Abstract
AIM: To investigate the effect of tetramethylpyrazine (ligustrazine, TMP) on the secretion of exocrine pancreas (and biliary).
METHODS: In in vivo study, we investigated the effect of TMP on the secretion of pancreatic-bile juice (PBJ) in rats. Using human pancreatic duct cell line, CAPAN-1, combined with the short-circuit current (ISC) technique we further studied the effect of TMP on the pancreatic anion secretion.
RESULTS: Administration of TMP (80 mg/kg, ip) significantly increased the secretion of PBJ (P < 0.05), but the pH of PBJ and the secretion of pancreatic protein were not significantly affected. Basolateral addition of TMP produced a dose-dependent increase in ISC (EC50 = 1.56 mmol/L), which contained a fast transient ISC response followed by a slow decay. Apical application of Cl- channel blockers, DPC (1 mmol/L), decreased the response by about 67.1% (P < 0.001), whereas amiloride (100 μmol/L), a epithelial sodium channel blockers, had no effect. Removal of extracellular HCO3- abolished TMP-induced increase in ISC by about 74.4% (P < 0.001), but the removal of external Cl- did not. Pretreatment with phosphodiesterase inhibitor, IBMX (0.5 mmol/L), decreased the TMP-induced ISC by 91% (P < 0.001).
CONCLUSION: TMP could stimulate the secretion of PBJ, especially pancreatic ductal HCO3- secretion via cAMP or cGMP-dependent pathway. It need further study to investigate the roles of cAMP or cGMP in the effect of TMP on the secretion of exocrine pancreas.
INTRODUCTION
Tetramethylpyrazine (TMP, C8H12N2, molecular mass 136.20 u, also known as ligustrazine) is an active alkaloid contained in the rhizome of Chuanxiong[1]. TMP has been widely used for the treatment of patients with cardiovascular and cerebrovascular diseases[1-5]. Its proposed pharmacological actions include antagonizing calcium mobilization[6], inhibiting platelet aggregation[7] and increasing intracellular cAMP level by inhibiting phosphodiesterase activity[8]. Recently it has been reported that TMP has antioxidant effect, reducing free radical generation[9,10] and decreasing nitric oxide production[11,12]. However, the effect of TMP on the pancreatic exocrine secretion are unknown. Since TMP is known to activate cAMP, it may act similarly to secretin, a physiological regulator of pancreatic secretion by elevating intracellular cAMP to act on pancreatic epithelial CFTR, which is a cAMP/cGMP-regulated Cl- channel[13,14] and involved in pancreatic HCO3- secretion. In the present study, we investigated the effect of TMP on the secretion of pancreatic-bile juice (PBJ), which includes both pancreatic protein and HCO3-. We also undertook the present study using the short-circuit current (ISC) technique to investigate TMP effect on HCO3- secretion by human pancreatic duct cell line, CAPAN-1, which retains most of the properties of pancreatic duct cells[15,16].
MATERIALS AND METHODS
Materials
Hydrochloride tetramethylpyrazine was purchased from the First Chengdu pharmaceutical Factory (Chengdu, China). RPMI 1640 medium and fetal bovine serum, trypsin-EDTA were supplied by Gibco Laboratories (New York). Hanks’ balanced salt solution (HBSS), diphenylamine-2, 2’-dicarboxylic acid (DPC), glucose, calcium gluconate, N-2-hydroxethypiperazine-N’-2-ethanessulfonic acid (HEPES), sodium bicarbonate, DMEG, penicillin-streptomycin (P/S) and Bradford reagent were supplied by Sigma Chemical (St.Louis, MO). Calcium chloride, magnesium sulfate, potassium chloride, sodium chloride, were obtained from Merck (Darmstadt, Germany). Potassium gluconate and sodium gluconate were from BDH Chemicals (Poole, England). Tris was from Amersham Biosciences (Stockholm, Sweden).
Methods
Animal preparation Animal experimentation was conducted according to institutional guidelines. Adult male Sprague-Dawley rats, weighing 220-280 g, were housed under controlled temperature (23 °C) and fasted for 12 h with free access to water before surgery. Midline abdominal incisions were made under xylazine and ketamine anesthesia (13 and 87 mg/kg body weight, respectively, im), followed by insertion of a polyethylene tube into the proximal duodenum for diversion of PBJ to the duodenum. A pancreatic duct cannula was made by inserting a polyethylene tube at the junction between the pancreatic duct and the duodenal wall for collection of PBJ[17-19].
Collection and measurement of exocrine pancreatic-bile secretions The animals were divided into two groups randomly: TMP group (TMP 80 mg/kg, ip. pH2.3) and control group (9 g/L NaCl solution, ip. pH2.3). After a 30-min stabilization period, pancreatic-bile secretions were collected every 15 min for 90 min. The volume was measured by a 1 mL syringe and the pH value of PBJ was determined by a pH analyzer. 10 μL of PBJ was taken and diluted for pancreatic protein determination using spectra MAX 250 and Brandford reagent. The remaining undiluted PBJ was pumped into the duodenum via the duodenal cannula during the next collection period. Administrations were given after 2-time collections (30 min) and the second 15-min-secretion was the basal PBJ secretion.
Cell culture Experiments were performed on the human pancreatic duct cell line, CAPAN-1 at a passage of 50-58. Cells were grown in RPMI 1640 medium with 200 mL/L fetal bovine serum and 10 g/L penicillin-streptomycin (P/S) as described previously[20,21]. Briefly, a volume of 0.2 mL of the cell suspension (1.5 × 109/L) was plated onto each permeable support, which was made of a millipore filter and a silicon ring with a confined area of 0.45 cm2, floating on culture medium and incubated at 37 °C with 50 mL/L CO2 and 950 mL/L O2 for 7-8 d. The cells were used for ISC once the monolayers reached confluence.
Short-circuit current measurement The basic principles of the short-circuit current experiments performed in the present study were the same as previously described[20,21]. Monolayers grown on permeable supports were clamped vertically between two halves of the Ussing chamber and bathed in Krebs-Henseleit (K-H) solutions with following compositions (mmol/L): NaCl, 117; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 24.8; CaCl2, 2.56; Glucose, 11.1; with an osmalarity of 285 gassed with 950 mL/L O2 and 50 mL/L CO2. In some experiments, gluconate was used to replace Cl-. For HCO3- free solution, HEPES and Tris were used and the solution was gassed in air. All the electrodes were connected to the voltage-current clamp amplifier. The signal output from the amplifier was the ISC measured and recorded online by chart recorders. A 0.1 mV voltage pulse was applied intermittently across the epithelium and the transepithelial resistance was calculated from the corresponding current changes.
Statistical analysis
The data were collected and analyzed by SPSS statistical package 10.0. The results were expressed as ¯x ± S-x. Comparisons between groups of data were carried out using Student’s paired or unpaired t-test. Comparisons in one group of data were carried out using One-way ANOVA. A P-value less than 0.05 was considered to be statistically significant. EC50 values were determined by non-linear regression by GraphPad Prism software.
RESULTS
Effect of TMP on secretion of PBJ
Table 1 shows the volume of pancreatic-bile juice (PBJ) collected at different time points before and after intraperitoneal administration of TMP or 9 mL/L NaCl (control). Compared with the basal secretion, the PBJ secretion in TMP treated rats was increased by 7.0% (P < 0.05), 22.3% (P < 0.001), 14.3% (P < 0.01), 18.2% (P < 0.01) and 14.2% (P < 0.01) at 15, 30, 45, 60 and 75 min, respectively (n = 13) (Figure 1). However, the same treatment with of 9 mL/L NaCl solution did not produce significant effect (n = 12) (Figure 1). Protein content in PBJ and pH of PBJ were shown to have no differences between TMP-treated and control groups (data not shown).
Table 1.
Volume of secretion of pancreatic bile juice (¯x ± S -x, μL)
| Groups | TMP n = 13 | Control n = 12 | |
| Basal | 372.3 ± 22.2 | 342.5 ± 15.8 | |
| 15 min | 398.5 ± 23.3 | 326.7 ± 1.74 | |
| After | 30 min | 455.4 ± 18. ± 8 | 344.2 ± 14.5 |
| administration | 45 min | 425.4 ± 19.8 | 331.7 ± 17.5 |
| 60 min | 440.0 ± 25.8 | 357.5 ± 18.8 | |
| 75 min | 425.0 ± 25.2 | 342.5 ± 18.2 |
Figure 1.
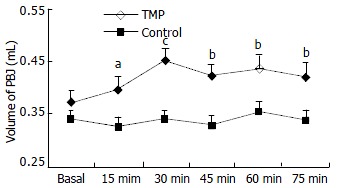
Effect of TMP on Volume of pancreatic-bile juice secretion. The volume of pancreatic-bile juice secretion collected before (basal) and 15, 30, 45, 60 and 75 min after intraperitoneal administration of TMP (80 mg/kg) and 9 mL/L NaCl in rats. The results were expressed as ¯x ± S-x. aP < 0.05, bP < 0.01, cP < 0.001, as compared to basal values.
Effect of TMP on CAPAN-1 cell line
The CAPAN-1 monolayer clamped in Ussing chambers bathing with normal K-HS (Cl-/HCO3--containing) exhibited a potential difference of 0.52 ± 0.04 mV, basal ISC of 5.70 ± 0.53 μA/cm2 and transmembrane resistance of 93.6 ± 4.5 Ωcm2 (n = 77). In Cl--free (n = 9) and HCO3--free (n = 9) K-H solution, the transepithelial potential difference was 0.62 ± 0.15 mV and 0.86 ± 0.28 mV and the basal ISC was 1.90 ± 0.50 mA/μm2 and 3.98 ± 1.32 μA/cm2, respectively, and the resistance of the monolayers were 208.6 ± 33.6 Ωcm2 and 161.8 ± 25.6 Ωcm2.
Basolateral addition of TMP (0.05, 0.15, 0.5, 1.5, 5 and 10 mmol/L) produced a dose-dependent increase in ISC. EC50 was about 1.56 mmol/L (Figure 2). TMP (5 mmol/L)-induced ISC increase was biphasic: A fast transient peak followed by a slow decay. The total transported charges for 15 min (the area under the curve of the TMP-induced ISC response) were about 1.06 ± 0.99 mC/cm2 (n = 27, Figure 3A and Figure 3C). TMP-induced current increase was inhibited by 67.1% after apical pretreatment of 1 mmol/L DPC, a non-specific Cl- channel blockers (n = 8, P < 0.001) (Figure 3B and Figure 3C). However apical application of epithelial sodium channel blockers, amiloride (100 μmol/L) did not affect the TMP-produced response (n = 3, Figure 3C). After the removal of external HCO3-, TMP-induced ISC was blocked by 74.4% (n = 9, P < 0.001) (Figure 3D), but the removal of external Cl- did not reduce but increase the TMP-induced ISC (n = 8, P < 0.05) (Figure 3D). Pretreatment of phosphodiesterase inhibitor, IBMX (0.5 mmol/L) for 15 min decreased the TMP-induced ISC by 91% (n = 4, P < 0.001) (Figure 4A and Figure 4B).
Figure 2.
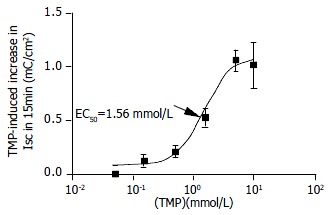
Concentration-response curve for TMP-induced ISC in CAPAN-1 cells. Different concentrations of TMP were added to basalateral side. Each data point was obtained from at least 4 individual experiments. Arrow shows EC50.
Figure 3.
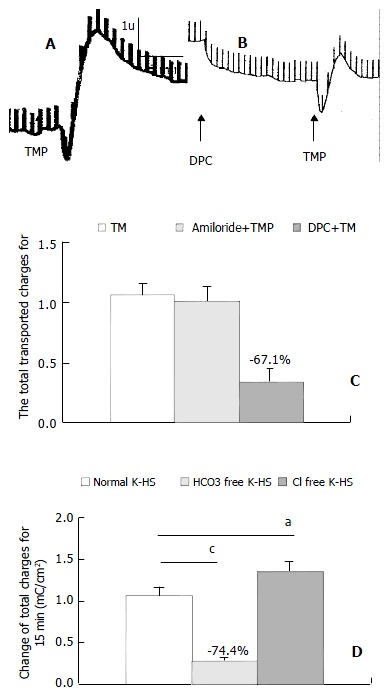
TMP-induced HCO3- secretion in CAPAN-1 cells. Comparison of TMP (5 mmoL/L)-induced ISC (mC/cm2) obtained in control (A, C), pretreatment with DPC (1 mmoL/L) (B, C), Amiloride 100 umoL/L (C), HCO3--free solution (D), Cl--free solution (D), Values are ¯x ± S-x, aP < 0.05, cP < 0.001.
Figure 4.
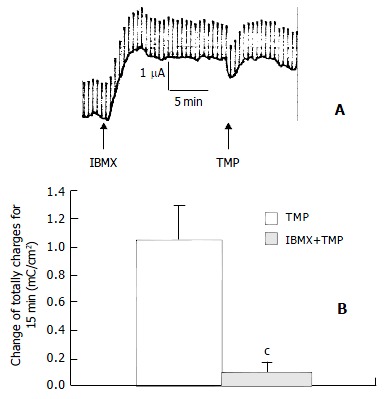
Pretreatment with IBMX decreased TMP-induced ISC by 91%. Values are ¯x ± S-x, cP < 0.001
DISCUSSION
While TMP has been widely used clinically for treating cardiovascular disorders and cerebrovascular diseases[1-5], little is known about its effect on pancreatic secretion. In the present study, intraperitoneal application of TMP increased secretion of pancreatic-bile juice (PBJ), but the protein content and pH value were not affected. It is known that PBJ consists of both bile and pancreatic juice, the volume of the basal secretion (or agonist induced) of pancreatic juice is normally very low, only about 9-18 μL in 15 min in rat[17,18], as compared to that of the liver. Although TMP increased the secretion of PBJ by 26-83 μL in 15 min, it was difficult to assess the contribution to exocrine pancreatic secretion from our experiments in vivo. Therefore, CAPAN-1 cell line was used to further demonstrate the effect of TMP on the pancreatic secretion. Our data suggested that TMP could directly stimulate HCO3- secretion in human pancreatic duct cells.
According to the HCO3- secretory model[22], the intracellular HCO3- was accumulated from the tissue fluid via the basolateral membrane[23-25] and mainly secreted by a Cl-/HCO3- exchanger[26,27] and/or directly via cAMP/cGMP-dependent Cl- channel (CFTR)[13,26,28]. Recently, it was reported that CFTR could also secrete HCO3- as Cl-/HCO3-exchanger[29,30]. The TMP- increased ISC in CAPAN-1 could be blocked by Cl- channel blockers, as well as phosphodiesterase inhibitors, which were known to increase cAMP and cGMP, and abolished by removal of extracellular HCO3-, suggesting that TMP can stimulate pancreatic HCO3- secretion via CFTR since it has been shown to be activated by both cAMP and cGMP. The TMP-induced increase in the volume of PBJ observed in the rats could be due to an increase in pancreatic HCO3- secretion, as well as bile secretion, leading to enhanced water secretion. However, the inability of TMP to increase the pH of PBJ appeared to contradict to its observed effect on the increase in HCO3- secretion. This could be explained by two possible reasons. One is that the mechanism of HCO3- secretion in human pancreatic duct cells was different from that in rats since the HCO3- concentration of pancreatic juice in rats was only 70 mmol/L while in human was 145 mmol/L[22]. The other is that the HCO3- secreted by the pancreas might be diluted by bile juice and thus having less prominent effect on pH. It is interesting to note that TMP could stimulate HCO3- secretion that could be inhibited by extracellular Cl- as evidenced by the increase in the TMP-induced ISC observed in the absence of extracellular Cl-. However, the mechanism for the observed inhibition of HCO3- secretion by extracellular Cl- remains to be elucidated.
In conclusion, the present in vivo and in vitro results suggest that TMP can promote the secretion of PBJ in rats and HCO3- secretion in human pancreatic duct cells, which may be beneficial to improving digestive function.
Footnotes
Edited by Wang XL
Supported by Innovation and Technology Funds of Hong Kong and strategic Program of the Chinese University of Hong Kong
References
- 1.Zheng HZ, Dong ZH, Yu J. Chuanxiong in: Zhongyao XiandaiYanjiu Yu Yingyong. 1st editor. Beijing: Xueyuan Publisher; 1997. pp. 629–682. [Google Scholar]
- 2.Liu LS, Chen MQ, Zeng GY, Zhou BF. [A forty-year study on hypertension] Zhongguo Yixue Kexueyuan Xuebao. 2002;24:401–408. [PubMed] [Google Scholar]
- 3.Cai Y, Ren M, Yang R. [Observation on curative effect of acute ischemic cerebrovascular disease treated with different dosage of ligustrazine] Zhongguo Zhongxiyi Jiehe Zazhi. 2000;20:747–749. [PubMed] [Google Scholar]
- 4.Liao F. Herbs of activating blood circulation to remove blood stasis. Clin Hemorheol Microcirc. 2000;23:127–131. [PubMed] [Google Scholar]
- 5.Huang RJ, Liao CX, Chen DZ. Effect of tetramethylpyrazine on endothelin, von Willebrand factor and thromboxane A2 during cardiopulmonary bypass in patients of congenital heart disease with pulmonary hypertension. Zhongguo Zhongxiyi Jiehe Zazhi. 2003;23:268–271. [PubMed] [Google Scholar]
- 6.Zou LY, Hao XM, Zhang GQ, Zhang M, Guo JH, Liu TF. Effect of tetramethyl pyrazine on L-type calcium channel in rat ventricular myocytes. Can J Physiol Pharmacol. 2001;79:621–626. [PubMed] [Google Scholar]
- 7.Sheu JR, Kan YC, Hung WC, Lin CH, Yen MH. The antiplatelet activity of tetramethylpyrazine is mediated through activation of NO synthase. Life Sci. 2000;67:937–947. doi: 10.1016/s0024-3205(00)00686-x. [DOI] [PubMed] [Google Scholar]
- 8.Lin CI, Wu SL, Tao PL, Chen HM, Wei J. The role of cyclic AMP and phosphodiesterase activity in the mechanism of action of tetramethylpyrazine on human and dog cardiac and dog coronary arterial tissues. J Pharm Pharmacol. 1993;45:963–966. doi: 10.1111/j.2042-7158.1993.tb05636.x. [DOI] [PubMed] [Google Scholar]
- 9.Pei L, Wang J. [Protective effect of tetramethylpyrazine on red blood cells during autotransfusion] Zhonghua Yixue Zazhi. 2002;82:322–324. [PubMed] [Google Scholar]
- 10.Shih YH, Wu SL, Chiou WF, Ku HH, Ko TL, Fu YS. Protective effects of tetramethylpyrazine on kainate-induced excitotoxicity in hippocampal culture. Neuroreport. 2002;13:515–519. doi: 10.1097/00001756-200203250-00032. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Wei T, Hou J, Li G, Yu S, Xin W. Tetramethylpyrazine scavenges superoxide anion and decreases nitric oxide production in human polymorphonuclear leukocytes. Life Sci. 2003;72:2465–2472. doi: 10.1016/s0024-3205(03)00139-5. [DOI] [PubMed] [Google Scholar]
- 12.Wu CC, Liao MH, Chen SJ, Yen MH. Tetramethylpyradizine prevents inducible NO synthase expression and improves survival in rodent models of endotoxic shock. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:435–444. doi: 10.1007/s002109900046. [DOI] [PubMed] [Google Scholar]
- 13.Kulaksiz H, Schmid A, Hönscheid M, Eissele R, Klempnauer J, Cetin Y. Guanylin in the human pancreas: A novel luminocrine regulatory pathway of electrolyte secretion via cGMP and CFTR in the ductal system. Histochem Cell Biol. 2001;115:131–145. doi: 10.1007/s004180000244. [DOI] [PubMed] [Google Scholar]
- 14.Gray M, O'Reilly C, Winpenny J, Argent B. Anion interactions with CFTR and consequences for HCO3- transport in secretory epithelia. J Korean Med Sci. 2000;15 Suppl:S12–S15. doi: 10.3346/jkms.2000.15.S.S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shumaker H, Amlal H, Frizzell R, Ulrich CD, Soleimani M. CFTR drives Na+-nHCO-3 cotransport in pancreatic duct cells: A basis for defective HCO-3 secretion in CF. Am J Physiol. 1999;276:C16–C25. doi: 10.1152/ajpcell.1999.276.1.C16. [DOI] [PubMed] [Google Scholar]
- 16.Lohi H, Kujala M, Kerkelä E, Saarialho-Kere U, Kestilä M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 2000;70:102–112. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- 17.Li JP, Chang TM, Chey WY. Roles of 5-HT receptors in the release and action of secretin on pancreatic secretion in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G595–G602. doi: 10.1152/ajpgi.2001.280.4.G595. [DOI] [PubMed] [Google Scholar]
- 18.Li JP, Lee KY, Chang TM, Chey WY. MEK inhibits secretin release and pancreatic secretion: roles of secretin-releasing peptide and somatostatin. Am J Physiol Gastrointest Liver Physiol. 2001;280:G890–G896. doi: 10.1152/ajpgi.2001.280.5.G890. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Wu XY, Zhu JX, Owyang C. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am J Physiol Gastrointest Liver Physiol. 2001;281:G916–G923. doi: 10.1152/ajpgi.2001.281.4.G916. [DOI] [PubMed] [Google Scholar]
- 20.Zhu JX, Lo PS, Zhao WC, Tang N, Zhou Q, Rowlands DK, Gou YL, Chung YW, Chan HC. Bak Foong Pills stimulate anion secretion across normal and cystic fibrosis pancreatic duct epithelia. Cell Biol Int. 2002;26:1011–1018. doi: 10.1006/cbir.2002.0960. [DOI] [PubMed] [Google Scholar]
- 21.Cheng HS, Wong WS, Chan KT, Wang XF, Wang ZD, Chan HC. Modulation of Ca2+-dependent anion secretion by protein kinase C in normal and cystic fibrosis pancreatic duct cells. Biochim Biophys Acta. 1999;1418:31–38. doi: 10.1016/s0005-2736(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 22.Sohma Y, Gray MA, Imai Y, Argent BE. HCO3- transport in a mathematical model of the pancreatic ductal epithelium. J Membr Biol. 2000;176:77–100. doi: 10.1007/s00232001077. [DOI] [PubMed] [Google Scholar]
- 23.Satoh H, Moriyama N, Hara C, Yamada H, Horita S, Kunimi M, Tsukamoto K, Iso-O N, Inatomi J, Kawakami H, et al. Localization of Na+-HCO-3 cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol. 2003;284:C729–C737. doi: 10.1152/ajpcell.00166.2002. [DOI] [PubMed] [Google Scholar]
- 24.Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na (+)-HCO (-) (3) cotransporter in human pancreas. Am J Physiol. 1999;277:G487–G494. doi: 10.1152/ajpgi.1999.277.2.G487. [DOI] [PubMed] [Google Scholar]
- 25.Ishiguro H, Naruse S, San Román JI, Case M, Steward MC. Pancreatic ductal bicarbonate secretion: past, present and future. JOP. 2001;2:192–197. [PubMed] [Google Scholar]
- 26.Sohma Y, Gray MA, Imai Y, Argent BE. 150 mM HCO3 (-)--how does the pancreas do it Clues from computer modelling of the duct cell. JOP. 2001;2:198–202. [PubMed] [Google Scholar]
- 27.Novak I. Keeping up with bicarbonate. J Physiol. 2000;528 Pt 2:235. doi: 10.1111/j.1469-7793.2000.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishiguro H, Naruse S, Kitagawa M, Mabuchi T, Kondo T, Hayakawa T, Case RM, Steward MC. Chloride transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol. 2002;539:175–189. doi: 10.1113/jphysiol.2001.012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO3- transport in mutations associated with cystic fibrosis. Nature. 2001;410:94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi JY, Lee MG, Ko S, Muallem S. Cl (-)-dependent HCO3- transport by cystic fibrosis transmembrane conductance regulator. JOP. 2001;2:243–246. [PubMed] [Google Scholar]


