Abstract
AIM: To investigate multidetector CT (MDCT) findings of hepatocelluar carcinoma (HCC)- associated hepatic arteriovenous shunt (HAVS) and to evaluate their clinical significance.
METHODS: Thin-slice and dynamic enhancement MDCT of HAVS was performed on 56 patients with HCC. MDCT findings, including those of portal veins, hepatic veins, superior mesenteric veins, splenic veins, HCC foci, liver parenchyma without HCC foci, spleens, and thromboses in portal veins and hepatic veins, were all confirmed by digital subtract angiography and analyzed.
RESULTS: MDCT demonstrated earlier enhancement of main portal trunks and/or the first order branches than that of superior mesenteric veins or splenic veins (n = 31). One patient had strong early enhancement of left hepatic vein with thromboses in left hepatic vein and upper part of inferior vena cava and 1 patient had transient patchy enhancement peripheral to HCC foci in late hepatic arterial phase among them. It demonstrated stronger opacification of main portal trunks and/or the first order branches than that of superior mesenteric veins or splenic veins (n = 18), and earlier enhancement of the second order and smaller branches of portal veins than that of main portal trunks (n = 4), stronger opacification of the second order and smaller branches of portal veins than that of main portal trunks (n = 3), with transient patchy enhancement (n = 3) or wedge-shaped enhancement (n = 4) peripheral to HCC foci in late hepatic arterial phase. Enhancement degree of HCC foci was all decreased. As for 49 patients with severe or moderate shunts, enhancement degree of liver parenchyma without HCC foci was increased with heterogeneous density, but enhancement degree of spleens was decreased. There were thromboses in main portal trunks and/or the first order branches in 32 patients.
CONCLUSION: The main MDCT findings of HCC-associated HAVS are earlier enhancement and stronger opacification of portal veins and/or hepatic veins. Understanding of these findings will contribute to the diagnosis and prognosis of the disease and improve therapy for the patients.
INTRODUCTION
Hepatic arteriovenous shunt (HAVS) is the communication between hepatic artery or its branches and portal vein or hepatic vein, forming hepatic artery portal venous shunt (HAPVS) or hepatic artery hepatic venous shunt (HAHVS) respectively. Hepatocelluar carcinoma (HCC) is the most common condition associated with HAVS because of its easy invasion of portal vein and hepatic vein. HAVS could result in direct blood flow between hepatic artery and portal vein or hepatic vein, which may cause severe portal hypertension and consequently, splenomegaly, ascites, and esophagogastric varices and bleeding, accelerating intrahepatic dissemination and extrahepatic metastasis of carcinoma cells[1,2]. Understanding of CT findings of HCC-associated HAVS is of significant clinical implications. Multidetector CT (MDCT) could contribute to the diagnosis of HAVS associated with HCC due to its fast scanning and improved image resolution and quality[3]. The purpose of this study was to examine MDCT findings of HCC-associated HAVS and to evaluate their clinical significance.
MATERIALS AND METHODS
Clinical data
Fifty-six patients (49 men and 7 women, range 29-73 years, mean age 49.8 years) with HCC-associated HAVS were included in the present study. The diagnosis of HCC was based on the results of percutaneous needle biopsy (n = 5) or laboratory testings, including elevated serum alpha-fetoprotein level, in combination with imaging appearance and follow-up images (n = 51) according to the diagnostic criteria for HCC formulated by Chinese National Association of Anticancer Committee.
MDCT
MDCT scanning was performed with a LightSpeed QX/i MDCT scanner (General Electronic Medical System, Milwaukee, USA). Multidetector row helical technique was applied to the scanning in cranial to caudal direction. Plain scanning of the liver was carried out first. This was followed by enhancement scanning of 2.5 mm axial section performed at 15 s, 25 s and 65 s after injection of contrast media for early hepatic arterial phase, late hepatic arterial phase and portal venous phase image acquisition respectively. A total of 100 mL contrast medium (Ultravist 300, Schering Pharmacy, Guangzhou, China; or Iopamiro 300, Bracco S.P.A., Milano, Italy) was administered to each patient, with a power injector at a rate of 3.5 mL·s-1 through a catheter placed in the peripheral vein of the antecubital fossa.
Digital subtract angiography (DSA)
DSA was performed using a TOSHIBA Digital 1000 MAX (Toshiba Corporation, Tokyo, Japan) within 2 wk of MDCT examination. A catheter was introduced via the right femoral artery by the Seldinger technique. The celiac (6 patients) or selective hepatic (50 patients) DSA was carried out with a catheter placed in the celiac trunk or in the proper hepatic artery and 40 mL Ultravist 300 (Schering Pharmacy, Guangzhou, China) or Iopamiro 300 (Bracco S.P.A., Milano, Italy) was injected with a power injector at a rate of 5.0 mL·s-1. Serial anterior-posterior images were obtained at 1 every 2 s for the first 8 s and a slower rate thereafter.
Diagnostic criterion for HAVS
Diagnostic criteria for HAPVS[4] were the earlier enhancement of main portal trunk and/or its first order branches than that of superior mesenteric vein or splenic vein, or stronger opacification of main portal trunk and/or its first order branches than that of superior mesenteric vein or splenic vein; or earlier enhancement of the second order and smaller branches of portal veins than that of main portal trunk, or stronger opacification of the second order and smaller branches of portal veins than that of main portal trunk. Diagnostic criteria for HAHVS were the earlier enhancement and stronger opacification of hepatic vein, approaching the density of enhanced aorta. The diagnosis of HAVS based on MDCT was confirmed by DSA.
Determination of shunting types and degrees of HAVS
According to the location of shunting, HAVS was divided into three types. The central HAVS was the shunting located in porta hepatis with earlier enhancement of main portal trunk and/or the first order branches, or hepatic veins at early hepatic arterial phase. The peripheral HAVS was the shunting located in peripheral liver parenchyma with earlier enhancement of the second order and smaller branches of portal vein, and transient patchy or wedge-shaped enhancement peripheral to HCC foci at late hepatic arterial phase. The mixed HAVS showed both central and peripheral HAVS.
According to the time of appearance of HAVS on images, HAVS was divided into three degrees. The severe HAVS showed opacification of main portal trunk and/or the first order branches, or hepatic veins, with enhancement of hepatic arterial and its branches at early hepatic arterial phase, without enhancement or with early enhancement of HCC foci. The moderate HAVS showed opacification of main portal trunk and/or the first order branches, or hepatic veins, with middle or late enhancement of HCC foci at late hepatic arterial phase. The mild HAVS showed opacification of the second order and smaller branches of portal veins at late hepatic arterial phase, with transient patchy or wedge-shaped enhancement peripheral to HCC foci.
Image analysis
Image analysis included examination of the shunting types and degrees of HAVS with or without thromboses in portal veins and/or hepatic veins; locations, gross pathologic patterns and enhancement of HCC; enhancement of liver parenchyma without HCC foci, spleens, superior mesenteric veins and splenic veins.
RESULTS
Earlier enhancement and stronger opacification of portal veins and hepatic veins
Earlier enhancement and stronger opacification of portal veins and hepatic veins were the major MDCT findings of HAVS. Their relations with shunting types and degrees of HAVS are shown in Table 1.
Table 1.
MDCT findings of earlier enhancement and stronger opacification of portal veins and hepatic veins and their relations with shunting types and degrees of HAVS
| MDCT findings |
Shunting patterns |
Shunting degrees |
||||
| Central | Peripheral | Mixed | Severe | Moderate | Mild | |
| Earlier enhancement of MPT and/or the first order branches than that of SMV or SV | 30 | 0 | 1a | 31 | 0 | 0 |
| Stronger opacification of MPT and/or the first order branches than that of SMV or SV | 18 | 0 | 0 | 11 | 7 | 0 |
| Earlier enhancement of the second order and smaller branches of PV than that of MPT | 0 | 4 | 0 | 0 | 0 | 4 |
| Stronger opacification of the second order and smaller branches of PV than that of MPT | 0 | 3 | 0 | 0 | 0 | 3 |
| Earlier enhancement and stronger opacification of HV, approaching density of enhanced aorta | 1b | 0 | 0 | 1b | 0 | 0 |
a: mixed HAPVS, with transient patchy enhancement peripheral to HCC foci at late hepatic arterial phase at the same time. b:HAHVS + HAPVS, combined with earlier enhancement of main portal trunk and the first order branches than that of superior mesenteric vein. MPT = main portal trunk, SMV = superior mesenteric vein, SV = splenic vein, HV = hepatic vein.
There was transient patchy (n = 3, Figure 1) or wedge-shaped (n = 4, Figure 2) enhancement peripheral to HCC foci at late hepatic arterial phase in patients with mild and peripheral HAVS, in addition to earlier enhancement of the second order and smaller branches of portal veins than that of main portal trunks, or stronger opacification of the second order and smaller branches of portal veins than that of main portal trunks.
Figure 1.
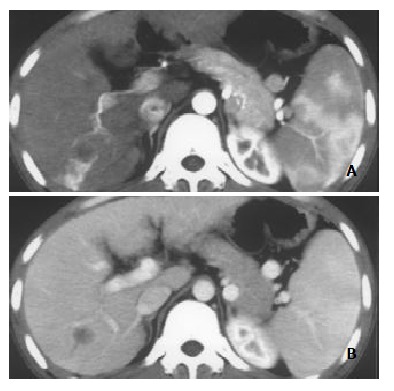
Nodular pattern of HCC with mild and peripheral HAVS. A: Transient patchy enhancement lateral to HCC foci at late hepatic arterial phase; B: becoming isoattenuation at portal vein phase.
Figure 2.
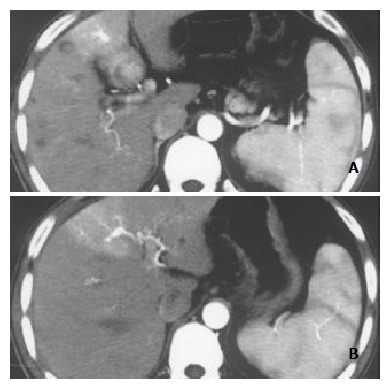
Nodular pattern of HCC accompanied by mild and peripheral HAVS. Stronger opacification of the third order portal vein branches than that of main portal trunk at late hepatic arterial phase with transient wedge-shaped enhancement lateral to HCC foci (A, B).
Thromboses in portal veins and hepatic veins
Thromboses in portal veins and hepatic veins were seen in all cases of central HAVS. Thirty-two patients had thromboses in portal veins, including 18 patients with thromboses in main portal trunks and the first order branches (Figure 3), 11 patients with thromboses in the right first order branches and 3 patients with thromboses in the left first order branches. One patient with mixed HAVS had thromboses in the left hepatic vein and upper part of inferior vena cava.
Figure 3.
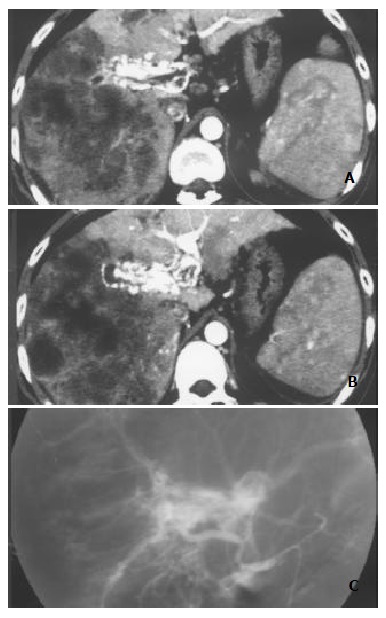
Massive and nodular pattern of HCC associated with severe and central HAVS. Earlier enhancement and stronger opacification of main portal trunk and the left and right first order branches with thromboses in them were shown. Enhancement degree of HCC foci was decreased, and enhancement degree of liver parenchyma without HCC foci was increased with heterogeneous density (A and B). DSA finding of the same patient (C).
Location, gross pathologic pattern and enhancement of HCC
HCC was located in different parts of liver parenchyma. Of the 49 patients with severe or moderate HAVS, 40 had foci adjacent to porta hepatis and 1 patient had foci nearby the secondary porta hepatis. Gross pathologic patterns included massive pattern (n = 17), nodular pattern (n = 11), massive and nodular pattern (n = 23) and diffuse pattern (n = 5). The enhancement of HCC foci was all decreased.
Enhancement of liver parenchyma without HCC foci and spleens
Liver parenchyma without HCC foci showed transient patchy (n = 3) or wedge-shaped (n = 4) enhancement peripheral to HCC foci at late hepatic arterial phase in 7 patients with mild and peripheral HAVS. In the 49 patients with severe or moderate and central HAVS, the enhancement degree of liver parenchyma without HCC foci was increased and heterogeneous, and enhancement degree of the spleen was all decreased (Figure 4).
Figure 4.
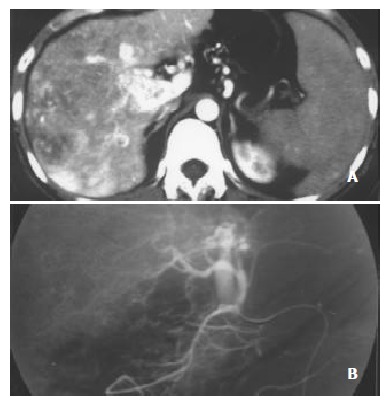
Massive pattern of HCC complicated with severe, central and slight, peripheral HAVS. Earlier enhancement and stronger opacification of main portal trunk with small thromboses in it were seen, with patchy enhancement internal and lateral to HCC foci. A: Enhancement degrees of HCC foci and spleen were decreased, enhancement degree of liver parenchyma without HCC foci was increased with heterogeneous density; B: DSA finding of the same patient.
DISCUSSION
Mechanism of formation of HAVS associated with HCC and MDCT findings
The formation of HAVS might be attributed to complex anatomy and various pathological conditions. Anatomically, hepatic artery and portal vein respectively branch and converge into hepatic sinuses, followed by entering into central vein and hepatic veins before into system circulation. Hence, there were abundant anastomoses between hepatic artery and portal vein[5,6]. The pathological conditions may include followings. With continued progression of HCC adjacent to porta hepatis, it might directly invade and destroy portal veins and/or hepatic veins, forming tumor thromboses in them or growing along the venous wall. The tumor thromboses are vascularized by arterial network nourished by hepatic artery branches around the vein and are growing up. The enlarged and dilated hepatic artery branches might become the main supplying arteries of the tumor thromboses and their blood flow might enter directly into portal veins and/or hepatic veins which act as the efferent vessels, resulting in transvasal HAVS. In our serial, HCC foci of 40 patients were adjacent to porta hepatis and 1 patient adjacent to the secondary porta hepatis with apparent invasion of main portal trunks and/or the first order branches or hepatic veins. Irregular, tortuous and dilated arterial networks were seen around the porta hepatis or the secondary porta hepatis, which were confirmed by DSA as the supplying arteries of HAVS. The direct invasion and destruction of main portal trunks and/or the first order branches, or hepatic veins, were the main causes of transvasal HAVS. It was specially the case for HCC in internal left hepatic lobe where HAVS might occur even if HCC focus was small. The transvasal HAVS had severe shunts shown as an earlier enhancement of main portal trunks and/or the first order branches, or hepatic veins than that of superior mesenteric veins or splenic veins at early hepatic arterial phase (n = 31), or as stronger opacification of main portal trunks and/or the first order branches than that of superior mesenteric veins or splenic veins (n = 11), but livers and spleens were ischemia due to "stolen blood" by HAVS. HCC might thus show no or little enhancement, and enhancement of the spleen decreased.
Blood flow of portal veins might be obstructed due to compression of the first order branches of portal vein by HCC or carcinoma thromboses in the first order branches of portal vein. The compensative hyperplasia of vessel plexus around larger biliary ducts in the central part of liver might result in opening of hepatopetal collateral vessels and formation of transplexal HAPVS. In our study, 7 patients with moderate shunts manifested as stronger opacification of main portal trunks and/or the first order branches than that of superior mesenteric veins or splenic veins at late hepatic arterial phase with MDCT.
Compression and invasion of branches of hepatic veins by HCC might obstruct hepatic veins and cause hypertension of hepatic sinuses. The portal veins might thus become their efferent vessels and receive blood supply of hepatic arteries directly when pressure of hepatic sinuses was higher than that of portal veins, resulting in transsinusoidal HAPVS. Subsequently, functional blood flow of portal veins in this area decreased and blood flow of hepatic arteries increased as a compensation, aggravating transsinusoidal HAPVS[6]. In addition, because tumor vessel had no muscular layer and its capability of regulating blood flow by systolic function of vessel wall was poor, the normal branches of hepatic arteries around HCC would preferably take in compensatively increased hepatic arterial blood due to obstruct of hepatic veins, resulting in further intensifying of transsinusoidal HAPVS. On MDCT, it had mild shunts shown as an earlier enhancement of the second order and smaller branches of portal veins than that of main portal trunks (n = 4), or stronger opacification of the second order and smaller branches of portal veins than that of main portal trunks (n = 3) at late hepatic arterial phase, with transient patchy or wedge-shaped enhancement peripheral to HCC foci. In our series, 7 cases of peripheral HAPVS and 1 case of mixed HAPVS showed these findings.
Diagnosis and differential diagnosis of HCC- associated HAVS
It could be seen from above analyses that there seemed no much difficulty in diagnosis of severe or moderate and central HAVS associated with HCC. However, mild HAVS and peripheral HAVS should be differentiated from hepatic perfusion abnormalities of physiological conditions and other pathological causes[7-14].
Hepatic perfusion abnormalities caused by physiological conditions such as origin variety of segment or subsegment hepatic artery, aberrant biliary bladder vein or gastric vein were only shown as local transient hepatic parenchyma hyperattenuation at hepatic arterial phase with no abnormality at portal vein phase and no HCC foci[15]. HAPVS in hepatic hemangioma manifested as a wedge-shaped or irregular homogenous hyperattenuation in the liver parenchyma adjacent to the tumor at hepatic arterial phase, becoming isoattenuation or slight hyperattenuation, and hemangioma itself tending to show rapid enhancement at portal vein phase, specially for hemangioma of 2-3 cm in diameter or less[16-18]. Hepatic adenoma and focal nodular hyperplasia appeared as homogenous enhancement at hepatic arterial phase, being hypoattenuation at delayed time phase. Abnormal perfusion in cirrhotic liver had the typical wedge-shaped and homogeneous appearance with or without internal linear branching structures at hepatic arterial phase, returning to isoattenuation or slight hyperattenuation at portal vein phase[5]. The site of abnormal perfusion associated with thrombosis in portal vein was conformed to respective portal vein distribution. Hepatic metastasis with abundant blood supply, liver infection, Budd-Chiari syndrome, changes after transjugular intrahepatic portosystemic shunt (TIPS), HAVS following liver biopsy, abnormal perfusion resulted from acute biliary bladder inflammation and acute pancreas inflammation all had their own MDCT features. With the help of clinical materials, they could be differentiated from mild and peripheral HAVS[5,19-26].
Clinical significance of diagnosis of HCC- associated HAVS by MDCT
Diagnosis of HAVS was mainly based on transcatheter hepatic angiography (including DSA) in the past. However, a proportion of patients could not undergo transcatheter hepatic angiography due to restriction of equipment conditions and technology, cost and invasive examination etc., resulting in missed diagnosis of HAVS and loss of treatment opportunity[24,27].
MDCT is a breakthrough in medical imaging examination technology. Equipped with a multidetector array, MDCT can perform multislice data acquisition simultaneously, which greatly reduces the time of volume scanning. In addition, image quality is improved due to increased image resolution and clarity. MDCT could therefore offer thin-slice and dynamic enhancement scanning of liver at early hepatic arterial phase, late hepatic arterial phase and portal venous phase and provide a convenient, fast and noninvasive new technology for examination of HAVS associated with HCC[3,28].
Clinically, correct interpretation of MDCT findings of HCC-associated HAVS could assist in making right diagnosis and prognosis and working out effective therapeutic strategy. Forty-nine patients with severe or moderate and central HAVS in our series underwent transcatheter supplying artery embolism of HAVS under the guidance of MDCT information and their esophagogastric varix bleeding, ascites and stubborn diarrhea were all brought under control timely. Moreover, MDCT provides a new technology for the study of mechanism of HAVS formation. With MDCT, shunting locations, types and degrees of HAVS can be determined, and mechanism of HAVS formation can be estimated. These may form the basis for comprehensive therapy of HCC and embolism of HAVS. Our study suggested that transvasal, transplexal and transsinusoidal HAVS might be the causes of severe, moderate and mild HAVS respectively, and enlarged and dilated nourishing artery manifested as irregular arterial network, originating from proximal proper hepatic artery, might be the main supplying artery of severe and central HAVS. Therefore, in embolism of HAVS, superselective embolism of nourishing artery rather than hepatic artery trunk was performed. If HAVS was supplied by many arteries, they should be embolized respectively. In addition, embolism agent should arrive at the end of nourishing artery to achieve permanent embolism and reduce the possibility of recanalization and recurrence of HAVS[29-33]. Our above-mentioned forty-nine patients with severe or moderate and central HAVS were all completely embolized by absolute ethanol combined with spring steel coil via 4.0F catheter or 3.0F microcatheter superselective embolism without recanalization and recurrence of HAVS by follow-up MDCT examinations.
In conclusion, our study investigated the complex MDCT findings of HAVS associated with HCC. The classification of shunting degrees into severe, moderate and mild shunts according to appearing time of HAVS at early or late hepatic arterial phase can contribute to the diagnosis and treatment of patients. It is also noted that transvasal, transplexal, transsinusoidal HAVS may be behind the formation of severe, moderate and mild HAVS respectively.
Footnotes
Edited by Liu HX and Wang XL
References
- 1.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo WP, Zhang HX, Wang ZM, Wang YQ, Ni DH, Li WX, Guan Y. DSA analysis of hepatic arteriovenous fistula concurrent with hepatic cancer and its clinical significance. World J Gastroenterol. 2000;6:872–876. doi: 10.3748/wjg.v6.i6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortele KJ, McTavish J, Ros PR. Current techniques of computed tomography. Helical CT, multidetector CT, and 3D reconstruction. Clin Liver Dis. 2002;6:29–52. doi: 10.1016/s1089-3261(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen JH, Chai JW, Huang CL, Hung HC, Shen WC, Lee SK. Proximal arterioportal shunting associated with hepatocellular carcinoma: features revealed by dynamic helical CT. AJR. Am J Roentgenol. 1999;172:403–407. doi: 10.2214/ajr.172.2.9930792. [DOI] [PubMed] [Google Scholar]
- 5.Kim TK, Choi BI, Han JK, Chung JW, Park JH, Han MC. Nontumorous arterioportal shunt mimicking hypervascular tumor in cirrhotic liver: two-phase spiral CT findings. Radiology. 1998;208:597–603. doi: 10.1148/radiology.208.3.9722834. [DOI] [PubMed] [Google Scholar]
- 6.Nagino M, Nimura Y, Kamiya J, Kanai M, Hayakawa N, Yamamoto H. Immediate increase in arterial blood flow in embolized hepatic segments after portal vein embolization: CT demonstration. AJR. Am J Roentgenol. 1998;171:1037–1039. doi: 10.2214/ajr.171.4.9762992. [DOI] [PubMed] [Google Scholar]
- 7.Luo TY, Shi B, Li YM, Lu FJ, Yuan SW, Yan M, Wu JQ. A study on the transient hepatic abnormal enhancement in the hepatic arterial phase during dynamic contrast-enhanced spiral CT. Zhonghua Fangshexue Zazhi. 2003;37:258–263. [Google Scholar]
- 8.Quiroga S, Sebastià C, Pallisa E, Castellà E, Pérez-Lafuente M, Alvarez-Castells A. Improved diagnosis of hepatic perfusion disorders: value of hepatic arterial phase imaging during helical CT. Radiographics. 2001;21:65–81; questionnaire 288-94. doi: 10.1148/radiographics.21.1.g01ja0165. [DOI] [PubMed] [Google Scholar]
- 9.Gryspeerdt S, Van Hoe L, Marchal G, Baert AL. Evaluation of hepatic perfusion disorders with double-phase spiral CT. Radiographics. 1997;17:337–348. doi: 10.1148/radiographics.17.2.9084076. [DOI] [PubMed] [Google Scholar]
- 10.Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol. 2001;3:1–15. doi: 10.3348/kjr.2002.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park CM, Cha SH, Kim DH, Choi JA, Cha IH, Kim YH, Chung KB, Suh WH. Hepatic arterioportal shunts not directly related to hepatocellular carcinoma: findings on CT during hepatic arteriography, CT arterial portography and dual phase spiral CT. Clin Radiol. 2000;55:465–470. doi: 10.1053/crad.2000.0477. [DOI] [PubMed] [Google Scholar]
- 12.Chen WP, Chen JH, Hwang JI, Tsai JW, Chen JS, Hung SW, Su YG, Lee SK. Spectrum of transient hepatic attenuation differences in biphasic helical CT. AJR. Am J Roentgenol. 1999;172:419–424. doi: 10.2214/ajr.172.2.9930795. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Wu PH, Mo YX, Lin HG, Zheng L, Li JQ, Lu LX, Ruan CM, Chen L. CT arterial portography and CT hepatic arteriography in detection of micro liver cancer. World J Gastroenterol. 1999;5:225–227. doi: 10.3748/wjg.v5.i3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Wu PH, Lin HG, Li JQ, Mo YX, Zheng L, Lu LX, Ruan CM, Chen L. Findings of non-pathologic perfusion defects by CT arterial portography and non-pathologic enhancement of CT hepatic arteriography. World J Gastroenterol. 1998;4:513–515. doi: 10.3748/wjg.v4.i6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon KH, Matsui O, Kadoya M, Yoshigawa J, Gabata T, Arai K. Pseudolesion in segments II and III of the liver on CT during arterial portography caused by aberrant right gastric venous drainage. J Comput Assist Tomogr. 1999;23:306–309. doi: 10.1097/00004728-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Kim KW, Kim TK, Han JK, Kim AY, Lee HJ, Choi BI. Hepatic hemangiomas with arterioportal shunt: findings at two-phase CT. Radiology. 2001;219:707–711. doi: 10.1148/radiology.219.3.r01ma05707. [DOI] [PubMed] [Google Scholar]
- 17.Vilgrain V, Boulos L, Vullierme MP, Denys A, Terris B, Menu Y. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics. 2000;20:379–397. doi: 10.1148/radiographics.20.2.g00mc01379. [DOI] [PubMed] [Google Scholar]
- 18.Naganuma H, Ishida H, Konno K, Hamashima Y, Komatsuda T, Ishida J, Masamune O. Hepatic hemangioma with arterioportal shunts. Abdom Imaging. 1999;24:42–46. doi: 10.1007/s002619900438. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki M, Furukawa A, Murata K, Morita R. Transient hepatic attenuation difference (THAD) in patients without neoplasm: frequency, shape, distribution, and causes. Radiat Med. 1999;17:91–96. [PubMed] [Google Scholar]
- 20.Lee WK, Stuckey S. Arterioportal fistula following liver biopsy demonstrated by lipiodol computed tomography. Clin Radiol. 2000;55:489–491. doi: 10.1053/crad.2000.0068. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Ishida H, Konno K, Komatsuda T, Hamashima Y, Naganuma H, Ohyama Y. Longstanding arterioportal fistula after laparoscopic liver biopsy. Abdom Imaging. 1999;24:383–385. doi: 10.1007/s002619900519. [DOI] [PubMed] [Google Scholar]
- 22.Lim JH, Lee SJ, Lee WJ, Lim HK, Choo SW, Choo IW. Iodized oil retention due to postbiopsy arterioportal shunt: A false positive lesion in the investigation of hepatocellular carcinoma. Abdom Imaging. 1999;24:165–170. doi: 10.1007/s002619900468. [DOI] [PubMed] [Google Scholar]
- 23.Thampanitchawong P, Piratvisuth T. Liver biopsy: complications and risk factors. World J Gastroenterol. 1999;5:301–304. doi: 10.3748/wjg.v5.i4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JH, Chen WP, Huang CL, Shen WC. Dynamic helical CT as a novel technique for diagnosing hepatic perfusion disorders. Hepatogastroenterology. 1999;46:303–307. [PubMed] [Google Scholar]
- 25.Quiroga S, Sebastià MC, Moreiras M, Pallisa E, Rius JM, Alvarez-Castells A. Intrahepatic arterioportal shunt: helical CT findings. Eur Radiol. 1999;9:1126–1130. doi: 10.1007/s003300050805. [DOI] [PubMed] [Google Scholar]
- 26.Arita T, Matsunaga N, Takano K, Hara A, Fujita T, Honjo K. Hepatic perfusion abnormalities in acute pancreatitis: CT appearance and clinical importance. Abdom Imaging. 1999;24:157–162. doi: 10.1007/s002619900466. [DOI] [PubMed] [Google Scholar]
- 27.Chen JH, Huang CL, Hwang JI, Lee SK, Shen WC. Dynamic helical biphasic CT emerges as a potential tool for the diagnosis of proximal arterioportal shunting. Hepatogastroenterology. 1999;46:1791–1797. [PubMed] [Google Scholar]
- 28.Takahashi S, Murakami T, Takamura M, Kim T, Hori M, Narumi Y, Nakamura H, Kudo M. Multi-detector row helical CT angiography of hepatic vessels: depiction with dual-arterial phase acquisition during single breath hold. Radiology. 2002;222:81–88. doi: 10.1148/radiol.2221010326. [DOI] [PubMed] [Google Scholar]
- 29.Guan SH, Dan H, Jiang ZB, Huang MS, Zhu KS, Li ZR, Meng XC. Transmicrocatheter local injection of ethanol to treat hepatocellular carcinoma with high flow arteriovenous shunts. Zhonghua Fangshexue Zazhi. 2002;36:997–1000. [Google Scholar]
- 30.Luo PF, Chen XM, Zhang LM, Zhou ZJ, Fu L, Wei ZH. The management of arteriovenous shunting in hepatocellularcarcinoma. Zhonghua Fangshexue Zazhi. 2002;36:114–117. [Google Scholar]
- 31.Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, Zhou XD, Ye SL. Multimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal vein. World J Gastroenterol. 2001;7:28–32. doi: 10.3748/wjg.v7.i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Wu PH, Li JQ, Zhang WZ, Lin HG, Zhang YQ. Segmental transcatheter arterial embolization for primary hepatocellular carcinoma. World J Gastroenterol. 1998;4:511–512. doi: 10.3748/wjg.v4.i6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan J, Ten GJ, He SC, Guo JH, Yang DP, Wang GY. Arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 1998;4:33–37. doi: 10.3748/wjg.v4.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]


