Abstract
AIM: To select valuable ultrasonographic predictors for the evaluation of hepatic inflammation and fibrosis degree in chronic hepatitis, and to study the value of ultrasonography in the evaluation of liver fibrosis and compensated liver cirrhosis in comparison with serology and histology.
METHODS: Forty-four ultrasonographic variables were analyzed and screened using color Doppler ultrasound system in 225 patients with chronic viral hepatitis and compensated liver cirrhosis. The valuable ultrasonographic predictors were selected on the basis of a comparison with histopathological findings. The value of ultrasonography and serology in the evaluation of liver fibrosis degree and the diagnosis of compensated liver cirrhosis was also studied and compared. Meanwhile, the influencing factors on ultrasonographic diagnosis of compensated liver cirrhosis were also analyzed.
RESULTS: By statistical analysis, the maximum velocity of portal vein and the degree of gall-bladder wall smoothness were selected as the valuable predictors for the inflammation grade (G), while liver surface, hepatic parenchymal echo pattern, and the wall thickness of gall-bladder were selected as the valuable predictors for the fibrosis stage (S). Three S-related independent ultrasonographyic predictors and three routine serum fibrosis markers (HA, HPCIII and CIV) were used to discriminate variables for the comparison of ultrasonography with serology. The diagnostic accuracy of ultrasonography in moderate fibrosis was higher than that of serology (P < 0.01), while there were no significant differences in the general diagnostic accuracy of fibrosis as well as between mild and severe fibrosis (P < 0.05). There were no significant differences between ultrasonography and serology in the diagnosis of compensated liver cirrhosis. However, the diagnostic accuracy of ultrasonography was higher in inactive liver cirrhosis and lower in active cirrhosis than that of serology (both P < 0.05). False positive and false negative results where found when the diagnosis of compensated liver cirrhosis was made by ultrasonography.
CONCLUSION: There are different ultrasonographic predictors for the evaluation of hepatic inflammation grade and fibrosis stage of chronic hepatitis. Both ultrasonography and serology have their own advantages and disadvantages in the evaluation of liver fibrosis and compensated liver cirrhosis. Combined application of the two methods is hopeful to improve the diagnostic accuracy.
INTRODUCTION
Viral hepatitis is one of the most common and prevalent infectious diseases in China. It was found that 25%-40% of the patients with chronic viral hepatitis would become liver cirrhosis or even hepatocellular carcinoma[1,2]. Prompt and effective treatment could postpone or interrupt the development of chronic hepatitis into liver cirrhosis. Accurate estimation of the disease severity is helpful for the evaluation of the therapeutic effect and the prognosis of the disease. At present, there are three ways for this purpose, namely histology, serology and imaging. Liver histological diagnosis based on needle biopsy is the gold standard to evaluate the degree of hepatic inflammation and fibrosis. However, liver biopsy can not be performed routinely at clinical settings becouse of its invasiveness. In addition, it is well known that liver parenchymal damage in chronic hepatitis and cirrhosis is not uniform. Sample errors were commonly encountered[3-5]. Serological examination is a non-invasive routine method. However, serological markers of fibrosis with high specificity have not been available yet in addition to the lack of organ specificity of these markers[6-8]. Imging technologies such as ultrasonography, computed tomography, magnetic resonance imaging could provide useful information on the morphological changes of the liver, and are important in the evaluation of chronic liver diseases[9-15]. Among them, ultrasonography has become the most common and valuable method because of its low cost, easy performance and high acceptability by the patient. It could provide not only valuable information on the morphological changes of the liver but also liver hemodynamics by color Doppler flow imaging[16-19].
It was reported that evaluating liver morphology and hemodynamics in patients with cirrhosis and portal hypertension had an important value for the estimation of the disease severity and prognosis[17-21]. As the disease progresses from chronic hepatitis to cirrhosis, a variety of intra- and extrahepatic abnormalities would occur. Using multiple ultrasonographic variables to evaluate morphological and hemodynamic changes of the liver, gallbladder and the spleen could be expected to reveal these abnormalites and improve the diagnostic ability of ultrasound[15,22,23].
In this study, 44 ultrasonographyic variables were analyzed and screened using color Doppler ultrasoud system in 225 patients with chronic viral hepatitis and compensated liver cirrhosis. The results were compared with histological findings based on the assessment criteria of the imflammation grade and fibrosis stage for chronic hepatitis, which were proposed by the academic meeting of Chinese Medical Association on epidemic and parasitosis in 1995 (95-Protocol)[24]. The valuable predictors were selected by statistical analysis. The value of ultrasonography in the evaluation of fibrosis and compensated liver cirrhosis was investigated and compared with serology.
MATERIALS AND METHODS
Patients selected
Two hundred and twenty-five patients with chronic viral hepatitis and compensated liver cirrhosis were treated in our hospital from 1996 to 1999. Among them, 199 were males and 26 were females, their average age was 30.5 years (range from 10 to 57 years). The pathogenic diagnoses were: hepatitis B in 208 patients, hepatitis C in 10 patients, coinfection of hepatitis B and C in 3 patients, coinfection of hepatitis B and E in 2 patients, hepatitis B plus A and hepatitis B plus D in 1 patient, respectively. Clinical diagnoses were mild chronic hepatitis in 78 patients, moderate chronic hepatitis in 114 patients, severe chronic hepatitis in 12 patients, and cirrhosis in 21 patients. No clinical manifestations of decompensated liver cirrhosis were found in all the patients.
Fifty-one healthy volunteers were examined as a control group. There were 26 males and 25 females, their average age was 27.8 years (range from 18 to 76 years). Serological and biochemical tests were normal.
Ultrasonographic examination
Using Esaote AU4 color Doppler system with 3.5-5.0 MHz curved probe, ultrasonographic examination was performed for the patients fasted for about 8 h 12-24 h after liver biopsy. First, sonographic findings of the liver, spleen and gallbladder, such as size, form, surface, internal echo, and diameter of vessels were observed and measured. Second, blood flow parameters of the hepatic and splenic vessels were measured by color Doppler flow imaging with the patient holding his/her breath at the end of a quiet breathing. Sample volume was adjusted slightly smaller than the diameter of vessels, and the sample angle was less than 60 degrees. Doppler spectrum with 3-5 stable heart cycles was selected for the measurement, which was performed at least two times and the average value was used.
Forty-four ultrasonographyic variables were analyzed, including the largest oblige diameter (RLOD) and anterior-posterior diameter (RLAP) of the right liver lobe, longitudinal diameter and anterior-posterior diameter of the left liver lobe (LLL, LLAP), the caudate liver lobe (CLL, CLAP), the ratio of CLL/LLL, transverse diameter (SPT) and longitudinal diameter (SPL) of the spleen, liver surface (Lsur) and hepatic parenchymal echo pattern (LE), size (GBsiz) and wall thickness (GBT) of the gall-bladder, degree of gall-bladder wall smoothness (GBsm), complication of gall-bladder stone (GBst) and gall-bladder pulypus (GBP), umbilical vein patent (U); diameter of the main portal vein trunk (PVD), right portal vein (RPVD) and left portal vein (LPVD); maximum and mean blood flow velocity of the main portal vein trunk (PVmax, PVmea), right portal vein (RPVD, RPVmax) and left portal vein (LPVmax, LPVmea), blood flow volume of the main portal vein trunk (PVF), right portal vein (RPVF) and left portal vein (LPVF); congestive index of the portal vein (CI); diameter (HVD) and Doppler waveform (HVW) of the hepatic vein; diameter (HAD), peak systolic blood flow velocity (HAS), blood flow volume (HAF) and resistant index (HARI) of the hepatic artery; diameter (SPAD), peak systolic blood flow velocity (SPAS), blood flow volume (SPAF), and resistant index (SPARI) of the splenic artery; diameter (SPVD) and maximum blood flow velocity (SPVmax) of the splenic vein, and the ratio of SPVF/PVF.
The measurements and evaluation of the parameters referred to the methods described in the literature[1,4-6]. Liver surface and the degree of gall-bladder wall smoothness were classified into 3 grades, namely smooth, less smooth and rough. Hepatic parenchymal echo patterns were classified into 5 grades according to the distribution and echogenicity of the parenchymal echo texture as the renal parenchymal echo was used as the contrast.
Examination of serum fibrosis markers
One hundred and ninety-seven cases underwent ultrasonographic and serologic examinations successively. Among them, 48 had compensated cirrhosis, and the rest had chronic hepatitis. The serum fibrosis markers including hyalurionic acid (HA), human procollagen III (HPCIII) and collagen type IV (CIV) were tested using radioimmunological method. The normal ranges were HA (57 ± 27 ng/L), HPCIII (≤ 120 μg/L)and CIV (38.22 ± 11.88 μg/L)
Histological study
All the patients underwent liver biopsy. The tissue sections were stained with HE and reticular fibers respectively. Histological study was performed according to the 95-Protocol[24], in which inflammation grade (G) and fibrosis stage (S) were classified from G1 to G4, and S0 to S4, respectively, based on the various degrees of inflammation or fibrosis. Furthermore, G1-2, G3 and G4 were defined as mild, moderate and severe inflammation, respectively, while S0-2, S3 and S4 were defined as mild, moderate and severe fibrosis, respectively. S4 also meant early or definite cirrhosis.
Quantitative assessment of liver fibrosis
Quantitative assessment of liver fibrosis was performed in 107 liver specimens with reticular fiber stain using an imagine analysis system (KONTRON IBAS2.5, German). The images were amplified by 400 times. Fibrosis degree was expressed as the ratio of fibrous area to the section area. Three to five fields of vision were selected randomly at the peripheral and center of the section for the average value of the ratio.
Statistical analysis
SPSS 9.0 software was used for this study. Numerical variables were described as mean ± standard deviation. For the comparison of means in different groups, univariate analysis and Student-Newman-Keuls test were used. While for the categorical variables, non-parameter analysis and χ² test were used for the comparison. A P value less than 0.05 was considered statistically significant. Spearman’s rank correlation coefficient was used for the correlation analysis. As for the selection of useful variables from categorical and numerical data, stepwise discriminant analysis and multiple linear regression analysis were used, respectively.
RESULTS
Screening for valuable ultrasonographic predictors
Histological diagnosis of the case group is shown in Table 1.
Table 1.
Histological results in case group
| Inflammation grade (G) | No. subjects | Fibrosis stage (S) | No. subjects |
| 0 | 0 | 0 | 7 |
| 1 | 31 | 1 | 54 |
| 2 | 85 | 2 | 66 |
| 3 | 85 | 3 | 52 |
| 4 | 24 | 4 | 46 |
| Total | 225 | 225 |
According to univariate analysis, there were 21 variables with significant difference (P < 0.05) among groups classified by the inflammation grade. While there were 19 variables with significant difference (P < 0.05) among groups classified by the fibrosis stage (Table 2 and Table 3). When stepwise correlation analysis was used, 20 variables were correlated with G (P < 0.01), and 18 variables were correlated with S (P < 0.01).
Table 2.
Univariate analysis of quantitative variables
| Variables |
Grouped by G |
Grouped by G |
||
| F value | P value | F value | P value | |
| PVmax | 29.17 | < 0.001 | 19.38 | < 0.001 |
| GBT | 24.03 | < 0.001 | 14.60 | < 0.001 |
| PVD | 20.43 | < 0.001 | 18.07 | < 0.001 |
| PVmea | 18.69 | < 0.001 | 11.28 | < 0.001 |
| HVD | 16.88 | < 0.001 | 20.21 | < 0.001 |
| RPVmax | 14.24 | < 0.001 | 7.18 | < 0.001 |
| SPL | 11.57 | < 0.001 | 14.87 | < 0.001 |
| SPVD | 9.47 | < 0.001 | 12.76 | < 0.001 |
| SPT | 9.28 | < 0.001 | 11.18 | < 0.001 |
| CI | 5.22 | < 0.01 | 3.96 | < 0.01 |
| RPVD | 5.30 | < 0.01 | 3.43 | < 0.01 |
| LLL | 2.99 | < 0.05 | 3.41 | < 0.05 |
| SPVF | 2.72 | < 0.05 | 4.97 | < 0.01 |
| SPAD | 4.20 | < 0.01 | 2.29 | < 0.05 |
Table 3.
Non-parametric analysis of categorical variables
| Variables |
Grouped by G |
Grouped by G |
||
| χ² value | P value | χ² value | P value | |
| LE | 50.17 | < 0.001 | 91.34 | < 0.001 |
| GBsiz | 22.18 | < 0.001 | 21.00 | < 0.001 |
| HVW | 22.14 | < 0.001 | 33.34 | < 0.001 |
| GBsm | 20.07 | < 0.001 | 78.99 | < 0.001 |
| Lsur | 18.31 | < 0.001 | 66.92 | < 0.001 |
| U | 13.66 | < 0.01 | 32.07 | < 0.001 |
| GBst | 8.45 | < 0.05 | 6.06 | > 0.05 |
The correlation coefficient between the quantitative analysis results of liver fibrosis and S was 0.76 (P < 0.001). The correlation coefficient between G and S was 0.75 (P < 0.001).
Twenty-two ultrasonographic variables associated with G and 19 variables associated with S were selected on the basis of univariate and correlation analysis results (Table 4), and used as the preliminary valuable variables for further analysis. When compared between case group and control group, there were significant differences in these selected variables (P < 0.05).
Table 4.
Ultrasonographic variables with significant correlation with G and S
| Ultrasonographic variables | |
| Associated with G (22 items) | GBsm, PVmax, GBT, LE, PVD, HVD, PVmea, RPVmax, SPL, SPT, SPVD, HVW, GBsiz, Lsur, CI, RPVD, RPVmea, U, LLL, GBst, SPVF and SPAD |
| Associated with S (19 items) | GBT, LE, GBsm, PVD, PVmax, Lsur, HVD, PVmea, RPVmax, SPL, SPT, SPVD, HVW, GBsiz, CI, U, LLL, SPVF and RPVD |
Stepwise discriminant analysis was used for further selection of valuable predictors. GBsm, PVmax were finally selected as independent predictors which were significantly correlated to G, while the independent predictors of LE, GBT and Lsur were significantly correlated to S.
Comparison of ultrasonography and serology in evaluation of fibrosis degree and diagnosis of compensated cirrhosis
Evaluation of fibrosis degree Liver fibrosis was divided into mild, moderate and severe fibrosis on the basis of fibrosis stage according to the 95-Protocol. The case distribution was mild fibrosis in 127 patients, moderate fibrosis in 52 patients, and severe fibrosis in 46 patients.
Three independent ultrasonographyic predictors associated with S (LE, GBT, Lsur) and three serum fibrosis markers (HA, HPCIII and CIV) were taken as discriminating variables for the comparison.
The correlation coefficients of LE, GBT and Lsur were 0.63, 0.58 and 0.5, respectively (P < 0.001). While the correlation coefficients of HA, HPCIII and CIV were 0.60, 0.46 and 0.50, respectively (P < 0.001).
The comparison between ultrasonographic variables and serologic fibrosis markers for the evaluation of fibrosis degree is shown in Table 5.
Table 5.
Diagnostic accuracy of ultrasonography and serology for the evaluation of fibrosis degrees (%)
| Mild fibrosis | Moderate fibrosis | Severe fibrosis | |
| Serology | 79.5 | 19.1b | 66.7 |
| Ultrasonography | 74.5 | 46.8b | 62.5 |
P < 0.01 serology vs ultrasonography.
The diagnostic accuracy of ultrasonography and serology was higher in mild liver fibrosis than in severe and moderate liver fibrosis. There were no significant differences between ultrasonography and serology in the general diagnostic accuracy of fibrosis and diagnostic accuracy of mild and severe fibrosis (P > 0.05). However, the diagnostic accuracy of ultrasonography in moderate fibrosis was higher than that of serology (P < 0.01).
Diagnosis of compensated cirrhosis
Among the 48 patients with compensated cirrhosis, 46 patients had hepatitis-related cirrhosis, and 2 patients had cholestatic cirrhosis. The fibrosis stage of all these patients was S4. All of them had no symptoms of decompensated cirrhosis.
The diagnostic accuracy of ultrasonography for compensated cirrhosis was 80.7%, while that of serology was 79.7%. There was no significant difference between them. However, ultrasonography had a lower sensitivity (62.5% vs 72.9%) and a higher specificity (86.6% vs 81.9%) compared with serology (P < 0.05).
According to the 95-Protocol, it would be regarded as active cirrhosis when G > 2 and S = 4, while it was regarded as inactive cirrhosis when G ≤ 2 and S = 4. In our patient group, there were 41 patients with active cirrhosis and 7 patients with inactive cirrhosis. Among the 7 patients with inactive cirrhosis, the clinical diagnoses were mild chronic hepatitis in 3 patients, moderate chronic hepatitis in 2 patients, and liver cirrhosis in 2 patients.
For the evaluation of inactive liver cirrhosis, the diagnostic accuracy of ultrasonography was higher than that of serology (100% vs 42.9%, P < 0.05). However, the diagnostic accuracy of ultrasonography was lower than that of serology in active cirrhosis (56.1% vs 78.0%, P < 0.05).
Ultrasonographic, histological and clinical figures of compensated cirrhosis with false positive and false negative results on ultrasonography
False negative results of cirrhosis on ultrasonography Eighteen patients who were predicted as mild or moderate fibrosis by ultrasonography were finally diagnosed as cirrhosis by histology. Among them, 9 patients were diagnosed as moderate chronic hepatitis, 5 patients as severe chronic hepatitis and 4 patients as liver cirrhosis according to their clinical manifestations.
The ultrasonographic features were coarse and homogenous hepatic parenchymal echo patterns (Figure 1). All of these patients had active liver cirrhosis with fine and sparse fibrotic septa (Figure 2) or small and uniform pseudolobular nodules on histology. Serologic tests showed that liver dysfunction was evident. The serum level of fibrosis markers in these patients increased obviously (HA: 408.06 ± 219.26, HPC3: 217.78 ± 84.96, and IVC: 210.28 ± 181.88).
Figure 1.

Active cirrhosis. Ultrasonography showed that the hepatic parenchymal echo became coarse and dot-shaped, and the distribution was still homogenous.
Figure 2.
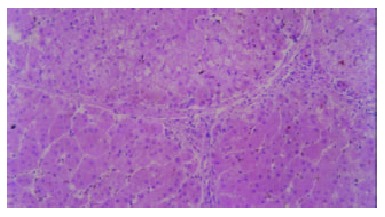
Active cirrhosis. Small and sparse fibroitc septa were shown on histology. (HE stain × 100).
False positive results of cirrhosis on ultrasonography Twenty patients who were predicted as liver cirrhosis by ultrsonography were finally diagnosed as mild (n = 6) and moderate (n = 14) fibrosis by histology. Among them, 3 patients were diagnosed as mild chronic hepatitis, 12 patients as moderate chronic hepatitis, 1 patient as severe chronic hepatitis and 4 patients as liver cirrhosis according to their clinical manifestations.
The ultrasonographic and histological features were as follows. Hepatic parenchymal echo patterns became coarse and heterogeneous with speckled hyper- and hypoechoic areas (Figure 3), the correspondent histological findings were fatty degeneration foci heterogeneously distributed within the sections in addition to the inflammation and fibrosis changes (Figure 4), and changes of liver cirrhosis were not found. Hepatic parenchymal echoes were strip-shaped and coarse (Figure 5), the correspondent histology showed wide and compact fibrotic septa (Figure 6), lobular generation was not evident.
Figure 3.

Chronic viral hepatitis. On ultrasonography, the hepatic parenchymal echo became coarse and heterogeneous with speckled hyper- and hypoechoic areas.
Figure 4.

Chronic viral hepatitis. On histology, focal fatty degeneration foci were shown. (HE stain, × 40).
Figure 5.
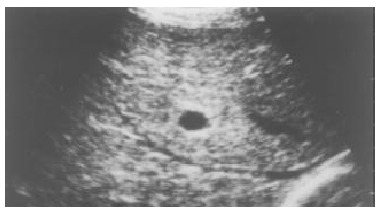
Chronic viral hepatitis. Ultrasonography showed the hepatic parenchymal echo to be strip-shaped and coarse.
Figure 6.
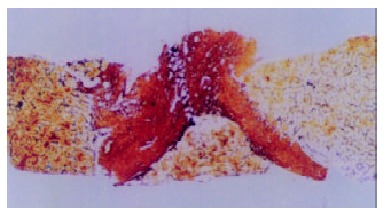
Chronic viral hepatitis. Wide and compact fibrotic septum was shown histologically. (Reticular fiber stain, × 40).
DISCUSSION
Among the multiple ultrasonographic parameters, PVmax and GBsm were selected as the independent predictors for the evaluation of fibrosis stage, while LE, Lsur and GBT were the independent predictors for the evaluation of inflammation grade. The results of this study showed that the portal venous velocity decreased and the wall of gallbladder became rough with the progress of fibrosis stage. While as the aggravation of inflammation grade, the liver parenchymal echo became coarse and heterogeneous, liver surface was rough and irregular, and the gallbladder wall became thick.
The decrease of portal venous velocity might relate to the increase of portal venous resistance. It was reported that patients with acute hepatitis and fulminant hepatitis developed portal hypertension with the aggravation of hepatic inflammation degrees[25,26].
There have been only a few reports about the relationship between the hemodynamic changes of portal vein and the histological changes in chronic hepatitis[22,27]. Aube et al[22] considered that the decrease of portal venous velocity was closely correlated with the histological degree of fibrosis. Our investigation showed that the decrease of portal venous velocity was significantly correlated not only with fibrosis stage but also with inflammation grade. The difference in the histological evaluating protocol used, and the difference in the sample size might partially account for the discrepancy.
The development of liver fibrosis into cirrhosis is a gradient course. There are non-specific findings on imaging. However, as the progresses of liver fibrosis reached to a certain degree, the liver pathological changes would become obvious. The acoustic interfaces would increase and the acoustic impedance between fibrotic tissue and other hepatic tissues would become large. On ultrasonography, the echo pattern of liver parenchymal would become coarse and echogenic, and the liver surface would become irregular. In this sense, the ultrasonographic features of liver parenchymal echo and liver surface would directly reflect the fibrotic changes in chronic hepatitis and liver cirrhosis, and thus playing an important role in the evaluation of fibrosis degree.
There is a close relationship between biliary system and the liver in histogenesis, anatomy and function. Viral hepatitis is often associated with biliary disorders. It was reported that abnormal ultrasonographic findings of the gallbladder such as wall thickening and double-edge sign, and abnormal size of the gallbladder and gallbladder stones were frequent in patients with hepatitis and liver cirrhosis[28-31]. The mechanism underlying the gallbladder disorders in viral hepatitis is still unclear. Some factors have been considered to be related with it[31,32] such as direct invasiveness of hepatitis virus, secondary infections, immunity injury, and edema of the gallbladder wall due to portal hypertension, circumfluence obstruction of the gallbladder vein, etc.
Ultrasonography and serology are both non-invasive methods in the evaluation of liver fibrosis and cirrhosis. However, their viewing aspects are different. The former is given priority to the reflection of morphological changes, while the latter reflects the function and metabolic changes. The knowledge about what are the similarities and differences between them in evaluating the liver fibrosis degree and diagnosis of cirrhosis is rarely known at present.
In our investigation, three independent ultrasonographic predictors correlated with fibrosis stage were chosen and compared with three serologic variables. Results showed that the correlation coefficients of ultrasonographic variables were similar to those of serological variables. There was the same tendency that the diagnostic accuracy of mild liver fibrosis was higher than that of moderate and severe fibrosis in both ultrasonography and serology, and the diagnostic accuracy of moderate liver cirrhosis was higher in ultrasonography. There were no significant differences between ultrasonography and serology in evaluating mild and severe liver fibrosis.
Referring to the diagnosis of compensated liver cirrhosis, there was no significant difference between ultrasonography and serology. However, the diagnostic sensitivity of ultrasonography was lower than that of serology, but its diagnostic specificity was higher than that of serology. It suggested that serological results should be mainly consulted for the early detection of pathological changes, while for the sake of exceptional diagnosis of cirrhosis, doctors had better to consult the results of ultrasonography in clinical practice.
Although the general diagnostic accuracy of compensated liver cirrhosis by ultrasonography and serology was similar, they were different in evaluating active and inactive stage cirrhosis. Results in our study showed that the diagnostic accuracy of ultrasonography for evaluating active stage cirrhosis was lower than that of serology with a high possibility of false-positive results, while the diagnostic accuracy for evaluating inactive stage cirrhosis was higher by ultrasonography than by serology. This difference might reflect the different features of the two modalities.
The serum fibrosis markers might reflect the activity of liver fibrosis and cirrhosis. When there was extensive inflammation in the liver, the level of serum fibrosis markers would raise with the active aggradation and degradation of the extracellular stroma. However, even if there was extensive fibrosis in the inactive stage cirrhosis, the level of serum fibrosis markers would be normal without active aggradation of the extracellular stroma[7]. Our results also illuminated that the level of serum fibrosis variables was distinctly higher in active stage cirrhosis than in inactive stage cirrhosis. Ultrasonography reflected the morphological changes of liver fibrosis and cirrhosis. When the accumulation of tissue morphological changes reached a certain degree, ultrasonography could depict these changes to a certain extent even if it was in the inactive fibrosis stage. Nevertheless, for the active fibrosis course at the level of cell and molecule, ultrasonography might fail to detect these fine morphological changes, and thus could not accurately evaluate the state of the disease.
It was inferred from our investigation that it was important to combine the two modalities for the evaluation of liver fibrosis and cirrhosis in clinical practice. When the level of serum fibrosis markers was normal, and the liver function damage was mild, liver cirrhosis should not be easily excluded with the distinct findings of cirrhosis showed by ultrasonography. The inactive stage cirrhosis might be possible at this situation. While the level of serum fibrosis markers rose obviously, and the liver function damage was severe, diagnosis of active stage cirrhosis should be considered with a long history of hepatitis viral infection even if there were no typical findings of cirrhosis on ultrasonography.
These results indicated that ultrasonography and serology both had their own advantages and disadvantages in the evaluation of liver fibrosis and liver cirrhosis.
The limitation of our investigation was that the case number of inactive stage cirrhosis was small. It was only a preliminary study and should be conducted more profoundly with a large sample size.
Ultrasonography is valuable in the evaluation of liver cirrhosis because of its low cost, easy performance, and high acceptability by the patients. However it is not a specific method and the diagnostic accuracy is still to be improved. Several factors could affect the diagnostic accuracy such as extra- and intraobserver variability, technical level of the operator, interference of obesity, ascites and intestinal gas, and modulation of the apparatus.
False positive and negative results might appear when diagnosis of compensated liver cirrhosis was made by ultrasonography. In patients with active stage liver cirrhosis, ultrasonography could not depict the abnormalities caused by histological changes such as fine and sparse fibrotic septa or small and uniform pseudolobular nodules, thus false negative diagnosis might be made. However, high level of serum fibrotic markers might be valuable for the accurate diagnosis.
In some patients with a relative long history of chronic hepatitis, liver active inflammation and inactive phase occurred by turns, which made the pathological changes of the liver more complicated. The changes such as focal fatty degeneration or wide and compact fibrotic septa might cause coarse and heterogeneous parenchyma echo patterns in the liver, leading to a false positive diagnosis of liver cirrhosis on ultrasonography. The possible mechanisms might be assumed as follows. The acoustic interfaces between different liver tissues changed and the scatters increased because of focal fatty degeneration of the liver cells, thus causing focal echo attenuation and heterogeneous parenchyma echo patterns in the liver. When the fibrotic septa became wide and compact, the acoustic interfaces also became large and the acoustic impedance increased, causing coarse parenchyma echo patterns in the liver.
Hepatic pathological changes are often heterogeneous in patients with chronic hepatitis and liver cirrhosis. Sampling errors are liable. Gaiani et al[23] reported that thirty-two patients considered as liver cirrhosis by ultrasonography were identified as chronic hepatitis by histology. However, eight of these patients showed clinical manifestations of decompensated cirrhosis in the follow-up for half a year later. It indicated that ultrasonography could make up the deficiency of histology in the diagnosis of liver cirrhosis in certain situations.
In summary, there are different ultrasonographic predictors for the evaluation of hepatic inflammation grade and fibrosis stage of chronic hepatitis. Both ultrasonography and serology have their own advantages and disadvantages in the evaluation of liver fibrosis and compensated liver cirrhosis. False positive and negative results may occur in the diagnosis of compensated liver cirrhosis by ultrasonography. Combined application of ultrasonography and serology can contribute to the improvement in their diagnostic accuracy.
Footnotes
Edited by Zhang JZ and Wang XL
Supported by the Medical Science Foundation of Guangdong Province, No. A1999198
References
- 1.Wang BE. Liver fibrosis: diagnosis and evaluation of disease severity. Zhonghua Ganzangbing Zazhi. 1998;6:193–194. [Google Scholar]
- 2.Yang XB, Huang ZM, Wang JH. The drug therapy of liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2002;10:956–957. [Google Scholar]
- 3.Cui DL, Yao XX. Serum test of liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:683–684. [Google Scholar]
- 4.Yao SK, Yin F. The early diagnosis of liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:681–683. [Google Scholar]
- 5.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 6.Bai WY, Yao XX, Feng LY. Researching of liver fibrosis: status in quo. Shijie Huaren Xiaohua Zazhi. 2000;8:1267–1268. [Google Scholar]
- 7.Luo KX. Serodiagnosis of liver fibrosis. Linchuang Gandanbing Zazhi. 1996;12:1–2. [Google Scholar]
- 8.Jing B, Li YB. Diagnostic strategy for liver fibrosis. Zhonghua Xiaohua Zazhi. 1997;17:170–172. [Google Scholar]
- 9.Harisinghani MG, Hahn PF. Computed tomography and magnetic resonance imaging evaluation of liver cancer. Gastroenterol Clin North Am. 2002;31:759–776, vi. doi: 10.1016/s0889-8553(02)00028-6. [DOI] [PubMed] [Google Scholar]
- 10.Martin DR. Magnetic resonance imaging of diffuse liver diseases. Top Magn Reson Imaging. 2002;13:151–163. doi: 10.1097/00002142-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Mitchell DG, Ito K, Kim JH, Pasqualin D, Rubin R. Hepatic iron deposition on magnetic resonance imaging: correlation with inflammatory activity. J Comput Assist Tomogr. 2002;26:988–993. doi: 10.1097/00004728-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Hung CH, Lu SN, Wang JH, Lee CM, Chen TM, Tung HD, Chen CH, Huang WS, Changchien CS. Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J Gastroenterol. 2003;38:153–157. doi: 10.1007/s005350300025. [DOI] [PubMed] [Google Scholar]
- 13.Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: Accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89–94. doi: 10.1148/radiol.2272020193. [DOI] [PubMed] [Google Scholar]
- 14.Filly RA, Reddy SG, Nalbandian AB, Lu Y, Callen PW. Sonographic evaluation of liver nodularity: Inspection of deep versus superficial surfaces of the liver. J Clin Ultrasound. 2002;30:399–407. doi: 10.1002/jcu.10095. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Wang B, Cao H. An ultrasound scoring system for the diagnosis of liver fibrosis and cirrhosis. Chin Med J ( Engl) 1999;112:1125–1128. [PubMed] [Google Scholar]
- 16.Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21:1023–1032; quiz 1023-1032;. doi: 10.7863/jum.2002.21.9.1023. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Noguera A, Montserrat E, Torrubia S, Villalba J. Doppler in hepatic cirrhosis and chronic hepatitis. Semin Ultrasound CT MR. 2002;23:19–36. doi: 10.1016/s0887-2171(02)90027-2. [DOI] [PubMed] [Google Scholar]
- 18.Arda K, Ofelli M, Calikoglu U, Olçer T, Cumhur T. Hepatic vein Doppler waveform changes in early stage (Child-Pugh A) chronic parenchymal liver disease. J Clin Ultrasound. 1997;25:15–19. doi: 10.1002/(sici)1097-0096(199701)25:1<15::aid-jcu3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Gorka W, al Mulla A, al Sebayel M, Altraif I, Gorka TS. Qualitative hepatic venous Doppler sonography versus portal flowmetry in predicting the severity of esophageal varices in hepatitis C cirrhosis. AJR. Am J Roentgenol. 1997;169:511–515. doi: 10.2214/ajr.169.2.9242766. [DOI] [PubMed] [Google Scholar]
- 20.Li XH, Wang L, Fang YW, Lu YK. Color Doppler evaluation for the hemodynamics of portal hypertension in liver cirrhosis. Shijie Huaren Xiaohua Zazhi. 1999;7:453–454. [Google Scholar]
- 21.Ohta M, Hashizume M, Kawanaka H, Akazawa K, Tomikawa M, Higashi H, Kishihara F, Tanoue K, Sugimachi K. Prognostic significance of hepatic vein waveform by Doppler ultrasonography in cirrhotic patients with portal hypertension. Am J Gastroenterol. 1995;90:1853–1857. [PubMed] [Google Scholar]
- 22.Aubé C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, Valsesia E, Pilette C, Rousselet MC, Bedossa P, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol. 1999;30:472–478. doi: 10.1016/s0168-8278(99)80107-x. [DOI] [PubMed] [Google Scholar]
- 23.Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D'Errico A, Zironi G, Grigioni W, Bolondi L. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27:979–985. doi: 10.1016/s0168-8278(97)80140-7. [DOI] [PubMed] [Google Scholar]
- 24.The academic meeting of Chinese Medical Association on Epidemic and Parasitosis in Beijing. The protocol for the prevention and treatment of viral hepatitis. Zhonghua Chuanranbing Zazhi. 1995;13:241–247. [Google Scholar]
- 25.Yang SS, Wu CH, Chen TK, Lee CL, Lai YC, Chen DS. Portal blood flow in acute hepatitis with and without ascites: A non-invasive measurement using an ultrasonic Doppler. J Gastroenterol Hepatol. 1995;10:36–41. doi: 10.1111/j.1440-1746.1995.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 26.Tai DI, Changchien CS, Chen CJ, Huang CS, Lo SK, Kuo CH. Changes in portal venous hemodynamics in patients with severe acute hepatitis over one year. J Clin Ultrasound. 2000;28:83–88. doi: 10.1002/(sici)1097-0096(200002)28:2<83::aid-jcu5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Koda M, Murawaki Y, Kawasaki H, Ikawa S. Portal blood velocity and portal blood flow in patients with chronic viral hepatitis: relation to histological liver fibrosis. Hepatogastroenterology. 1996;43:199–202. [PubMed] [Google Scholar]
- 28.Wang TF, Hwang SJ, Lee EY, Tsai YT, Lin HC, Li CP, Cheng HM, Liu HJ, Wang SS, Lee SD. Gall-bladder wall thickening in patients with liver cirrhosis. J Gastroenterol Hepatol. 1997;12:445–449. doi: 10.1111/j.1440-1746.1997.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 29.Dogra R, Singh J, Sharma MP. Enterically transmitted non-A, non-B hepatitis mimicking acute cholecystitis. Am J Gastroenterol. 1995;90:764–766. [PubMed] [Google Scholar]
- 30.Portincasa P, Moschetta A, Di Ciaula A, Palmieri VO, Milella M, Pastore G, Palasciano G. Changes of gallbladder and gastric dynamics in patients with acute hepatitis A. Eur J Clin Invest. 2001;31:617–622. doi: 10.1046/j.1365-2362.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- 31.Xiao SS. Ultrasonigraphic abnormalities of gallbladder in patients with viral hepatitis and liver cirrhosis: clinicalsignificance. Weichangbingxue He Ganbingxue Zazhi. 1996;5:77–79. [Google Scholar]
- 32.Yan FM, Chen AS, Hao F, Zhao XP, Gu CH, Zhao LB, Yang DL, Hao LJ. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6:805–811. doi: 10.3748/wjg.v6.i6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]


