Abstract
AIM: A myriad of healthful effects has been attributed to the probiotic lactic acid bacteria, perhaps the most controversial issue remains that of anticancer activity. This study was aimed at investigating the putative anti-cancer effects of lactic acid bacteria strains on the progression of colon tumor in 1,2-dimethylhydrazine (DMH)-treated animals.
METHODS: The strain of lactic acid bacteria used in this study was lactic acid bacteria NZ9000 that conformed to the characteristics of plasmid free. Sixty male Wistar rats were given subcutaneous injections of DMH at a dose of 40 mg/kg body wt or saline once a week for 10 wk. The rats were divided into 6 experimental groups. After the last DMH injection, animals in groups 1 and 4 were gavaged with 1 mL of lactic acid bacteria at a dose of 5 × 109 per day or vehicle until sacrifice at the end of week 22 or week 52. Animals in groups 1-3 were killed at the end of week 22 for histopathological examination. The whole period of experimental observation was 52 wk.
RESULTS: By the end of 22nd week, final average body weights of the rats treated with DMH alone and all animals receiving lactic acid bacteria were significantly decreased compared with the vehicle control (P < 0.05). No differences in tumor incidence, multiplicity, dimensions and stage in the colonic mucosa were observed among the groups. At week 52, the survival rate of the rats administered lactic acid bacteria was lower than that of the rats treated with DMH that were fed on control fluids of non-lactococcus lactis. The mean survival time of lactic acid bacteria-treated animals was 39 wk.
CONCLUSION: These results indicate that lactic acid bacteria lacks inhibitory effects on the progression of colon tumor in DMH-treated animals, and does not support the hypothesis that alteration of colonic flora may exert an influence on the progression of colon tumor.
INTRODUCTION
Epidemiological studies have provided evidences that the morbidity of colon cancer is influenced by diet. Several studies of humans and experimental animals suggested that the influence of diet was mediated by altering the metabolic activity of intestinal bacterial flora[1,2]. Some of these enteric bacteria are beneficial to the host and have been shown to exert antimutagenic and anticarcinogenic properties[3-5]. By definition, probiotic bacteria can beneficially affect the host by improving its intestinal microbial balance. Bacterial flora that ferment the dairy products (e.g. lactic acid bacteria) might be used for cancer chemoprevention[4].
Lactobacilli are one of the dominant species in the small intestine, and these micro-organisms presumably affect metabolic reactions occurring in this part of the gastrointestinal tract. Some metabolites of lactic bacteria, such as lactic acid and enzymes, have been reported to have some chemotherapeutic value[6]. Furthermore, several studies have also shown that consumption of bifidobacteria reduces colon cancer risk in carcinogen-treated animals, suggesting that consumption of certain bacteria has a beneficial effect on the balance of colon bacteria[7,8]. Although a myriad of healthful effects have been attributed to the probiotic lactic acid bacteria, perhaps the most controversial issue is its anticancer activity. How dietary components, including lactic acid bacteria, interact with genes contributes to tumor development
An autochthonous colon cancer model is useful to evaluate the clinical therapeutic efficacy of drugs for colorectal cancer[9,10]. The experimental carcinogen DMH has been used in the study of the effect of diet in experimental animals[1,11]. As the DMH model is known to closely parallel the human disease in term of disease presentation, gross and microscopic pathology[12], it is anticipated that DMH-induced colon tumor in rats would respond to chemotherapeutic drugs used in man[13]. Although clinical use of 5-fluorouracil (5-FU) derivatives was tested in the DMH model, few studies on the effect of lactic acid bacteria on chemically induced colon tumor progression have been reported.
Supplementation of diet with components possessing antimutagenic and anticarcinogenic properties might result in a significant decrease of tumor frequency. A number of studies indicate that administration of bifidobacteria or lactobacilli alone or in combination with fermentable carbohydrate (defined as a prebiotic) can alter colonic microflora populations and decrease the development of early preneoplastic lesions and tumors. This study was aimed at investigating the putative anti-cancer effects of lactic acid bacteria strains on the progression of colon tumor in DMH-treated animals.
MATERIALS AND METHODS
Materials
Animals and chemicals Male Wistar rats at 5 wk of age were obtained (Department of Animals, Chinese Academy of Medical Sciences, Beijing, China) and housed in plastic cages with wood chips for bedding in an animal room with a 12 h light/dark cycle at 22 ± 2 °C and 44% ± 5% relative humidity. The rats were fed on the basal diet. Water was available ad libitum, and body weight and food consumption were measured weekly during the experiments. DMH was purchased from Tokyo Kasei Co. (Tokyo, Japan).
Methods
Treatment protocol The experimental design is shown in Figure 1. One week after acclimatization, sixty rats of 6-week-old were randomly divided into six groups (10 rats/group). Animals in Groups 1 and 4 were given subcutaneous injections of DMH dissolved in normal saline solution (40 mg/kg body wt) once a week for 10 wk. Rats in groups 2 and 5 were injected 0.9% normal saline (vehicle) at the same time. After the last DMH treatment, the animals in groups 1 and 4 were additionally gavaged with 1 mL of lactic acid bacteria at a dose of 5 × 109 per day for 12 wk or until they were dead. Groups 2 and 5 were gavaged with 1 mL of protective reagents per day (the vehicle control). Groups 3 and 6 served as a carcinogen control. The course of treatments differed slightly from each experiment. All surviving animals were sacrificed under ether anesthesia at week 22 (Groups 1-3) and at the end of experiment (Groups 4-6), respectively. The whole period of experimental observation was 52 wk.
Figure 1.
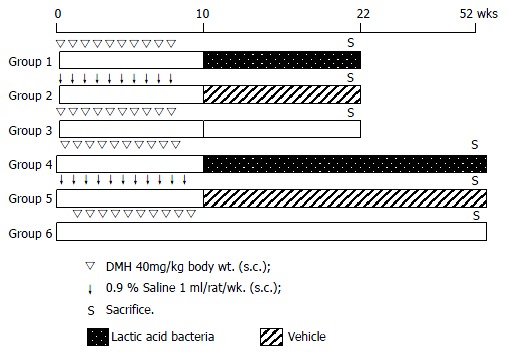
Experimental design.
Tissue processing All animals were autopsied. The colons were removed, flushed with saline, opened along the longitudinal median axis. Macroscopically, the number of tumors in each colon was counted. Tumor width (W) and length (L) were measured with calipers. Tumor volume (TV) was determined according to the following formula: TV = (L × W2)/2[14]. After the gross pathologic changes (number, dimensions and distribution of the tumors) were recorded, the colons were fixed flat between pieces of filter paper in 10% phosphate-buffered formalin. The liver and kidneys were removed and weighed. Other major organs (stomach, small intestine, spleen, lungs and lymph nodes) were also excised and fixed in 10% phosphate-buffered formalin solution. Afterward, all tissues were embedded in paraffin and used for sectioning. After the sections were stained with routine hematoxylin and eosin, the colons were divided into proximal, intermediate, and distal segments and examined for histopathological analysis.
Tumor staging Animals with DMH-induced colon cancer characteristically developed multiple tumors and each tumor was at a different histological stage[15]. Consequently, for the purpose of this experiment, animals were staged (Duke’s stage) with reference to a single index tumor, defined as the largest macroscopically and histologically identifiable colon tumor.
Preparation of lactococcus lactis The strain of L. lactis used in this study was lactic acid bacteria NZ9000 that conformed to the characteristics of plasmid free. The strain was obtained from French Academy of Agricultural Sciences. All cloning steps were done with E. coli Top10. E. coli was grown on Luria-Bertani (LB) medium and incubated at 37 °C under aeration. L. lactis was grown without shaking on M17 medium in which 1% (wt/vol.) glucose was added (M17-Glu) and incubated at 30 °C. When appropriate, antibiotics was added to the culture medium. For lactic acid bacteria strains, chloramphenicol was used at a final concentration of 10 μg/mL. Ampicillin was supplied at a concentration of 100 μg/mL in the case of E. coli. In the experiments, the lactic acid bacteria was induced with nisin as follows. An overnight culture of lactic acid bacteria was transferred into a fresh medium at a dilution of 1:50. After 3-4 h of incubation, 1 ng/mL of nisin (Sigma) was added to the culture, which was incubated for 3-4 h. For L. lactis, nisin induction was performed as described previously[16].
After incubation, lactic acid bacteria was concentrated to 5 × 109 cells/mL in PBS buffer (pH7.0). This concentration would be used as an oral dosage for a rat. PBS buffer (pH7.0) was used as a liquid protecting solution for lactic acid bacteria. The preparations were preserved at 4 °C for a week.
Statistical analysis
Statistical analyses were completed with SPSS 9.0 (Statistical Package for the Social Science). The significance of differences between the average values of the groups was analyzed using Cochran’s two-tailed Student’s t-test. The significance of differences in lesion incidences between the groups was assessed by χ² test. Rat mortality was analysed by the Log Rank method of Peto et al[17].
RESULTS
All rats in groups 1-3 and 5 survived to the final termination and maintained a relatively healthy appearance throughout the experiment. No signs of severe toxicity or diarrhea were observed in all animals given lactic acid bacteria. No tumors were found in vehicle-treated animals fed on the protective reagents. By the end of 22nd week, final average body weights of the rats treated with DMH alone and all animals receiving lactic acid bacteria were significantly decreased compared with the vehicle control (P < 0.05). Relative liver and kidney weights and food consumption were not significantly differ among the groups (shown in Table 1). Macroscopically, the distribution of colon tumors was predominantly observed in the proximal and middle colon at the end of 22nd week, there were no significant differences among the groups (data not shown). Histo-pathological examinations of adenocarcinomas in the proximal and middle colon showed that all were invasive through the muscularis mucosa and moderately differentiated.
Table 1.
Average final body weight, relative liver and kidney weights, and food consumption data (22wk)
| Group | Treatment | No. of rats | Final body Wt, (g) | Relative liverWt, (g) | Relative kidneyWt, (g) | Average food consumption (g/rat/day) |
| 1 | DMH + lactic acid | 10 | 389.6 ± 44.1e | 2.92 ± 0.24 | 0.54 ± 0.06 | 19.45 |
| 2 | Saline + vehicle | 10 | 439.5 ± 39.3 | 3.09 ± 0.35 | 0.56 ± 0.12 | 20.07 |
| 3 | DMH | 10 | 383.5 ± 19.2e | 3.10 ± 0.40 | 0.55 ± 0.07 | 18.48 |
a: Values are means ± SD, DMH, 1,2-dimethylhydrazine, b: Statistical significance:
P < 0.05, Student’s t-test, vs Group 2. c: Kidney weight values are totals for both kidneys.
Histo-pathological findings are summarized in Table 2. Colon epithelial lesions were divided into adenoma, carcinoma in situ and carcinoma. At the end of 22nd week, the incidence of colon tumor was not significantly affected by lactic acid bacteria. The mean tumor incidence per tumor-bearing rat was 3.11 in group 1 and 4.00 in group 3. Tumor volume was decreased in rats receiving lactic acid bacteria, however, the differences were also not significant compared with DMH- treated group. On the other hand, there was no significant difference in the incidence of metastasis (Duke’s stage) between the animals treated with DMH alone and those received lactic acid bacteria.
Table 2.
Colon tumor incidence, classification, multiplicity, tumor volume and stage in rats treated with DMH followed or not by lactic acid bacteria (22wk)a
| Treatment | Incidencen (%) | Total no.tumors | Adenoma No (%) | Carcinoma in situ No (%) | Carcinoma No (%) | Multiplicityb No. | Tumor volumemm3 |
Duke’s stagec |
||
| A | B | C | ||||||||
| DMH + lactic acid | 9 (90) | 28 | 12 (43) | 8 (28.5) | 8 (28.5) | 3.11 ± 2.00 | 1.64 ± 1.92 | 0 | 2 | 1 |
| DMH | 10 (100) | 40 | 27 (67.5) | 3 (7.5) | 10 (25) | 4.00 ± 2.96 | 4.31 ± 4.56 | 0 | 0 | 3 |
a: Values are means ± SD. b: No. of tumors/tumor-bearing rat. c: No. of rats with carcinoma were allocated to one of three tumor stages.
At the termination, the survival rate of rats in groups 4-6 is shown in Figure 2. None of the rats received L. lactis survived the full duration of the experiment, while the DMH-treated rats had a 20% survival rate. However, the survival rats were sacrificed and macroscopically visible metastases were found in their lungs and livers. There was no significant differene in the survival rate between the lactic acid bacteria treated rats and DMH treated rats. All rats in Group 5 (the vehicle control) survived. The mean survival in lactic acid bacteria-treated animals was 39 wk.
Figure 2.
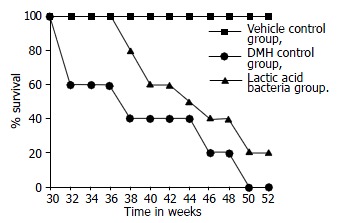
Survival rate of rats injected DMH followed or not by lactic acid bacteria and normal saline.
DISCUSSION
Oral administration of lactic acid bacteria has been shown to effectively reduce DNA damage in animals induced by chemical carcinogens, especially the damage of gastric and colonic mucosa in rats[7]. Certain strains of lactic acid bacteria have also been found to be able to prevent putative preneoplastic lesions induced by carcinogens and have antitumor activity[18,19]. However, findings of in current study do not support the suggestion that addition of lactic acid bacteria may protect against the progression of DMH-induced colon tumor in rats.
The exact change in tumor size or survival of the animals may be crucial for sensitivity determination of anticancer drugs. In this regard, our experimental results seem to be not ideal. The present data did not show any inhibitory effect of lactic acid bacteria on the dimension, multiplicity and invasion of colon tumors. By the end of 22nd week, although there was a reduction in colon tumor volume in animals received lactic acid bacteria, this difference was not statistically significant. The Duke’s staging system for human colorectal cancer provides accurate prognostic information. In other words, animals with less advanced diseases (stage A) can survive significantly longer than those with more advanced diseases (stage B and C), irrespective of treatment. In the present study, there was no significant difference in the incidence of metastases at necropsy in lactic acid bacteria-treated and untreated animals. Moreover, the survival rate of rats received lactic acid bacteria was lower than that of rats treated with DMH that were fed on non-lactic acid bacteria. The lower survival rate of rats administered L. lactis suggested that lactic acid bacteria had no protective role in decreasing DMH-induced mortality. Whether lactic acid bacteria has some promoting role in the progression of colon tumor is not clear.
In fact, the data from experimental studies indicated that ingestion of certain lactic cultures or their fermented dairy products could reduce the risk of certain types of cancer and inhibit tumor growth[20-22]. The animal experiments did indicate that feeding certain lactic cultures or fermented milk not only suppressed the incidence of DMH-induced colon carcinogenesis but also increased the survival rate of rats with chemically induced colon cancer[23]. These lactic cultures have been shown to possess antimutagenic properties[24], and the probiotic was given during the initiation and promotion phases. However, the antimutagenic activity of lactic acid-producing bacteria was suspected to reside in cell wall[25], as lactic acid itself had no antimutagenic effects[26]. In a previous study, the findings failed to demonstrate that bifidobacteria or lactobacilli administration alter colonic microflora had effects on the host[27], and no significant effect was observed when probiotic was given after the promotion phase. In the present study, the lower survival rate of the lactic acid bacteria group (Figure 2) suggested that lactic acid bacteria had no protective role in decreasing DMH-induced mortality. The observed results in survival rats might be due to the fact that lactic acid bacteria can not decrease faecal enzymes involved in formation of carcinogenesis. In addition, our studies failed to show a significant reduction in total number of colon tumors in rats. We believe that this indicates lactic acid bacteria lacks effects on colon cancer in rats. At present, the results from epidemiological studies do not appear to support the results from experimental studies of lactobacilli or lactic acid bacteria on colon cancer prevention or treatment. The reason for this is unclear but might be explained by differences in bacterial strains. The precise mechanisms by which lactic acid bacteria may inhibit colon cancer are presently unknown. However, many antitumor activities attributed to lactic cultures have been suggested to involve an enhanced immune response. Therefore, more work needs to be done to identify the specific strains and their antitumor effects and the mechanisms underlying these effects.
Human cancer-nude mice subcutaneous xenograft system as a sensitivity test for chemotherapeutic drugs was studied[28], but it has not been established that the system is a predictive model for screening anticancer drugs. Recently, investigators have been giving greater attention to more rigorous experimental endpoints, such as tumor regression[29-31]. In this respect, autochthonous colon cancer in rats may be suitable to disease oriented in vivo screening. DMH-induced rat colon tumor model might be a valuable for new therapeutic agents[9,10]. The fact that clinically used drugs such as 5FU could inhibit the growth of DMH-induced colon tumors and prolong the survival of their rodent hosts suggested a parallel in tumor sensitivity[13]. Therefore, colon tumors induced by DMH at present were the most popular models used in experimental oncology to study various aspects of the morphology, pathogenesis, prevention and treatment of colorectal cancer[9-13]. The animal model described in this study was a truly adjuvant model since the rats developed primary colon tumors in situ, which resembled the histopathologic features of human colon tumors. Moreover, DMH decreased animal body weight at the end of week 22, but had no effect on food intake, probably due to its aggressive effect on the mucosa and carcinogenicity.
In the present study, two possible reasons for the failure to alter rat colon tumor were put forward. One was that L. lactis lacked inhibitory effects because the initiation of DMH was too strong in our study. The other was that the dose of L. lactis might be inadequate to significantly alter colonic flora. It is clear that an optimal condition must be met for each inhibitor to be used for animal and possibly for human cancer prevention and treatment. Otherwise, the same substance, it might enhance the processes of growth of tumor instead of inhibiting. Therefore, it will be extremely difficult, to devise an optimal diet because of the wide variety of cancer inhibitors and enhancers present in human diet.
In conclusion, this study did not find any inhibitory effect in experimental colon tumor progression by addition of lactic acid bacteria after the promotion stage of carcinogenesis and thus does not support the hypothesis that alteration of colonic flora may exert an influence on the progression of DMH-induced colon tumor.
ACKNOWLEDGEMENTS
Authors are very grateful to Prof. Shoji Fukushima, Department of Pathology, Osaka City Medical School (Japan) for valuable discussion and comments, and Prof. Yanfeng Zhong, Department of Pathology, Beijing University Medical School for histopathological examination.
Footnotes
Edited by Zhao M and Wang XL
References
- 1.Balansky R, Gyosheva B, Ganchev G, Mircheva Z, Minkova S, Georgiev G. Inhibitory effects of freeze-dried milk fermented by selected Lactobacillus bulgaricus strains on carcinogenesis induced by 1,2-dimethylhydrazine in rats and by diethylnitrosamine in hamsters. Cancer Lett. 1999;147:125–137. doi: 10.1016/s0304-3835(99)00287-6. [DOI] [PubMed] [Google Scholar]
- 2.Femia AP, Luceri C, Dolara P, Giannini A, Biggeri A, Salvadori M, Clune Y, Collins KJ, Paglierani M, Caderni G. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002;23:1953–1960. doi: 10.1093/carcin/23.11.1953. [DOI] [PubMed] [Google Scholar]
- 3.Renner HW, Münzner R. The possible role of probiotics as dietary antimutagen. Mutat Res. 1991;262:239–245. doi: 10.1016/0165-7992(91)90090-q. [DOI] [PubMed] [Google Scholar]
- 4.Lidbeck A, Nord CE, Gustafsson JA, Rafter J. Lactobacilli, anticarcinogenic activities and human intestinal microflora. Eur J Cancer Prev. 1992;1:341–353. doi: 10.1097/00008469-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Vorob'eva LI, Abilev SK. [Antimutagenic properties of bacteria: A review] Prikl Biokhim Mikrobiol. 2002;38:115–127. [PubMed] [Google Scholar]
- 6.Shahani KM, Chandan RC. Nutritional and healthful aspects of cultured and culture-containing dairy foods. J Dairy Sci. 1979;62:1685–1694. doi: 10.3168/jds.S0022-0302(79)83481-5. [DOI] [PubMed] [Google Scholar]
- 7.Pool-Zobel BL, Neudecker C, Domizlaff I, Ji S, Schillinger U, Rumney C, Moretti M, Vilarini I, Scassellati-Sforzolini R, Rowland I. Lactobacillus- and bifidobacterium-mediated antigenotoxicity in the colon of rats. Nutr Cancer. 1996;26:365–380. doi: 10.1080/01635589609514492. [DOI] [PubMed] [Google Scholar]
- 8.Singh J, Rivenson A, Tomita M, Shimamura S, Ishibashi N, Reddy BS. Bifidobacterium longum, a lactic acid-producing intestinal bacterium inhibits colon cancer and modulates the intermediate biomarkers of colon carcinogenesis. Carcinogenesis. 1997;18:833–841. doi: 10.1093/carcin/18.4.833. [DOI] [PubMed] [Google Scholar]
- 9.Tsunoda A, Shibusawa M, Tsunoda Y, Yasuda N, Nakao K, Kusano M. A model for sensitivity determination of anticancer agents against chemically-induced colon cancer in rats. Anticancer Res. 1994;14:2637–2642. [PubMed] [Google Scholar]
- 10.Tsunoda A, Shibusawa M, Tsunoda Y, Yokoyama N, Nakao K, Kusano M, Nomura N, Nagayama S, Takechi T. Antitumor effect of S-1 on DMH induced colon cancer in rats. Anticancer Res. 1998;18:1137–1141. [PubMed] [Google Scholar]
- 11.Li W, Wanibuchi H, Salim EI, Wei M, Yamamoto S, Nishino H, Fukushima S. Inhibition by ginseng of 1,2-dimethylhydrazine induction of aberrant crypt foci in the rat colon. Nutr Cancer. 2000;36:66–73. doi: 10.1207/S15327914NC3601_10. [DOI] [PubMed] [Google Scholar]
- 12.LaMont JT, O'Gorman TA. Experimental colon cancer. Gastroenterology. 1978;75:1157–1169. [PubMed] [Google Scholar]
- 13.Danzi M, Lewin MR, Cruse JP, Clark CG. Combination chemotherapy with 5-fluorouracil (5FU) and 1, 3-bis (2-chloro-ethyl)-1-nitrosourea (BCNU) prolongs survival of rats with dimethylhydrazine-induced colon cancer. Gut. 1983;24:1041–1047. doi: 10.1136/gut.24.11.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon SS, Eto H, Lin CM, Nakamura H, Pawlik TM, Song SU, Tanabe KK. Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res. 1999;59:6251–6256. [PubMed] [Google Scholar]
- 15.Pozharisski KM. Morphology and morphogenesis of experimental epithelial tumors of the intestine. J Natl Cancer Inst. 1975;54:1115–1135. doi: 10.1093/jnci/54.5.1115. [DOI] [PubMed] [Google Scholar]
- 16.de Ruyter PG, Kuipers OP, Beerthuyzen MM, van Alen-Boerrigter I, de Vos WM. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol. 1996;178:3434–3439. doi: 10.1128/jb.178.12.3434-3439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelkar SM, Shenoy MA, Kaklij GS. Antitumor activity of lactic acid bacteria on a solid fibrosarcoma, sarcoma-180 and Ehrlich ascites carcinoma. Cancer Lett. 1988;42:73–77. doi: 10.1016/0304-3835(88)90241-8. [DOI] [PubMed] [Google Scholar]
- 19.Goldin BR, Gualtieri LJ, Moore RP. The effect of Lactobacillus GG on the initiation and promotion of DMH-induced intestinal tumors in the rat. Nutr Cancer. 1996;25:197–204. doi: 10.1080/01635589609514442. [DOI] [PubMed] [Google Scholar]
- 20.Bolognani F, Rumney CJ, Pool-Zobel BL, Rowland IR. Effect of lactobacilli, bifidobacteria and inulin on the formation of aberrant crypt foci in rats. Eur J Nutr. 2001;40:293–300. doi: 10.1007/s394-001-8359-7. [DOI] [PubMed] [Google Scholar]
- 21.Gallaher DD, Khil J. The effect of synbiotics on colon carcinogenesis in rats. J Nutr. 1999;129:1483S–1487S. doi: 10.1093/jn/129.7.1483S. [DOI] [PubMed] [Google Scholar]
- 22.Reddy BS. Possible mechanisms by which pro- and prebiotics influence colon carcinogenesis and tumor growth. J Nutr. 1999;129:1478S–1482S. doi: 10.1093/jn/129.7.1478S. [DOI] [PubMed] [Google Scholar]
- 23.Shackelford LA, Rao DR, Chawan CB, Pulusani SR. Effect of feeding fermented milk on the incidence of chemically induced colon tumors in rats. Nutr Cancer. 1983;5:159–164. doi: 10.1080/01635588309513793. [DOI] [PubMed] [Google Scholar]
- 24.Renner HW, Münzner R. The possible role of probiotics as dietary antimutagen. Mutat Res. 1991;262:239–245. doi: 10.1016/0165-7992(91)90090-q. [DOI] [PubMed] [Google Scholar]
- 25.Tejada-Simon MV, Pestka JJ. Proinflammatory cytokine and nitric oxide induction in murine macrophages by cell wall and cytoplasmic extracts of lactic acid bacteria. J Food Prot. 1999;62:1435–1444. doi: 10.4315/0362-028x-62.12.1435. [DOI] [PubMed] [Google Scholar]
- 26.Morita T, Takeda K, Okumura K. Evaluation of clastogenicity of formic acid, acetic acid and lactic acid on cultured mammalian cells. Mutat Res. 1990;240:195–202. doi: 10.1016/0165-1218(90)90058-a. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen OH, Jørgensen S, Pedersen K, Justesen T. Microbiological evaluation of jejunal aspirates and faecal samples after oral administration of bifidobacteria and lactic acid bacteria. J Appl Bacteriol. 1994;76:469–474. doi: 10.1111/j.1365-2672.1994.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 28.Boven E, Winograd B, Berger DP, Dumont MP, Braakhuis BJ, Fodstad O, Langdon S, Fiebig HH. Phase II preclinical drug screening in human tumor xenografts: A first European multicenter collaborative study. Cancer Res. 1992;52:5940–5947. [PubMed] [Google Scholar]
- 29.Gadducci A, Viacava P, Cosio S, Fanelli G, Fanucchi A, Cecchetti D, Cristofani R, Genazzani AR. Intratumoral microvessel density, response to chemotherapy and clinical outcome of patients with advanced ovarian carcinoma. Anticancer Res. 2003;23:549–556. [PubMed] [Google Scholar]
- 30.Snyder C, Harlan L, Knopf K, Potosky A, Kaplan R. Patterns of care for the treatment of bladder cancer. J Urol. 2003;169:1697–1701. doi: 10.1097/01.ju.0000056727.30546.b7. [DOI] [PubMed] [Google Scholar]
- 31.Raghavan D. Progress in the chemotherapy of metastatic cancer of the urinary tract. Cancer. 2003;97:2050–2055. doi: 10.1002/cncr.11280. [DOI] [PubMed] [Google Scholar]


