Abstract
AIM: After transarterial chemoembolization (TACE), the residual cancer cells are under extensive hypoxic or even anoxic environment. Hypoxia can lead to adaptive responses. For example, angiogenesis will help these cells survive. In this study, we examined the effect of TACE on angiogenesis and expression of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (b-FGF) and to assess their relevance to Walker-256 transplanted hepatoma.
METHODS: Male Wistar rats were inoculated with Walker-256 tumor in the left lobe of liver. Angiography and transarterial chemoembolization were performed at d14 after transplantation. Sixty rats bearing walker-256 transplanted hepatoma were randomly divided into control group, arterial infusion group and TACE group. Each group consisted of twenty rats. Normal saline, 5-FU, 5-FU and lipiodol were infused through hepatic artery respectively. Two weeks after the infusion, staining of factor VIII, VEGF and b-FGF was performed by immunohistochemistry method in routine paraffin-embeded sections. Microvessel density (MVD) was counted in endothelial cells with positive factor VIII. Their expression levels were analyzed in conjunction with the pathologic features.
RESULTS: While a smaller tumor volume was found in TACE group (F = 37.818, P < 0.001), no statistical differences between MVD and expression of VEGF and b-FGF were found among the 3 groups. MVD of the control group, chemotherapy group and chemoemoblization group was 80.84 ± 24.24, 83.05 ± 20.29 and 85.20 ± 23.91 (F = 0.193, P = 0.873), respectively. The positive expression of VEGF and b-FGF was 75%, 75%, 85% (χ² = 0.449, P = 0.799) and 30%, 25%, 30% (χ² = 0.141, P = 0.922), respectively. Statistical analysis revealed a positive correlation between the expression of VEGF and MVD (r = 0.552, P < 0.001).
CONCLUSION: There has been little influence of lipiodol chemoembolization on the formation of tumor angiogenesis, but the development of neovascularization and expression of VEGF play important roles in establishment of collateral circulation and reconstruction of blood supply of residual cancer tissue.
INTRODUCTION
Transarterial chemoembolization (TACE) has been widely practiced in the treatment of unresectable hepatocellular carcinoma (HCC)[1-5], but long-term TACE therapy was as yet unsatisfactory[6-8]. Histopathologic examination showed although TACE could induce significant necrosis, yet complete tumor necrosis was rare, the residual tumor cells remained viable in the peripheral region, which may play an important role in local recurrence or as a source of metastasis affecting the long-term efficacy of TACE[9-11].
Tissue in hypoxic environment is common in both experimental and human solid tumor[12]. Hypoxia cells have reduced metabolic rate, reduced transcription and translation, even cell cycle arrest. Hypoxia can inhibit cell division or even lead to cell death[13]. But body compensatory reaction can also lead to a wide range of responses including angiogenesis, anti-apoptosis gene expression and changes in metabolic rate at both systemic and cellular levels[14,15]. After TACE, the tumor cells are under extremely hypoxic or even anoxic environment. It is possible that some adaptive responses to hypoxia will help these cells survive, but to our knowledge, studies regarding these are very few.
Recent studies revealed that TACE might enhance the expression of VEGF in meningiomas[16,17]. In the study of hepatoma, TACE could enhance the expression of VEGF[18-21], but not the MVD level[22] or expression of b-FGF[19,22]. All these studies were retrospective.
Walker-256 transplanted hepatoma is a useful model for the study of cancer therapy[20,23,24]. In hepatic arteriography, the tumor is usually hypervascular and receives its blood supply almost exclusively from hepatic artery just as that seen in human HCC[23]. Our study was designed to observe the influence of TACE on the *expression of angiogenesis, VEGF and b-FGF and to assess their relevance to Walker-256 transplanted hepatoma.
MATERIALS AND METHODS
Walker-256 tumor inoculation into the rat livers
Walker-256 carcinoma cells (Cell Preserve Center, Wuhan University, Wuhan, P.R. China) were inoculated subcutaneously in intact rats. Male Wistar rats (200-250 g) were anesthetized with pentobarbital sodium (30 mg/kg ip), then the tumor was inoculated as described in our previous study[23]. Walker-256 carcinoma cells were inoculated into the subcapsular parenchyma of the left lobe of liver.
Experimental methods
Fourteen days after transplantion, a second laparotomy was performed. A 0.6 mm polyethylene catheter was inserted into the gastroduodenal artery and fixed by a suture. The common hepatic artery and right hepatic artery were temporarily ligated during injection of the drugs. In this study, sixty rats were randomly divided into three groups, twenty rats each group (Table 1).
Table 1.
Drug administration in the three groups
| Group | Drug administration |
| Control | 0.3 mL normal saline |
| Arterial infusion (AI group) | 5-fluorouracil (5-FU) 20 mg/kg |
| Transarterial chemoembolization (TACE group) | lipiodol ultra-fluis (Andre Guerbet Laboratories, Aulnay-Sous-Bios, France) 0.5 mL/kg and 5-FU 20 mg/kg |
Tumor volume and degree of tumor necrosis
MR images were performed on 1.5-Tesla system (Siemens Vision, Siemens, Germany) supplemented with a cervical coil. T1-weighted (TR/TE, 450/12 msec) and T2-weighted (TR/TE, 2800/96 msec) transverse SE images (slice thickness 2 mm) were obtained by acquisition times of 7:25 min and 6:16 min, respectively.
The tumor volume was determined by MR measurements of the largest and smallest diameters and calculated according to the following formula: Tumor volume (mm3) = largest diameter (mm) × [smallest diameter (mm)]2/2.
Fourteen days after TACE, hepatoma specimens were obtained and fixed in 10% formalin for 12 h, then embedded in paraffin according to routine procedures. The histological sections were stained with hematoxylin-eosin (HE) for observation under light microscopy and measurement of the extent of tumor necrosis.
Immunohistochemistry
Immunohistochemistry was performed in sections from a selected block of each specimen. 5-μm thick sections mounted on 3-aminopropyltrietoxysilane-coated slides were dewaxed in xylene and rehydrated at graded concentrations of alcohol. Endogenous peroxide was blocked with 0.6% H2O2 for 20 min. Then the sections were pretreated in a microwave oven in citrate buffer for 15 min at 95 °C. Thereafter, the slides were processed according to the standard methods with ABC kit. The primary antibody used was polyclonal antibody of von-Willebrand factor at dilution of 1:100, polyclonal antibodies to VEGF and b-FGF at dilution of 1:50. All these antibodies were from Santa Cruz Biotechnology; Santa Cruz, Calif USA. Diaminobenzine was used for the coloration development. Negative controls were generated by substituting the primary antibody with distilled water.
Determination of MVD
The tumors were frequently inhomogeneous in their microvessel density. For determination of MVD, the five most vascular areas within a section were chosen and counted under a light microscope with a 200-fold magnification as described by Weidner[25]. A brownstain structure clearly separated from the adjacent microvessels was regarded as a single countable microvessel. The average count was recorded as MVD for each case.
Determination of VEGF and b-FGF expression
The counting of VEGF and b-FGF immunoreactive cells was made by scanning and scoring of five high-power fields using 400 magnification. Positive VEGF and b-FGF were recognized as intensely stained in tumor cell cytoplasm.
The expression of VEGF was semi-quantitatively evaluated as follows. Those having positive staining less than 5% were regarded as -, between 5% and 15% as +, between 15% and 50% as 2+, and greater than 50% as 3+. The b-FGF immunostaining was evaluated by a qualitative method as follows. Positive staining less than 10% was regarded as negative, greater than 10% as positive.
Statistical analysis
Statistical analysis was carried out by SPSS packed program. Variance tests, χ² tests, Mann-Whitney test and linear regression test were used. P value < 0.05 based on a two-tailed test was considered statistically significant.
RESULTS
Histopathology findings in tumor
Hematoxylin-eosin (H&E) stained sections of the liver specimens showed poorly-differentiated carcinomas, which were apparently spherical or ovoid in shape. The tumor cells were arranged irregularly with hyperchromatosis, polymorphism and numerous mitoses. The mass was sharply demarcated from the surrounding normal parenchyma, its capsule was thin and composed of collagen fibers caused by tumor compression. The tumor showed inhomogeneous hypervascularization consisting mainly of small arteries and capillaries. Satellite nodules or portal vein tumor thrombus could be seen in some rats.
In TACE group, examination of the sections showed extensive central necrosis of the tumor in all cases. The central necrosis enlarged to moderate (16/20) or severe degree (4/20). Intra-tumoral hemorrhage or bile stases were seen in some, but the tumor cells tended to remain viable at the periphery of the nodules 2 wk after embolization therapy. We also found thick fibrous capsules with chronic inflammatory and cells infiltration. Various degrees of degeneration and necrosis were seen in normal hepatic parenchyma adjacent to the tumor. In the control and AI groups, the interior of the tumor had many viable tumor cells with spotty and scattered necrosis, the capsules were thin and composed of collagen fibers. Tumor volume and tumor growth rate before and after therapy are listed in Table 2.
Table 2.
Tumor volumes and tumor growth rate before and after therapy (¯x ± s)
| Group |
Tumor volume (mm3) |
Tumor growth rate (%) | |
| Before therapy | 2 wk after therapy | ||
| Control | 195.6 ± 65.9 | 963.6 ± 214.8 | 459.2 ± 52.7 |
| AI | 218.9 ± 48.7 | 868.9 ± 179.8 | 412.2 ± 18.9 |
| TACE | 210.2 ± 59.2 | 356.5 ± 78.4 | 173.4 ± 20.4 |
| F value | 0.272 | 37.818 | 197.932 |
| P value | 0.764 | < 0.001 | < 0.001 |
Microvessel counts
The von Willebrand Factor (Factor-VIII) antibody gave an intense staining of the vascular endothelial cells (Figure 1). A substantial inhomogeneity in microvessel counts was found in different areas in the same section. In TACE group, the positive factor VIII cells were found focusing in the newly formed tumor. The MVD of each group is shown in Table 3.
Figure 1.
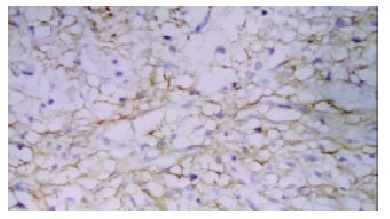
Positive factor-VIII endothelial cells in tumor nest (SABC × 100).
Table 3.
MVD counts, expression of VEGF and b-FGF in each group
| Group | n | MVD (¯x ± s) |
VEGF |
b-FGF |
|||||
| 0 | + | ++ | +++ | Mean | 0 | ||||
| Control | 20 | 80.80 ± 20.94 | 5 | 8 | 5 | 2 | 1.2 | 14 | 6 |
| AI | 20 | 83.55 ± 18.26 | 5 | 7 | 6 | 2 | 1.25 | 15 | 5 |
| TACE | 20 | 85.20 ± 23.91 | 3 | 9 | 5 | 3 | 1.4 | 14 | 6 |
| Statistic value | F = 0.193 | χ² = 0.449 | χ² = 0.141 | ||||||
| P value | P = 0.873 | P = 0.799 | P = 0.922 | ||||||
VEGF protein expression and its correlation with MVD
Immunohistochemical staining of VEGF showed strong immunoreactivity in tumor cells and vascular endothelial cells within the tumor tissues (Figure 2). In contrast, non-neoplastic hepatocytes showed weak staining. The staining of VEGF was inhomogeneous. In the sections after TACE, at the tumor nests, boundary of necrotic and non-necrotic area and pericapsular area, VEGF positive tumor cells were relatively easy seen. The expression of VEGF in each group is shown in Table 3.
Figure 2.
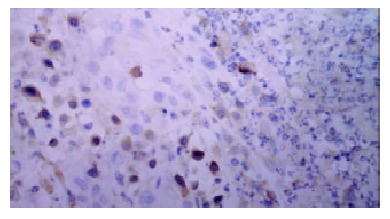
Positive VEGF cells in newly formed tumor nest after TACE (SABC × 200).
Linear regression analysis showed a clear correlation between tumor MVD and VEGF protein expression (r = 0.452, P < 0.01). The MVD counts in negative and positive VEGF groups were 67.86 ± 11.66 and 87.36 ± 22.97 (per high power field), respectively (Table 4).
Table 4.
MVD count in negative and positive VEGF groups after TACE
| Expression of VEGF | n | MVD (per high power field) (¯x ± s) |
| Negative group | 13 | 67.86 ± 11.66 |
| Positive group | 47 | 87.36 ± 22.97 |
| T value | 2.543 | |
| P value | 0.014 |
b-FGF protein expression and its correlation with MVD
b-FGF protein was identified sporadically in tumor tissues. No statistical differences were found between these groups (P = 0.922) (Table 3). There was no correlation between b-FGF and MVD counts (r = 0.036, P = 0.786).
DISCUSSION
Angiogenesis is an inherent property of tumor. It is important in its development and progression. Among many solid tumors, the degree of tumor angiogenesis was closely related to its biological behaviors, which reflect its ability for infiltration, recurrence and metastasis[26-30]. Intratumoral microvessel density (MVD) is commonly used to assess angiogenic activity. Defining and measuring MVD in the residual tumor cells after TACE might help judge the blood supply of residual tumor tissue and prognosticate the activity of these cells to a certain extent indirectly[31].
TACE could hardly abolish tumor blood supply. The possible causes included: incomplete embolization or partial recanalization of the artery, presence of intrahepatic porto-arterial shunt, formation of collateral circulation, and opening of potential communicating vessels[9,32,33]. The present study suggested that neovascularization and expression of VEGF might be another important factor. At the newly formed tumor nests, abundant factor VIII and positive VEGF cells could be found, and slight necrosis was seen in regions that had abundant neovasculature. These suggest that neovascularization plays an important role in the formation of collateral circulation, and relatively abundant blood supply will help tumor cells escape from the damage by TACE and increase the opportunity of survival.
Some authors considered that TACE might increase the chance of tumor recurrence or metastasis[20,34,35], which might be related to hypoxia caused by TACE and increased level of angiogenesis due to hypoxia[36-38]. The present study showed that MVD of TACE group was slightly higher than that of the control group and AI group, but no statistical difference was found, suggesting that TACE would not increase the level of tumor angiogenesis. Although the exact mechanism is currently unclear, we consider the possible explanation would be that the tumor cells were in an extreme hypoxia or even anoxia environment for a longer duration after TACE, which resulted in extensive necrosis and apoptosis of timorous and endothelial cells and decrease of secretion of angiogenic factor and weakening of the paracrine effect.
The intratumoral distribution of tumor neovasculature was inhomogeneous after embolization therapy. The newly formed blood vessels were less in the center of tumor but much more in the periphery and subcapsular area. This phenomenon might be related to the characteristics of tumor blood supply. Although blood supply of the tumor mainly came from hepatic artery, the peripheral area also received portal blood. There were potential collateral circulations both between the artery-portal veins and between the lobular arteries. After TACE, the portal blood supply increased with opening of collateral circulation, the blood supply of the peripheral region of the tumor restored more easily.
A balance between angiogenic and angiostatic factors regulates the angiogenesis. These factors may be produced either by tumor cells or host cells. The most commonly studied angiogenic factors were VEGF and b-FGF. VEGF could stimulate endothelia cells to proliferate, migrate, and alter their patterns of gene expression, increase microvascular hyperpermeability, extravasate plasma proteins into extravascular space and induce plasma-derived matrix[39,40]. VEGF played a key role in tumoral angiogenesis, it could induce neovascularization and was considered to be crucial in tumor biology[41,42]. b-FGF had synergistic effects with VEGF[43].
In the present study, the expression of VEGF was slightly higher in TACE group than in the control and AI groups, but no statistical difference was found. There was a significant correlation between MVD and VEGF expression. MVD counts in positive VEGF group were much higher than in negative VEGF group. At the newly formed tumor nests, the boundary of necrotic and non-necrotic area and the subcapsular area, positive VEGF tumor cells were more abundant and this was consistent with the distribution of neovasculature. Taken together, the results in the current study strongly suggested that VEGF played a key role in the formation of tumor neovasculature after TACE.
In conclusion, the influence of lipiodol chemoembolization on formation of tumor angiogenesis is relatively limited, but the development of neovascularization and expression of VEGF play important roles in establishing collateral circulation and reconstructing blood supply of residual cancer tissue.
Footnotes
Edited by Wu XN and WangXL
Supported by the National Natural Science Foundation of China, No.39770839
References
- 1.Rose DM, Chapman WC, Brockenbrough AT, Wright JK, Rose AT, Meranze S, Mazer M, Blair T, Blanke CD, Debelak JP, et al. Transcatheter arterial chemoembolization as primary treatment for hepatocellular carcinoma. Am J Surg. 1999;177:405–410. doi: 10.1016/s0002-9610(99)00069-0. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Liu Q, He J, Yang J, Yang G, Wu M. The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma. Cancer. 2000;89:2606–2612. [PubMed] [Google Scholar]
- 3.Poyanli A, Rozaneş I, Acunaş B, Sencer S. Palliative treatment of hepatocellular carcinoma by chemoembolization. Acta Radiol. 2001;42:602–607. doi: 10.1080/028418501127347278. [DOI] [PubMed] [Google Scholar]
- 4.Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, Guo YP, Lin XJ, Li GH. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002;8:74–78. doi: 10.3748/wjg.v8.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saccheri S, Lovaria A, Sangiovanni A, Nicolini A, De Fazio C, Ronchi G, Fasani P, Del Ninno E, Colombo M. Segmental transcatheter arterial chemoembolization treatment in patients with cirrhosis and inoperable hepatocellular carcinomas. J Vasc Interv Radiol. 2002;13:995–999. doi: 10.1016/s1051-0443(07)61863-6. [DOI] [PubMed] [Google Scholar]
- 6.Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Ueno K, Miyazono N, Inoue H, Nishida H, Kanetsuki I, Nakajo M. Transcatheter arterial chemoembolization therapy using iodized oil for patients with unresectable hepatocellular carcinoma: evaluation of three kinds of regimens and analysis of prognostic factors. Cancer. 2000;88:1574–1581. [PubMed] [Google Scholar]
- 8.Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747–1752. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]
- 9.Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994;73:2259–2267. doi: 10.1002/1097-0142(19940501)73:9<2259::aid-cncr2820730905>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, Ren Z, Xia J. [Appraisal of postoperative transcatheter arterial chemoembolization (TACE) for prevention and treatment of hepatocellular carcinoma recurrence] Zhonghua Zhongliu Zazhi. 2000;22:315–317. [PubMed] [Google Scholar]
- 11.Lee JK, Chung YH, Song BC, Shin JW, Choi WB, Yang SH, Yoon HK, Sung KB, Lee YS, Suh DJ. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2002;17:52–58. doi: 10.1046/j.1440-1746.2002.02664.x. [DOI] [PubMed] [Google Scholar]
- 12.Harrington EA, Fanidi A, Evan GI. Oncogenes and cell death. Curr Opin Genet Dev. 1994;4:120–129. doi: 10.1016/0959-437x(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 13.Schmaltz C, Hardenbergh PH, Wells A, Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol. 1998;18:2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 15.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 16.Jensen RL, Soleau S, Bhayani MK, Christiansen D. Expression of hypoxia inducible factor-1 alpha and correlation with preoperative embolization of meningiomas. J Neurosurg. 2002;97:658–667. doi: 10.3171/jns.2002.97.3.0658. [DOI] [PubMed] [Google Scholar]
- 17.Park K, Kim JH, Nam DH, Lee JI, Kim JS, Hong SC, Shin HJ, Eoh W, Park K. Vascular endothelial growth factor expression under ischemic stress in human meningiomas. Neurosci Lett. 2000;283:45–48. doi: 10.1016/s0304-3940(00)00904-6. [DOI] [PubMed] [Google Scholar]
- 18.An FQ, Matsuda M, Fujii H, Matsumoto Y. Expression of vascular endothelial growth factor in surgical specimens of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2000;126:153–160. doi: 10.1007/s004320050025. [DOI] [PubMed] [Google Scholar]
- 19.Shao G, Wang J, Zhou K, Yan Z. [Intratumoral microvessel density and expression of vascular endothelial growth factor in hepatocellular carcinoma after chemoembolization] Zhonghua Ganzangbing Zazhi. 2002;10:170–173. [PubMed] [Google Scholar]
- 20.Guo WJ, Li J, Ling WL, Bai YR, Zhang WZ, Cheng YF, Gu WH, Zhuang JY. Influence of hepatic arterial blockage on blood perfusion and VEGF, MMP-1 expression of implanted liver cancer in rats. World J Gastroenterol. 2002;8:476–479. doi: 10.3748/wjg.v8.i3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki M, Shimoda T, Itoh H, Kaiho T, Iinuma K, Koyama T, Nakagawa K, Andoh K, Anbiru S, Ohtawa S. Enhancement of cytotoxicity of doxorubicin by verapamil in the hepatic artery infusion for liver tumors in rats. Cancer. 1993;72:349–354. doi: 10.1002/1097-0142(19930715)72:2<349::aid-cncr2820720207>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Shao G, Wang J, Zhou K, Yan Z. [Intratumoral microvessel density and expression of vascular endothelial growth factor in hepatocellular carcinoma after chemoembolization] Zhonghua Ganzangbing Zazhi. 2002;10:170–173. [PubMed] [Google Scholar]
- 23.Li X, Zheng CS, Feng GS, Zhuo CK, Zhao JG, Liu X. An implantable rat liver tumor model for experimental transarterial chemoembolization therapy and its imaging features. World J Gastroenterol. 2002;8:1035–1039. doi: 10.3748/wjg.v8.i6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang R, Rescorla FJ, Reilly CR, Faught PR, Sanghvi NT, Lumeng L, Franklin TD, Grosfeld JL. A reproducible rat liver cancer model for experimental therapy: introducing a technique of intrahepatic tumor implantation. J Surg Res. 1992;52:193–198. doi: 10.1016/0022-4804(92)90072-8. [DOI] [PubMed] [Google Scholar]
- 25.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 26.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 27.Takeda A, Stoeltzing O, Ahmad SA, Reinmuth N, Liu W, Parikh A, Fan F, Akagi M, Ellis LM. Role of angiogenesis in the development and growth of liver metastasis. Ann Surg Oncol. 2002;9:610–616. doi: 10.1007/BF02574475. [DOI] [PubMed] [Google Scholar]
- 28.Koide N, Nishio A, Hiraguri M, Shimada K, Shimozawa N, Hanazaki K, Kajikawa S, Adachi W, Amano J. Cell proliferation, apoptosis and angiogenesis in gastric cancer and its hepatic metastases. Hepatogastroenterology. 2002;49:869–873. [PubMed] [Google Scholar]
- 29.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997;3:1222–1227. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- 31.Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566–1577. doi: 10.1038/sj.bjc.6600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui O, Kawamura I, Takashima T. Occurrence of an intrahepatic porto-arterial shunt after hepatic artery embolization with Gelfoam powder in rats and rabbits. Acta Radiol Diagn ( Stockh) 1986;27:119–122. doi: 10.1177/028418518602700124. [DOI] [PubMed] [Google Scholar]
- 33.Demachi H, Matsui O, Takashima T. Scanning electron microscopy of intrahepatic microvasculature casts following experimental hepatic artery embolization. Cardiovasc Intervent Radiol. 1991;14:158–162. doi: 10.1007/BF02577719. [DOI] [PubMed] [Google Scholar]
- 34.Liou TC, Shih SC, Kao CR, Chou SY, Lin SC, Wang HY. Pulmonary metastasis of hepatocellular carcinoma associated with transarterial chemoembolization. J Hepatol. 1995;23:563–568. doi: 10.1016/0168-8278(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 35.Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381–388. doi: 10.1016/s1072-7515(00)00700-6. [DOI] [PubMed] [Google Scholar]
- 36.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 37.Höckel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol. 2001;28:36–41. [PubMed] [Google Scholar]
- 38.Giatromanolaki A, Harris AL. Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res. 2001;21:4317–4324. [PubMed] [Google Scholar]
- 39.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 40.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 41.Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061–1065. doi: 10.5858/2000-124-1061-IEOVEG. [DOI] [PubMed] [Google Scholar]
- 42.O'Byrne KJ, Dalgleish AG, Browning MJ, Steward WP, Harris AL. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur J Cancer. 2000;36:151–169. doi: 10.1016/s0959-8049(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 43.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:II365–II371. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]


