpetuation of IBD. The increasing attention given to the ecosystem of the gut may help define the antigens responsible for immune reactivity and provide opportunities toward application of antigen-specific therapeutic interventions such as induction of tolerance. Further investigation into probiotic agents and their mechanisms is especially appealing as a way to provide alternative therapies to decrease the inflammatory response. Antibodies to an oligomannose epitope of Saccharomyces cerevisiae demonstrated in 60%-70% of the patients with Crohn’s disease. The origin and clinicopathological role of ASCA have not been clarified. The sporadic information about ASCA positivity in patients suffering from gluten sensitive enteropathy in the literature suggests another occurrence.
We examined the ASCA’s occurrence in our patients and compared it with the clinical picture of the Crohn’s disease. The results supported the theory that ASCA positivity correlated with small intestinal Crohn’s disease and in these cases both IgG and IgA type antibodies were proved. The relatively high incidence of ASCA in GSE was unexplained but indicated further surveys to elucidate it as it was definitely more than accidental[22]. The antibodies in the sera of the analyzed ASCA positive cases proved a systemic immune response against Saccharomyces cerevisiae and suggested the end of the oral tolerance against the yeast’s antigens. The diet restriction (elemental diet, total parenteral nutrition, and fecal diversion) may ameliorate the status of the patients with Crohn’s disease. It can also be speculated that the yeast-free diet as a part of the therapy for the ASCA positive patients can be reasonable, moreover the permanent “forbidding” of the yeast can be an acceptable alternative in case of getting well.
Footnotes
Edited by Xu XQ and Wang XL
References
- 1.Scoglio R, Di Pasquale G, Pagano G, Lucanto MC, Magazzù G, Sferlazzas C. Is intestinal biopsy always needed for diagnosis of celiac disease? Am J Gastroenterol. 2003;98:1325–1331. doi: 10.1111/j.1572-0241.2003.07455.x. [DOI] [PubMed] [Google Scholar]
- 2.Barnes RM, Allan S, Taylor-Robinson CH, Finn R, Johnson PM. Serum antibodies reactive with Saccharomyces cerevisiae in inflammatory bowel disease: is IgA antibody a marker for Crohn's disease? Int Arch Allergy Appl Immunol. 1990;92:9–15. doi: 10.1159/000235217. [DOI] [PubMed] [Google Scholar]
- 3.Falchuk KR, Isselbacher KJ. Circulating antibodies to bovine albumin in ulcerative colitis and Crohn's disease. Characterization of the antibody response. Gastroenterology. 1976;70:5–8. [PubMed] [Google Scholar]
- 4.Hoffenberg EJ, Fidanza S, Sauaia A. Serologic testing for inflammatory bowel disease. J Pediatr. 1999;134:447–452. doi: 10.1016/s0022-3476(99)70202-7. [DOI] [PubMed] [Google Scholar]
- 5.Knoflach P, Park BH, Cunningham R, Weiser MM, Albini B. Serum antibodies to cow's milk proteins in ulcerative colitis and Crohn's disease. Gastroenterology. 1987;92:479–485. doi: 10.1016/0016-5085(87)90145-4. [DOI] [PubMed] [Google Scholar]
- 6.Main J, McKenzie H, Yeaman GR, Kerr MA, Robson D, Pennington CR, Parratt D. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 1988;297:1105–1106. doi: 10.1136/bmj.297.6656.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giaffer MH, Clark A, Holdsworth CD. Antibodies to Saccharomyces cerevisiae in patients with Crohn's disease and their possible pathogenic importance. Gut. 1992;33:1071–1075. doi: 10.1136/gut.33.8.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinton JF, Sendid B, Reumaux D, Duthilleul P, Cortot A, Grandbastien B, Charrier G, Targan SR, Colombel JF, Poulain D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barclay GR, McKenzie H, Pennington J, Parratt D, Pennington CR. The effect of dietary yeast on the activity of stable chronic Crohn's disease. Scand J Gastroenterol. 1992;27:196–200. doi: 10.3109/00365529208999948. [DOI] [PubMed] [Google Scholar]
- 10.Vidrich A, Lee J, James E, Cobb L, Targan S. Segregation of pANCA antigenic recognition by DNase treatment of neutrophils: ulcerative colitis, type I autoimmune hepatitis, and primary scle-rosing cholangitis. J Clin Immunol. 1995;15:293–299. doi: 10.1007/BF01541319. [DOI] [PubMed] [Google Scholar]
- 11.Domer J, Elkins K, Ennist D, Baker P. Modulation of immune responses by surface polysaccharides of Candida albicans. Rev Infect Dis. 1988;10 Suppl 2:S419–S422. doi: 10.1093/cid/10.supplement_2.s419. [DOI] [PubMed] [Google Scholar]
- 12.Trinel PA, Plancke Y, Gerold P, Jouault T, Delplace F, Schwarz RT, Strecker G, Poulain D. The Candida albicans phospholipomannan is a family of glycolipids presenting phosphoinositolmannosides with long linear chains of beta-1, 2-linked mannose residues. J Biol Chem. 1999;274:30520–30526. doi: 10.1074/jbc.274.43.30520. [DOI] [PubMed] [Google Scholar]
- 13.Janusz MJ, Austen KF, Czop JK. Isolation of a yeast heptaglucoside that inhibits monocyte phagocytosis of zymosan particles. J Immunol. 1989;142:959–965. [PubMed] [Google Scholar]
- 14.Jouault T, Lepage G, Bernigaud A, Trinel PA, Fradin C, Wieruszeski JM, Strecker G, Poulain D. Beta-1,2-linked oligomannosides from Candida albicans act as signals for tumor necrosis factor alpha production. Infect Immun. 1995;63:2378–2381. doi: 10.1128/iai.63.6.2378-2381.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heelan BT, Allan S, Barnes RM. Identification of a 200-kDa glycoprotein antigen of Saccharomyces cerevisiae. Immunol Lett. 1991;28:181–185. doi: 10.1016/0165-2478(91)90001-q. [DOI] [PubMed] [Google Scholar]
- 16.Sander U, Kunze I, Bröker M, Kunze G. Humoral immune response to a 200-kDa glycoprotein antigen of Saccharomyces cerevisiae is common in man. Immunol Lett. 1998;61:113–117. doi: 10.1016/s0165-2478(98)00003-0. [DOI] [PubMed] [Google Scholar]
- 17.Sendid B, Colombel JF, Jacquinot PM, Faille C, Fruit J, Cortot A, Lucidarme D, Camus D, Poulain D. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin Diagn Lab Immunol. 1996;3:219–226. doi: 10.1128/cdli.3.2.219-226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan CA. Oligosaccharide signals: from plant defense to parasite offense. Proc Natl Acad Sci USA. 1994;91:1–2. doi: 10.1073/pnas.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–181. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 20.Spellberg B, Edwards JE. Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 21.Winston BW, Krein PM, Mowat C, Huang Y. Cytokine-induced macrophage differentiation: a tale of 2 genes. Clin Invest Med. 1999;22:236–255. [PubMed] [Google Scholar]
- 22.Damoiseaux JG, Bouten B, Linders AM, Austen J, Roozendaal C, Russel MG, Forget PP, Tervaert JW. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies for inflammatory bowel disease: high prevalence in patients with celiac disease. J Clin Immunol. 2002;22:281–288. doi: 10.1023/a:1019926121972. [DOI] [PubMed] [Google Scholar]


