Abstract
AIM: To investigate telomerase activity and hTERT, TP-1 expression and their relationships in esophageal squamous cell carcinoma (ESCC).
METHODS: Telomerase activity was measured in 60 ESCC tissues using telomeric repeat amplification protocol (TRAP) assay by silver staining. In situ hybridization was used for detecting hTERT and TP-1mRNA.
RESULTS: The telomerase activity was detected in 83.3% of ESCC tissues. The difference of telomerase activity was significant between well and poorly cancer differentiated lesions (P < 0.05). The positive rate of telomerase activity was higher in patients with lymphatic metastasis than in patients without lymphatic metastasis. In cancer tissues hTERT mRNA expression was 75% and TP-1 mRNA expression was 71.7%. The expression of hTERT, TP-1 mRNA in well and poorly differentiated carcinoma was not significant. The expression of hTERT mRNA was correlated with telomerase activity, but TP-1 mRNA expression was not correlated with it.
CONCLUSION: Telomerase activity and hTERT, TP-1 mRNA expression are up-regulated in ESCC. Telomerase activity in ESCC is correlated with lymphatic metastasis and cancer differentiation. Telomerase activity may be used as a prognostic marker in ESCC. hTERT mRNA expression is correlated with telomerase activity. Enhanced hTERT mRNA expression may initially comprehend the telomerase activity level, but it is less sensitive than TRAP assay.
INTRODUCTION
Repetitive telomere sequences are present at the ends of eukaryotic chromosomes that protect the ends from damage and rearrangement. Progressive shortening of telomeric sequences is associated with cell division owing to the end replication involved in DNA replication. Telomerase is a special type of reverse transcriptase that stabilizes the telomeric ends of chomosome by adding TTAGGG repeats onto the chromosome ends. In humans, telomerase is composed of at least two components. hTR containing the template for reverse transcription[1], and telomerase associated proteins. Telomerase associated protein consists of hTP-1[2,3] and human telomerase reverse transcriptase (hTERT). hTERT is thought to be a enzyme’s catalytic subunit. In human, telomerase was active during embryonic development, and it was active in adult germ-line tissues, immortal cell[4,5] and most malignant tumors[6,7]. We have reported that telomerase activities were high both in esophageal squamous cell carcinoma (ESCC) and in their preneoplasia lesions[8]. This study was to examine telomerase activity, expression of hTERT mRNA, and hTP-1 mRNA in ESCC tissues and to analyze the relationship between teolmerase activity and its associated proteins.
MATERIALS AND METHODS
Materials
Esophageal squamous cell carcinoma tissues from 60 patients (40 men and 20 female, aged from 34 to 70 years) undergone surgical resection in Tumor Hospital of Medical College Shantou University from 1999 to 2001 were analyzed. All fresh tissues were taken immediately after operation and stored at -70 °C.
TRAP-silver staining for telomerase activity
We used TRAPEZE telomerase detection kit (Intergen Company). After addition of 10-20 μL telomerase assay lysis buffer (1 × CHAPS), the cells were lysed on ice. The lysate was incubated on ice for 30 min and then centrifuged at 12000 r/min for 20 min at 4 °C. The supernatant was collected and the protein concentration was determined by standard procedures (BCA protein assay). A volume of 0.6 μg protein equivalent was added to 48 μL reaction solution containing TRAP buffer, dNTP Mix, TS primer, RP primer, K1 primer and 2 units Taq polymerase. PCR condition was 33 cycles at 94 °C for 30 s, at 59 °C for 30 s. PCR products were loaded and run on 12.5% non-denaturing polyacrylamide gel. After electrophoresis, the gel was stained with silver.
In situ hybridization for hTERT and TP-1 mRNA expression
Samples were frozen and serially cut into 5 μm thick sections. One section was stained by hematoxylin and eosin (HE) for microscopic examination. Another was detected for hTERT and TP-1 mRNA expression. Expression of hTERT mRNA and hTP-1 mRNA was detected by digoxigenin-labeked gene probe which form a commercial kit (Boster Company, China) according to the manufacturer’s instructions. The hTERT oligonucleotides probes were 5’-AGTCAGGCTGGGCCTCAGAGAGCTGAGTAGGAAGG-3’, 5’-GCATGTACGGCTGGAGGTCTGTCAAGGTAGAGACG-3’, and 5’-AGCCAAGGTTCCAGGCAGCTCACTGACCCT-3’. The hTP-1 oligonucleotides probes were 5’-ATATCTGAGTGGGTAGATACATGCTGATGT-3’, 5’-GTCAGATAGACCAAGACAGTGCGGCCTGGCCTGGC-3’, and 5’-AGCCAAGGTTCCAGGCAGCTCACTGACCCT-3’. The positive expression showed brown staining signals in cytoplasm. The positive cancer cells constituted more than 75% of all cancer cells on the section were defined as a score of 3 + (strong), about 25%-75% of positive cells had a score of 2 + (moderate), and less than 25% had a score of 1 + (weak). The score of - (negative) had no positive cancer cell.
Controls
TRAP telomerase activity analysis: Esophageal cancer cell line EC109 was used as positive telomerase control, which was identified to be telomerase positive by our laboratory. Negative control was to perform a TRAPEZE kit assay with 1 × CHAPS lysis buffer substituted for the cell extract. Heat-treatment of each sample by incubating at 85 °C for 20 min prior to TRAPEZE kit assay to inactivate telomerase served as a heat inactivation control. The TRAPEZE primer Mix contains internal control, which produce a 36 bp band (S-IC) in every lane to monitor PCR inhibition. If the extract with telomerase activity, a ladder of products with 6 bp increment starting at 50 bp nucleotide and a 36 bp internal control band could be seen. If the extract was telomerase negative, there was only a 36 bp internal control band (S-IC) (Figure 1).
Figure 1.
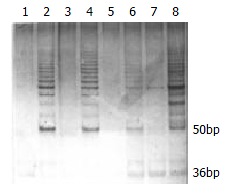
Carcinoma telomerase activity detected by TRAP-silver assay and separated on a 12.5% polyacrylamide gel. Lane 1: primer-dimer/PCR contamination control, Lane 2: telomerase positive control, Lanes 4, 6 and 8: telomerase positive samples, Lanes 3, 5 and 7: heat-treated controls.
In situ hybridization: Positive control section was provided with the kit. Negative control included using incubation solution instead of the probe or the sections digested with ribonucleases (RNase) (10 mg•L-1) before hTERT or hTP-1 detection.
Statistical analysis
Statistical significance was tested using rectified χ² test and exact method.
RESULTS
Relationship between telomerase activity and cancer differentiation
Histopathologically, the study population (n = 60) was divided into 3 categories according to cancer differentiation as follows: grade I (n = 15), grade II (n = 33), and grade III (n = 12). In 60 cases of esophageal SCC, telomerase activity was detected in 50 cases. The positive rate was 83.3%. As showed in Table 1, the positive rates of telomerase activity were low in grade I and progressively increased from grade I to grade III. The significant difference of telomerase activity rate was found between grade I and grade II (χ² = 4.597, P < 0.05), while, there was no significant differences between grade II and grade III (χ² = 0.263, P > 0.05).
Table 1.
Relationship between differentiation degree and telomerase activity in 60 cases of ESCC
| Tumor stage | Sample (n) | Positive (n) | Positive rate (%) | P value |
| I | 15 | 9 | 60 | |
| II | 33 | 30 | 90.9 | P < 0.05 |
| III | 12 | 11 | 91.6 |
Relationship between telomerase activity and lymphatic metastasis
There were 41 patients with lymph node metastasis out of the 60 patients. Telomerase activity rates were higher in patients with lymphatic metastasis (90.2%) than in those without (63.2%) (Table 2), and this difference was significant (χ² = 4.68, P < 0.05).
Table 2.
Relationship between telomerase activity and lymphatic metastasis
| Sample (n) | Lymphatic metastasis |
Telomerase activity |
P value | |
| + | - | |||
| 41 | + | 37 | 4 | P < 0.05 |
| 19 | - | 12 | 7 | |
Expression of hTERT and hTP-1 mRNA in esophageal squamous cell cacinoma
The positive rates of hTERT and hTP-1 mRNA expression were 75% and 71.7% respectively. The expression of hTERT and hTP-1 in well-differentiated carcinoma was limited to the basaloid cell nests (Figure 2). In the poorly-differentiated carcinoma, most tumor cells showed diffuse or occasionally focal expression (Figure 3). As showed in Table 3, the positive intensity and the positive rate of hTERT and hTP-1 expression progressively increased from grade I to grade III, but no significant differences existed between them (hTERT: χ² = 4.95, P > 0.05; TP-1: χ² = 3.49, P > 0.05).
Figure 2.
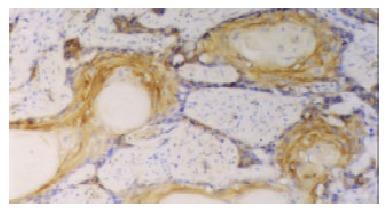
Esophageal squamous cell carcinoma (grade I). expression of hTERT mRNA was limited to the basaloid cell nests (40 ×).
Figure 3.
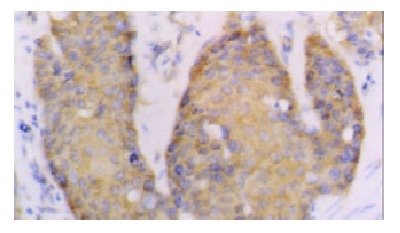
Poorly-differentiated esophageal squamous cell carcinoma. The expression of TP-1 mRNA was diffuse in cancer cells (40 ×).
Table 3.
Intensity of hTERT and hTP-1 mRNA expression in esophageal carcinoma tissues
| Tumor stage |
hTERT |
P value |
hTP-1 |
P value | |||||||||
| n | - | + | ++ | +++ | Positive rate (%) | - | + | ++ | +++ | Positive rate (%) | |||
| I | 15 | 7 | 4 | 3 | 1 | 53.3 | 7 | 5 | 3 | 0 | 53.3 | ||
| II | 33 | 6 | 7 | 11 | 9 | 81.8 | P > 0.05 | 8 | 6 | 12 | 7 | 75.8 | P > 0.05 |
| III | 12 | 2 | 2 | 4 | 4 | 83.3 | 2 | 2 | 5 | 3 | 83.3 | ||
Relationship between hTERT, hTP-1 expression and telomerase activity
Relationship between hTERT and hTP-1 expression and telomerase activity are summarized in Table 4. By statistical analyses, the expression of hTERT mRNA was correlated with telomerase activity (χ² = 5.76, P < 0.05), but the expression of hTP-1 mRNA was no correlated with telomerase activity (χ² = 1.64, P > 0.05).
Table 4.
Relationship between hTERT, hTP-1 expression and telomerase activity
| Telomerase activity |
hTERT |
P value |
TP-1 |
P value | ||
| + | - | + | - | |||
| + | 41 | 9 | P < 0.05 | 38 | 12 | P > 0.05 |
| - | 4 | 6 | 5 | 5 | ||
DISCUSSION
Telomerase is a ribonucleoprotein reverse transcriptase that utilizes its own RNA template for the addition of telomeric sequences to chromosomal ends in order to maintain telomeric length. In vivo and in vitro studies suggested that telomerase was associated with cellular immortality and malignance, indicating that activation of telomerase might play an important role in tumorigenesis and immortalization. Telomerase activation has been demonstrated in many types of human tumors, including tumors in breast[9], nasopharynx[10,11], stomach[12-14], prostate[15], urinary bladder[16], skin[17], cervix[18], lung[19,20] and brain[21]. Some scientists suggested that telomerase activity was a diagnostic and prognostic marker for malignant tumors[22,23]. Previous research suggested that the positive rate of telomerase activity in esophageal carcinoma was 79%-87%, but there were different conclusions regarding the relationship between telomerase activity and cancer differentiation in esophageal cacinoma. Asai et al[24] reported that well-differentiated cancer had higher detectable telomerase activity, but Ikeguchi et al[25] found the opposite result. Zhao et al[26] concluded that telomerase activity had no correlation with cancer differentiation. Our present study results suggested that the detectable rate of telomerase activity was gradually increased from well-differentiated carcinoma to poorly-differentiated one. The difference between grade I and grade II was significant, while the difference between grade II and grade III was not significant. This result gave more evidences that telomerase activity was correlated with differentiation in esophageal SCC. The presence of telomerase activity in esophageal SCC with lymphatic metastasis suggested that the telomerase activity in patients with lymphatic metastasis was higher than that in patients without lymphatic metastasis.
Collins et al[27,28] first purified tetrahymena telomease protein p80. The p80 components could be specifically cross-linked to telomerase RNA. Then, the mammalian (mouse and human) homology of p80 was found, and termed telomerase-associated protein 1 (TP-1)[29]. The sequence of TP-1 showed most homology to tetrahymena p80. In vitro experiments suggested that TP-1 interacted specifically with telomerase RNA and that TP-1 was associated with telomerase activity. However, expression of TP-1 did not reflect the level of telomerase activity[30-32]. In the present study, we found that TP-1 expression was high in esophageal squamous cell carcinoma, but it was not correlated with telomerase activity. The specific mechanism by which the protein participated in telomerase function has not been defined.
Two related proteins, Est2p from the yeast Saccharomyces cerevisiae and p123 from the ciliate Euplotes aediculatus, have been identified as the catalytic subunits of telomerase in their respective species[33-36]. Est2 was first identified as a gene required for telomere maintenance in yeast[37] and was essential for telomerase activity. Then, a human gene, hEST2/hTERT (human telomerase reverse transcriptase), sharing significant sequence similarity with telomerase catalytic subunit genes of lower eukaryotes was cloned and the protein was identified[38]. Studies suggested that hTERT expression was correlated with telomerase activity. The expression of hTERT was up-regulated concomitantly with the activation of telomerase during the immortalization of cultured cells and down-regulated during in vivo cellular differentiation[39]. In vitro, changes in the sequence of hTERT amino acid might reduce telomerase activity, and transfer of hTERT into normal human cell might resurrect telomease[40]. Designing a ribozyme targeting hTERT also reduced telomerase activity[41]. However, some studies revealed that the levels of hTERT expression and detectable telomerase activity were not concomitant[42-48]. The present experiment suggested that hTERT mRNA expression was correlated with telomeras activity in esophageal SCC. But we found that the positive rate of hTERT mRNA expression was lower than detectable telomerase activity. The positive intensity and the positive rate of hTERT expression were progressively increased from grade I to grade III, but the differences between them was not significant. In 50 detectable telomerase activity cases, just 41 cases expressed hTERT mRNA, 9 cases had negatively expression of hTERT mRNA. There were 4 hTERT positive cases without detectable telomerase activity. It indicated that hTERT gene expression was an important factor to resurrect telomease, but not the only one. There was a possibility that other mechanisms were intervened to modulate telomerase activity. The reasons why the tissue had expression of hTERT mRNA without detectable telomerase activity are not clear. Following might be the explanations. Posttranscriptional modification of telomerase subunits would modulate telomerase activity, and there were some inhibitors in telomerase extract solution to reduce its activity. Using in situ hybiridization assay detected hTERT expression might initially comprehend telomerase activity level. However, the result of hTERT expression and detectable telomerase activity were not identical. It was less sensitive than TRAP assay.
In summary, detectable telomerase activity and telomerase subunit expression are high in esophageal squamous cell carcinoma. Telomerase activity is related to tumor differentiation and lymphatic metastasis, which may provide a new marker for evaluating the prognosis of patients with esophageal SCC. Expression of hTERT mRNA is correlated with telomerase activity, but the expression of TP-1 mRNA is not correlated with telomerase activity.
Footnotes
Edited by Zhang JZ and Wang XL
Supported by the Medical Science Research Foundation of Guangdong Province, No.A2001413
References
- 1.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 2.Nakayama J, Saito M, Nakamura H, Matsuura A, Ishikawa F. TLP1: A gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell. 1997;88:875–884. doi: 10.1016/s0092-8674(00)81933-9. [DOI] [PubMed] [Google Scholar]
- 3.Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]
- 4.Shen ZY, Xu LY, Chen MH, Shen J, Cai WJ, Zeng Y. Progressive transformation of immortalized esophageal epithelial cells. World J Gastroenterol. 2002;8:976–981. doi: 10.3748/wjg.v8.i6.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen ZY, Xu LY, Li EM, Cai WJ, Chen MH, Shen J, Zeng Y. Telomere and telomerase in the initial stage of immortalization of esophageal epithelial cell. World J Gastroenterol. 2002;8:357–362. doi: 10.3748/wjg.v8.i2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 7.Hiyama E, Hiyama K. Clinical utility of telomerase in cancer. Oncogene. 2002;21:643–649. doi: 10.1038/sj.onc.1205070. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Liang Y, Wu M, Xu L, Cai W. Telomerase activity analysis of esophageal carcinoma using microdissection-TRAP assay. Chin Med J ( Engl) 2002;115:1405–1408. [PubMed] [Google Scholar]
- 9.Hiyama E, Gollahon L, Kataoka T, Kuroi K, Yokoyama T, Gazdar AF, Hiyama K, Piatyszek MA, Shay JW. Telomerase activity in human breast tumors. J Natl Cancer Inst. 1996;88:116–122. doi: 10.1093/jnci/88.2.116. [DOI] [PubMed] [Google Scholar]
- 10.Cheng RY, Yuen PW, Nicholls JM, Zheng Z, Wei W, Sham JS, Yang XH, Cao L, Huang DP, Tsao SW. Telomerase activation in nasopharyngeal carcinomas. Br J Cancer. 1998;77:456–460. doi: 10.1038/bjc.1998.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsao SW, Zhang DK, Cheng RY, Wan TS. Telomerase activation in human cancers. Chin Med J ( Engl) 1998;111:745–750. [PubMed] [Google Scholar]
- 12.Hiyama E, Yokoyama T, Tatsumoto N, Hiyama K, Imamura Y, Murakami Y, Kodama T, Piatyszek MA, Shay JW, Matsuura Y. Telomerase activity in gastric cancer. Cancer Res. 1995;55:3258–3262. [PubMed] [Google Scholar]
- 13.Yakoob J, Hu GL, Fan XG, Zhang Z. Telomere, telomerase and digestive cancer. World J Gastroenterol. 1999;5:334–337. doi: 10.3748/wjg.v5.i4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhan WH, Ma JP, Peng JS, Gao JS, Cai SR, Wang JP, Zheng ZQ, Wang L. Telomerase activity in gastric cancer and its clinical implications. World J Gastroenterol. 1999;5:316–319. doi: 10.3748/wjg.v5.i4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommerfeld HJ, Meeker AK, Piatyszek MA, Bova GS, Shay JW, Coffey DS. Telomerase activity: A prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56:218–222. [PubMed] [Google Scholar]
- 16.Lin Y, Miyamoto H, Fujinami K, Uemura H, Hosaka M, Iwasaki Y, Kubota Y. Telomerase activity in human bladder cancer. Clin Cancer Res. 1996;2:929–932. [PubMed] [Google Scholar]
- 17.Taylor RS, Ramirez RD, Ogoshi M, Chaffins M, Piatyszek MA, Shay JW. Detection of telomerase activity in malignant and nonmalignant skin conditions. J Invest Dermatol. 1996;106:759–765. doi: 10.1111/1523-1747.ep12345811. [DOI] [PubMed] [Google Scholar]
- 18.Zhang DK, Ngan HY, Cheng RY, Cheung AN, Liu SS, Tsao SW. Clinical significance of telomerase activation and telomeric restriction fragment (TRF) in cervical cancer. Eur J Cancer. 1999;35:154–160. doi: 10.1016/s0959-8049(98)00303-7. [DOI] [PubMed] [Google Scholar]
- 19.Wu TC, Lin P, Hsu CP, Huang YJ, Chen CY, Chung WC, Lee H, Ko JL. Loss of telomerase activity may be a potential favorable prognostic marker in lung carcinomas. Lung Cancer. 2003;41:163–169. doi: 10.1016/s0169-5002(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 20.Hiyama K, Hiyama E, Ishioka S, Yamakido M, Inai K, Gazdar AF, Piatyszek MA, Shay JW. Telomerase activity in small-cell and non-small-cell lung cancers. J Natl Cancer Inst. 1995;87:895–902. doi: 10.1093/jnci/87.12.895. [DOI] [PubMed] [Google Scholar]
- 21.Sano T, Asai A, Mishima K, Fujimaki T, Kirino T. Telomerase activity in 144 brain tumours. Br J Cancer. 1998;77:1633–1637. doi: 10.1038/bjc.1998.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YL, Zhang ZS, Wu BP, Zhou DY. Early diagnosis for colorectal cancer in China. World J Gastroenterol. 2002;8:21–25. doi: 10.3748/wjg.v8.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asai A, Kiyozuka Y, Yoshida R, Fujii T, Hioki K, Tsubura A. Telomere length, telomerase activity and telomerase RNA expression in human esophageal cancer cells: correlation with cell proliferation, differentiation and chemosensitivity to anticancer drugs. Anticancer Res. 1998;18:1465–1472. [PubMed] [Google Scholar]
- 25.Ikeguchi M, Kaibara N. [Telomerase activity in esophageal squamous cell carcinoma and in normal esophageal epithelium adjacent to carcinoma] Nihon Rinsho. 1998;56:1176–1180. [PubMed] [Google Scholar]
- 26.Zhao CF, Chen CL. Detection of telomerase activity in tumor tissues from patients with esophageal carcinoma. Chin J Cancer. 2000;19:131–133. [Google Scholar]
- 27.Collins K, Kobayashi R, Greider CW. Purification of Tetrahymena telomerase and cloning of genes encoding the two protein components of the enzyme. Cell. 1995;81:677–686. doi: 10.1016/0092-8674(95)90529-4. [DOI] [PubMed] [Google Scholar]
- 28.Kickhoefer VA, Stephen AG, Harrington L, Robinson MO, Rome LH. Vaults and telomerase share a common subunit, TEP1. J Biol Chem. 1999;274:32712–32717. doi: 10.1074/jbc.274.46.32712. [DOI] [PubMed] [Google Scholar]
- 29.Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]
- 30.Harrington L, Zhou W, McPhail T, Oulton R, Yeung DS, Mar V, Bass MB, Robinson MO. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997;11:3109–3115. doi: 10.1101/gad.11.23.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakayama J, Saito M, Nakamura H, Matsuura A, Ishikawa F. TLP1: A gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell. 1997;88:875–884. doi: 10.1016/s0092-8674(00)81933-9. [DOI] [PubMed] [Google Scholar]
- 32.Saito T, Matsuda Y, Suzuki T, Hayashi A, Yuan X, Saito M, Nakayama J, Hori T, Ishikawa F. Comparative gene mapping of the human and mouse TEP1 genes, which encode one protein component of telomerases. Genomics. 1997;46:46–50. doi: 10.1006/geno.1997.5005. [DOI] [PubMed] [Google Scholar]
- 33.Counter CM, Meyerson M, Eaton EN, Weinberg RA. The catalytic subunit of yeast telomerase. Proc Natl Acad Sci USA. 1997;94:9202–9207. doi: 10.1073/pnas.94.17.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lingner J, Cech TR, Hughes TR, Lundblad V. Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc Natl Acad Sci USA. 1997;94:11190–11195. doi: 10.1073/pnas.94.21.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 36.Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 37.Lendvay TS, Morris DK, Sah J, Balasubramanian B, Lundblad V. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics. 1996;144:1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 39.Counter CM, Meyerson M, Eaton EN, Ellisen LW, Caddle SD, Haber DA, Weinberg RA. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–1222. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- 40.Ramakrishnan S, Eppenberger U, Mueller H, Shinkai Y, Narayanan R. Expression profile of the putative catalytic subunit of the telomerase gene. Cancer Res. 1998;58:622–625. [PubMed] [Google Scholar]
- 41.Hao ZM, Luo JY, Cheng J, Wang QY, Yang GX. Design of a ribozyme targeting human telomerase reverse transcriptase and cloning of it's gene. World J Gastroenterol. 2003;9:104–107. doi: 10.3748/wjg.v9.i1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–4172. [PubMed] [Google Scholar]
- 43.Liu K, Schoonmaker MM, Levine BL, June CH, Hodes RJ, Weng NP. Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. Proc Natl Acad Sci USA. 1999;96:5147–5152. doi: 10.1073/pnas.96.9.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hara T, Noma T, Yamashiro Y, Naito K, Nakazawa A. Quantitative analysis of telomerase activity and telomerase reverse transcriptase expression in renal cell carcinoma. Urol Res. 2001;29:1–6. doi: 10.1007/s002400000156. [DOI] [PubMed] [Google Scholar]
- 45.Kameshima H, Yagihashi A, Yajima T, Kobayashi D, Hirata K, Watanabe N. Expression of telomerase-associated genes: reflection of telomerase activity in gastric cancer. World J Surg. 2001;25:285–29, iv. doi: 10.1007/s002680020046. [DOI] [PubMed] [Google Scholar]
- 46.Zhang RG, Guo LX, Wang XW, Xie H. Telomerase inhibition and telomere loss in BEL-7404 human hepatoma cells treated with doxorubicin. World J Gastroenterol. 2002;8:827–831. doi: 10.3748/wjg.v8.i5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yajima T, Yagihashi A, Kameshima H, Kobayashi D, Hirata K, Watanabe N. Telomerase reverse transcriptase and telomeric-repeat binding factor protein 1 as regulators of telomerase activity in pancreatic cancer cells. Br J Cancer. 2001;85:752–757. doi: 10.1054/bjoc.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tominaga T, Kashimura H, Suzuki K, Nakahara A, Tanaka N, Noguchi M, Itabashi M, Ohkawa J. Telomerase activity and expression of human telomerase catalytic subunit gene in esophageal tissues. J Gastroenterol. 2002;37:418–427. doi: 10.1007/s005350200061. [DOI] [PubMed] [Google Scholar]


