Abstract
AIM: To investigate the expression and mutation of c-kit gene and its correlation with the clinical pathology and prognosis of gastrointestinal stromal tumors (GISTs).
METHODS: A total of 94 cases of GISTs, 10 leiomyomas and 2 schwannomas were studied for the expression of KIT by immunohistochemistry. The c-kit gene mutations in exon 11 of these specimens were detected by PCR-SSCP technique.
RESULTS: Of the 94 cases of GISTs, 91 (96.8%) expressed the KIT protein. Leiomyomas and schwannomas were negative for KIT. The c-kit gene mutations of exon 11 were found in 38 out of the 94 cases of GISTs (40.4%). The mutations involved point mutations (Val560-Asp, Ile563-Met), del 557-559 and 579ins12. No mutations were detectable in benign GISTs, leiomyomas or schwannomas. The patients with mutation-positive GISTs showed more frequent recurrences, invasion and metastasis in adjacent tissues than those with mutation-negative ones.
CONCLUSION: KIT is a useful marker for diagnosis of GISTs. Mutation of the c-kit gene may play a significant role in the pathogenesis of GISTs and may be associated with poor prognosis in patients with GISTs.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the human gastrointestinal tract that may occur in the entire gastrointestinal tract[1-4]. In recent years, much attention has focused on GISTs. Studies have shown that GISTs strongly express the KIT protein[1,5,6], a type III tyrosine-kinase receptor encoded by the c-kit proto-oncogene[7,8]. The c-kit gene located in the long arm of chromosome 4, is the cellular homologue of oncogene v-kit of the HZ4 feline sarcoma virus[9]. KIT, which is structurally related to the receptors for platelet-derived growth factor and colony-stimulating factor, consists of an extracellular domain, a transmembrane domain, a juxtamembrane domain and a kinase domain with an insert that splits the kinase domain[10]. KIT and its ligand, stem cell factor (SCF), are known to play crucial roles in the development of germ cells, melanocytes, mast cells and interstitial cells of Cajal[11].
Recently, activated c-kit mutations have been identified in GISTs. Most mutations were detected in the juxtamembrane domain (Lys-550 to Val-560)[1,12-14]. These mutations were related to a poorer prognosis. The purpose of this study was to examine the KIT expression and characterize the range of c-kit mutations in GISTs, and to evaluate the significance between c-kit mutations and prognostic factors.
MATERIALS AND METHODS
Patients
Ninety-four cases of GISTs (62 male, 32 female) diagnosed at Changhai Hospital with a median age of 53 years (ranging from 5-77) were included in the study between January 1991 and December 2002. Forty-six tumors were located in the stomach, thirty-two in the small bowel, ten in the large intestine, three in the esophagus and three in the omentum and mesentery. We used Lewin’s determination to separate benign from malignant lesions[15]. Sixty-seven cases of GISTs were malignant and 27 were benign. Of the patients in the malignant group, ten received reoperation due to recurrences, distant metastasis was found in 6 patients at the time of surgery, four to the liver and two to both the liver and peritoneum. The remaining patients were free of distant metastasis. One patient with liver metastasis died after operation. Twelve control tumors were also analyzed, including 10 leiomyomas and 2 schwannomas.
Immunohistochemistry
A rabbit polyclonal antibody against human KIT and an EnVision kit were purchased from DAKO. Immunohistochemistry was performed using the two-step technique. All specimens were fixed in 10% buffered formalin and embedded in paraffin. Four-μm thick sections were cut from the tissue blocks. The sections were deparaffinized and rehydrated, then treated using a microwave epitope retrieval technique with citrate buffer, pH6.0 at 85 °C for 3 min. After cooled at room temperature, the sections were washed in PBS (0.01 M, pH7.2) and incubated with the antibody against c-kit (1:100) at room temperature for 1 h. After washed in PBS, the sections were incubated with the EnVision compound at room temperature for 30 min. Staining was developed by immersing slides in 0.05% DAB with 0.33% hydrogen peroxide. All slides were counterstained with haematoxylin, dehydrated and mounted. PBS substituted for the primary antibody was used as the negative control.
DNA Extraction
DNA was extracted from formalin-fixed, paraffin-embedded tissues using standard methods with proteinase K digested and phenol/chloroform purified.
PCR-SSCP
Exon 11 of the c-kit gene was amplified by PCR using the following oligonucleotide primer pairs: sense primer 5’-AACTCAGCCTGTTTCTGG-3’ and antisense primer 5’-GATCTATTTTTCCCTTTCTC-3’. PCR was carried out with the following conditions: 50 μL total reaction volume, with 5 μL template, 5 μL of each oligonucleotide primer, 10 μL dNTP, 10 μL ddH2O, 2 μL Taq polymerase, 8 μL Mg2+ and 5 μL 10 °C PCR buffer. Cycling conditions were as follows: An initial penetration at 95 °C for 4 min, 38 cycles each at 94 °C for 1 min, at 56 °C for 1 min, at 72 °C for 1 min, followed by one cycle at 72 °C for 10 min. PCR products were visualized by gel electrophoresis in 1.7 g/L agarose. Then the PCR products were subjected to 8% non-denaturation polyacrylamide gel electrophoresis (aer: bis = 49:1) with 5% glycerin and silver nitrate staining.
DNA sequencing
PCR products that showed abnormal gel shift by PCR-SSCP were selected for sequencing after cloned into PMD18-T vector. The sequencing procedures were performed by Sangon Co., Shanghai.
Statistical methods
The data were analyzed with χ² test.
RESULTS
Immunohistochemistry
Immunohistochemical analyses revealed strong and diffuse KIT expression in 91 out of the 94 cases of GISTs. The positive signals were localized in cytoplasm and membrane (Figure 1 and Figure 2). Ninety-seven percent of malignant GISTs and ninety-six percent of benign GISTs were KIT positive. Compared with the benign group, some malignant GISTs showed weaker and focal positivity. There was no significant difference in the expression of KIT between benign and malignant GISTs (P > 0.05). Leiomyomas and schwannomas were negative for KIT.
Figure 1.
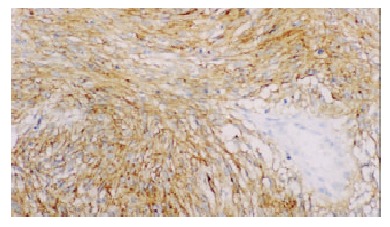
KIT staining in cytoplasm and membrane of GISTs (spindle type) × 200.
Figure 2.
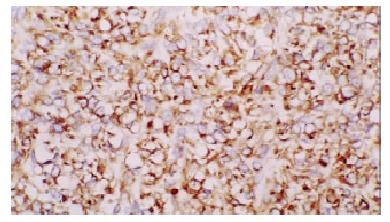
KIT staining in cytoplasm and membrane of GISTs (epithelioid type) × 200.
Evaluation of mutations in exon 11 of c-kit gene
Analysis of PCR-SSCP showed abnormal gel shifts in 38 out of the 67 (56.7%) malignant GISTs. No mutant bands were observed in benign GISTs, as well as in leiomyomas and schwannomas. Sequencing of 6 mutant bands revealed three types of mutations. One case showed point mutations (Val560-Asp and Ile563-Met), one case a 6-bp deletion involving codons 557 to 559, one case a 12-bp insertion at the codon 579 (Figure 3).
Figure 3.
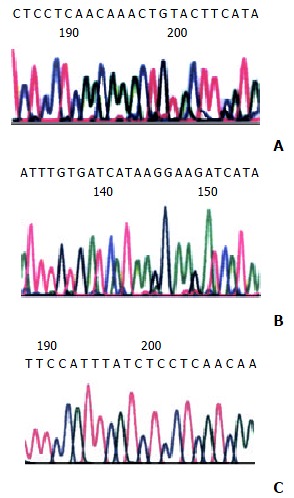
Sequence of the exon 11 of c-kit from the mutant bands obtained from the GISTs. A: 6-bp deletion involving codons 557 and 559 (GGAAGG), B: 12-bp insertion at codon 579 (CTTCCTTATGAT), C: two point mutations (Val 560-Asp, Ile 563-Met).
Correlation of c-kit mutations with clinicopathological parameters
Of the 38 cases of GISTs with c-kit mutations, six developed distant metastasis, eight had local recurrences and one died of liver metastasis. Only two recurrences were found in the remaining 56 cases of GISTs without c-kit mutations.
DISCUSSION
It has been reported that GISTs are strongly and nearly consistent KIT positive and mutations of the c-kit gene were observed in GISTs. In this study, we examined the sequences and expression of c-kit in the spectrum of GISTs, including benign and malignant variants from different sites. The expression and mutations of the c-kit gene were also evaluated in leiomyomas and schwannomas.
As this study has shown, 96.8% (91/94) GISTs strongly and diffusely expressed KIT irrespective of tumor location, histologic subtype and grade. Neither age nor sex was significantly correlated with the expression levels of KIT. The findings were in agreement with the previous studies[16-18]. No expression of KIT was found in leiomyomas and schwannomas. These results verified that KIT was a sensitive diagnostic marker for GISTs, but it could not be used as a prognostic index[19,20].
Recently, mutations of the c-kit gene were observed in GISTs. Most mutations were located at the juxtamembrane domain encoded by exon 11, especially between codons 550-560. Mutations of exon 11 were observed in 40.4% (38/94) of GISTs in our study, and other group observed mutations of exon 11 in 57%, 42% and 21% of GISTs[6,13,21]. The difference in mutation rates appeared to be due to the proportion of malignant GISTs, as suggested by our data and the previous reports. Some studies have shown that the mutant types including insertions or duplications, in addition to deletions and point mutations. These were consistent with our results. DNA sequencing showed that point mutations, deletions and insertions were found in our six GISTs and the range of the c-kit mutations was not only between codons 550-560 but also at codon 579. Although 96.8% of GISTs expressed KIT, only 40.4% of GISTs showed mutations in the juxtamembrane region of the c-kit gene. This indicated that some KIT-positive GISTs could occur without mutation of the c-kit gene or with mutations other than exon 11 of the c-kit gene. There was another possibility that some mutations of the NF-1 gene might result in the occurrence of KIT-positive GISTs[1,16,22]. Moreover, the mutations of exons 9, 13, 17, 14 and 15 in GISTs have been identified in recent years[23-25]. But mutations in these exons were found to be few compared with in exon 11[26,27]. These observations demonstrate that multiple mutations of c-kit, irrespective of domain-extracellular, juxtamembrane or kinase, are crucial tumorigenic events in GISTs.
Since c-kit mutations are commonly found in GISTs, how these mutations lead to kinase activation is a field of active investigation. KIT participates in complex networks of signal cascade proteins, and some of these proteins regulate KIT activation in positive or negative manners. Under normal conditions, KIT activation occurs when the receptor is bound to its ligand, a stem cell factor. Ligand-mediated KIT activation triggered various cell-signaling cascades that regulate cell behavior[28,29]. The c-kit mutations of these domains resulted in activation of kinase by allowing ligand-independent receptor dimerization[30-32]. In other words, the mutations in GISTs lead to structural changes of KIT oncoproteins that favor receptor oligomerization and cross-phosphorylation, even in the absence of ligand binding. Because activation of KIT is a ubiquitous oncogenic pathway in most GISTs and important to the growth of GISTs, it has become possible that patients with GISTs can be treated with STI571, a KIT tyrosine kinase inhibitor. STI571 is a 2-phenylaminopyrimidine that selectively inhibits protooncogenic and oncogenic forms of the ABL, PDGFR and c-kit tyrosine kinases[33,34]. STI571 has a potent activity against GIST cells grown in vitro and the majority of patients with malignant GIST have shown a benefit to treatment with STI571 in recent clinical trials[35-37].
Finally, we compared clinical outcome between the mutation-positive and negative GISTs, and found more frequent recurrences and poorer prognosis were related with mutation-positive GISTs. In our study, 6 out of the 38 patients with mutation-positive GISTs developed distant metastasis, 8 had local recurrences and 1 died of GIST, whereas only 2 out of the 56 patients with mutation-negative GISTs had recurrences. These findings are consistent with some other previous results[1,6,13,38,39], and indicate that the c-kit mutations seem to be related to poorer prognosis. But there is an opposite opinion recently. The results showed that these mutations occurred very early in the course of GISTs development and were of little prognostic importance in GISTs[40]. And now cytogenetic abnormalities have been detected in GISTs[41-43]. These findings suggest that molecular alterations and c-kit mutations are likely to be involved in determining the biologic behaviors of both benign and malignant GISTs.
Considering these findings, we conclude that most GISTs strongly and diffusely express KIT protein, and KIT is a useful and sensitive marker for diagnosis of GISTs. C-kit mutation is undoubtedly a pivotal event in GISTs and may be associated with poor prognosis. Evaluation of KIT mutation may have both prognostic and therapeutic significances as the new tyrosine kinase inhibitor (STI571) treatments are available. The correlation between c-kit mutations and clinical behaviors is far more complex than initially appreciated, and further studies are needed.
Footnotes
Edited by Zhang JZ and Wang XL
Supported by the National Natural Science Foundation of China, No.30070743
References
- 1.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, Sobin LH. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Shibata Y, Ueda T, Seki H, Yagihashi N. Gastrointestinal stromal tumour of the rectum. Eur J Gastroenterol Hepatol. 2001;13:283–286. doi: 10.1097/00042737-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M, Sobin LH. Gastrointestinal stromal tumors in the appendix: A clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol. 2001;25:1433–1437. doi: 10.1097/00000478-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: A sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728–734. [PubMed] [Google Scholar]
- 6.Taniguchi M, Nishida T, Hirota S, Isozaki K, Ito T, Nomura T, Matsuda H, Kitamura Y. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res. 1999;59:4297–4300. [PubMed] [Google Scholar]
- 7.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 8.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 9.Majumder S, Brown K, Qiu FH, Besmer P. c-kit protein, a transmembrane kinase: identification in tissues and characterization. Mol Cell Biol. 1988;8:4896–4903. doi: 10.1128/mcb.8.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirota S. Gastrointestinal stromal tumors: their origin and cause. Int J Clin Oncol. 2001;6:1–5. doi: 10.1007/pl00012072. [DOI] [PubMed] [Google Scholar]
- 11.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 12.Nakahara M, Isozaki K, Hirota S, Miyagawa J, Hase-Sawada N, Taniguchi M, Nishida T, Kanayama S, Kitamura Y, Shinomura Y, et al. A novel gain-of-function mutation of c-kit gene in gastrointestinal stromal tumors. Gastroenterology. 1998;115:1090–1095. doi: 10.1016/s0016-5085(98)70079-4. [DOI] [PubMed] [Google Scholar]
- 13.Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999;154:53–60. doi: 10.1016/S0002-9440(10)65250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 15.Lewin KJ, Riddell RH, Weinstein WM. Gastrointestinal pathology and its clinical implications.1 st editor. New York: Igaku-shoin; 1992. pp. 284–341. [Google Scholar]
- 16.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 17.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377–389. doi: 10.1097/00000478-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213–1220. doi: 10.1016/s0046-8177(99)90040-0. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa T, Matsuno Y, Shimoda T, Hirohashi S. Gastrointestinal stromal tumor: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum Pathol. 2002;33:669–676. doi: 10.1053/hupa.2002.124116. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 21.Moskaluk CA, Tian Q, Marshall CR, Rumpel CA, Franquemont DW, Frierson HF. Mutations of c-kit JM domain are found in a minority of human gastrointestinal stromal tumors. Oncogene. 1999;18:1897–1902. doi: 10.1038/sj.onc.1202496. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T, Wada I, Horiuchi H, Oka T, Machinami R. Multiple small intestinal stromal tumors with skeinoid fibers in association with neurofibromatosis 1 (von Recklinghausen's disease) Pathol Int. 1996;46:689–695. doi: 10.1111/j.1440-1827.1996.tb03673.x. [DOI] [PubMed] [Google Scholar]
- 23.Lux ML, Rubin BP, Biase TL, Chen CJ, Maclure T, Demetri G, Xiao S, Singer S, Fletcher CD, Fletcher JA. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol. 2000;156:791–795. doi: 10.1016/S0002-9440(10)64946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson J, Sjögren H, Meis-Kindblom JM, Stenman G, Aman P, Kindblom LG. The complexity of KIT gene mutations and chromosome rearrangements and their clinical correlation in gastrointestinal stromal (pacemaker cell) tumors. Am J Pathol. 2002;160:15–22. doi: 10.1016/S0002-9440(10)64343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, Hibbard MK, Chen CJ, Xiao S, Tuveson DA, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. [PubMed] [Google Scholar]
- 26.Lasota J, Wozniak A, Sarlomo-Rikala M, Rys J, Kordek R, Nassar A, Sobin LH, Miettinen M. Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors. A study of 200 cases. Am J Pathol. 2000;157:1091–1095. doi: 10.1016/S0002-9440(10)64623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota S, Nishida T, Isozaki K, Taniguchi M, Nakamura J, Okazaki T, Kitamura Y. Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. J Pathol. 2001;193:505–510. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH818>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 29.Taylor ML, Metcalfe DD. Kit signal transduction. Hematol Oncol Clin North Am. 2000;14:517–535. doi: 10.1016/s0889-8588(05)70294-x. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, Cunningham ME, Wang X, Ghosh I, Regan L, Longley BJ. Inhibition of spontaneous receptor phosphorylation by residues in a putative alpha-helix in the KIT intracellular juxtamembrane region. J Biol Chem. 1999;274:13399–13402. doi: 10.1074/jbc.274.19.13399. [DOI] [PubMed] [Google Scholar]
- 31.Isozaki K, Terris B, Belghiti J, Schiffmann S, Hirota S, Vanderwinden JM. Germline-activating mutation in the kinase domain of KIT gene in familial gastrointestinal stromal tumors. Am J Pathol. 2000;157:1581–1585. doi: 10.1016/S0002-9440(10)64795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–495. doi: 10.1053/hupa.2002.124124. [DOI] [PubMed] [Google Scholar]
- 33.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: A novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002;20:1692–1703. doi: 10.1200/JCO.2002.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 35.Tuveson DA, Willis NA, Jacks T, Griffin JD, Singer S, Fletcher CD, Fletcher JA, Demetri GD. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 36.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 37.van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, Martens M, Webb A, Sciot R, Van Glabbeke M, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: A phase I study. Lancet. 2001;358:1421–1423. doi: 10.1016/s0140-6736(01)06535-7. [DOI] [PubMed] [Google Scholar]
- 38.Ernst SI, Hubbs AE, Przygodzki RM, Emory TS, Sobin LH, O'Leary TJ. KIT mutation portends poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest. 1998;78:1633–1636. [PubMed] [Google Scholar]
- 39.Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: A review. Hum Pathol. 2002;33:478–483. doi: 10.1053/hupa.2002.124123. [DOI] [PubMed] [Google Scholar]
- 40.Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160:1567–1572. doi: 10.1016/S0002-9440(10)61103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.el-Rifai W, Sarlomo-Rikala M, Miettinen M, Knuutila S, Andersson LC. DNA copy number losses in chromosome 14: An early change in gastrointestinal stromal tumors. Cancer Res. 1996;56:3230–3233. [PubMed] [Google Scholar]
- 42.O'Leary T, Ernst S, Przygodzki R, Emory T, Sobin L. Loss of heterozygosity at 1p36 predicts poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest. 1999;79:1461–1467. [PubMed] [Google Scholar]
- 43.Gunawan B, Bergmann F, Höer J, Langer C, Schumpelick V, Becker H, Füzesi L. Biological and clinical significance of cytogenetic abnormalities in low-risk and high-risk gastrointestinal stromal tumors. Hum Pathol. 2002;33:316–321. doi: 10.1053/hupa.2002.32216. [DOI] [PubMed] [Google Scholar]


