Abstract
AIM: To investigate the effects of vitamin E succinate (VES) on the expression of c-jun gene and protein in human gastric cancer SGC-7901 cells.
METHODS: After SGC-7901 cells were treated with VES at different doses (5, 10, 20 mg•L-1) at different time, reverse transcription-PCR technique was used to detect the level of c-jun mRNA; Western Blot was applied to measure the expression of c-jun protein.
RESULTS: After the cells were treated with VES at 20 mg•L-1 for 3 h, the expression rapidly reached its maximum that was 3.5 times of UT control (P < 0.01). The level of c-jun mRNA was also increased following treatment of VES for 6 h. However, the expression after treatment of VES at 5 mg•L-1 for 24 h was 1.6 times compared with UT control (P < 0.01). Western blot analysis showed that the level of c-jun protein was obviously elevated in VES-treated SGC-7901 cells at 20 mg•L-1 for 3 h. The expression of c-jun protein was gradually increased after treatment of VES at 20 mg•L-1 for 3, 6, 12 and 24 h, respectively, with an evident time-effect relationship.
CONCLUSION: The levels of c-jun mRNA and protein in VES-treated SGC-7901 cells were increased in a dose- and time-dependent manner; the expression of c-jun was prolonged by VES, indicating that c-jun is involved in VES-induced apoptosis in SGC-7901 cells.
INTRODUCTION
RRR-α-tocopheryl succinate (vitamin E succinate, VES), a derivative of natural vitamin E, has been shown to be a potent growth inhibitor of various cancer cell types in vitro and in vivo[1-7]. Growth inhibition by VES is attributed to cell cycle blockage[4,8-10], induced cellular differentiation[11,12], increased expression of biologically active transforming growth factor-βs (TGF-βs) and their type II cell surface receptors[1,13,14] and the induction of apoptosis[15-18]. VES is noteworthy not only for its antiproliferative effects on tumor cells, but also for its non-toxic effect on normal cell types.
Gastric cancer is one of the most common tumors in China[19-28]. Up to date, the exact mechanisms of tumorigenesis is still unclear, but our previous studies showed that VES can block cell cycle, arrest DNA synthesis and induce apoptosis in human gastric cancer SGC-7901 cells, therefore inhibiting the cell growth[29-32]. In addition, our in vivo research in our laboratory demonstrated that VES inhibited benzo (a) pyrene (B (a) P)-induced forestomach carcinogenesis in female mice[33]. The exact mechanisms of apoptosis are not clearly known, but we found that VES can secrete and activate biologically active TGF-β and then TGF-β increases the kinase activity of c-jun N-terminal kinase (JNK) followed by phosphorylation of c-jun, and finally activated c-jun triggers apoptosis in human gastric cancer SGC-7901 cells[34]. In this study, the expression of c-jun mRNA was detected using reverse-transcription polymerase chain reaction (RT-PCR) technique and the level of c-jun protein was measured using western blot in order to further investigate the mechanisms of VES-triggered apoptosis.
MATERIALS AND METHODS
Materials
VES was purchased from Sigma Co. Ltd. RPMI 1640 media and TRIzol total RNA isolation kit were obtained from Gibco BRL, TITANIUMTM one-step RT-PCR kit from Clontech. Inc. c-jun (H79) rabbit polyclonal antibody was from Santa Cruz Biotechnologies.
Methods
Cell culture Human gastric cancer cell lines SGC-7901 were maintained in RPMI 1640 medium supplemented with 100 mL•L-1 fetal calf serum (FCS), 100 kU•L-1 penicillin, 100 mg•L-1 streptomycin and 2 mmol•L-1 L-glutamine under 50 mL•L-1 CO2 in a humidified incubator at 37 °C. SGC-7901 cells were incubated for different time periods in the presence of VES at 5, 10 and 20 mg•L-1 (VES was dissolved in absolute ethanol and diluted in RPMI 1640 complete condition media correspondingly to a final concentration of VES and 1 mL•L-1 ethanol), succinic acid, vitamin E and ethanol equivalents as vehicle (VEH) control and condition media only as untreated (UT) control.
RT-PCR After SGC-7901 cells were treated with VES for 3, 6 and 24 h, respectively, total cellular RNA was isolated by using TRIzol Reagent according to the manufacturer’s instructions. The concentration and purity of total RNA were determined by DUR 640 nucleic acid and protein analyzer (Beckman, USA). One-step RT-PCR was carried out following the manufacturer's instructions. RT-PCR mixture was heated 1 h at 50 °C for reverse transcription and 5 min at 95 °C for pre-denaturation, then into 34 PCR cycles of 30 s at 94 °C for denaturation, 30 s at 60 °C for annealing, 30 s at 72 °C for extension in PTC-100 programmable thermal controller (MJ Research, USA). The corresponding fragment of c-jun gene was amplified with specific primers synthesized[35]. β-actin gene was designed as an internal standard with purpose to remove false negative outcome (Table 1).
Table 1.
Nucleotide sequence and size of the expected PCR products for oligonucleotide primers used for RT-PCR
| Genes | Sequence | Size (bp) |
| c-jun | Upstream: 5'-GGAAACGACCTTCTATGACGAGCCC-3' | 315 |
| Downstream: 5'-GAACCCCTCCTGCTCATCTGTCAGG-3' | ||
| β-actin | Upstream: 5'-GTGGGCCGCTCTAGGCACCAA-3' | 540 |
| Downstream: 5'-CTCTTTGATGTCACGCACGATTTC-3' |
The amplified products were seperated in 20 g•L-1 agorose gel stained with ethidium bromide. After electrophoresis, the gel was observed and photographed under ultraviolet reflector. The density and area of each band were analyzed using ChemiImagerTM 4000 Digital System (Alpha Innotech Corporation, USA).
Western blot SGC-7901 cells treated with VES were harvested, washed in PBS and lyzed in lysis buffer containing 150 mmol•L-1 NaCl, 1 mL•L-1 NP-40, 5 mg•L-1 sodium deoxycholate, 1 g•L-1 SDS, 50 mmol•L-1 Tris (pH7.4), 1 mmol•L-1 DTT, 0.5 mmol•L-1 Na3VO4, 10 mmol•L-1 phenylmethylsulfonyl fluoride (PMSF), 10 mg•L-1 trypsin, 10 mg•L-1 aprotinin and 5 mg•L-1 leupeptin. Following the centrifugation of 12000 × g for 30 min at 4 °C, the amount of protein in the supernatant was determined using Biorad DC protein assay. Equal amount of protein was separated on 10% SDS-PAGE and transferred to nitrocellulose filter (Gibco BRL, USA) overnight. Blocked with 50 g•L-1 defatty milk, the filter was incubated with c-jun (H79) rabbit polyclonal antibody and horseradish peroxidase-conjugated IgG, finally developed with DAB.
Statistical analysis
The data were expressed as ¯x ± s. Statistical analysis was performed using student’s t-test. P < 0.05 was considered significant.
RESULTS
Effect of VES on the expression of c-jun mRNA in SGC-7901 cells
1 μg of total cellular RNA from groups of control, succinate, vitamin E, VES at 5, 10 and 20 mg•L-1 was added to amplify c-jun and β-actin genes by RT-PCR. Baseline expression of c-jun mRNA was observed in SGC-7901 cells (Figure 1). After the cells were treated with VES at 20 mg•L-1 for 3 h, the expression rapidly reached its maximum that was 3.5 times of UT control (P < 0.01). The level of c-jun mRNA was also increased following treatment of VES for 6 h. However, the expression after treatment of VES at 5 mg•L-1 for 24 h was 1.6-fold increase compared with UT control (P < 0.01), while there was no significant difference between 10 and 20 mg•L-1 VES groups and UT control group (Table 2).
Figure 1.
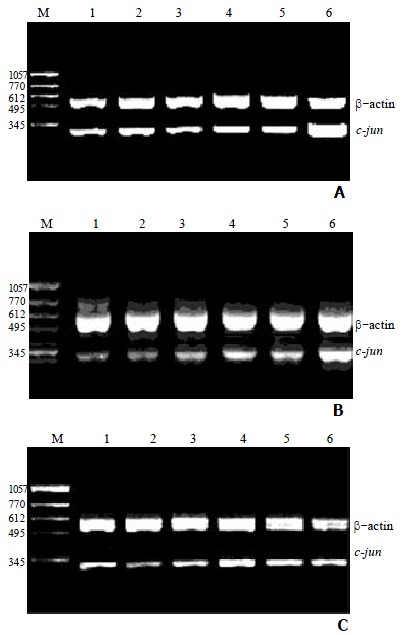
Effect of VES on the expression of c-jun mRNA in SGC-7901 cells for different time points by RT-PCR. A: treatment of VES for 3 h; B: treatment of VES for 6 h; C: treatment of VES for 24 h.1: UT control; 2: succinate; 3: vitamin E; 4: VES at 5 mg•L-1; 5: VES at 10 mg•L-1; 6: VES at 20 mg•L-1; M: molecular weight marker.
Table 2.
The relative expression of c-jun mRNA in SGC-7901 cells (n = 6, ¯x ± s)
| Groups |
Ratio of c-jun/β-actin |
||
| 3 h | 6 h | 24 h | |
| UT control | 0.469 ± 0.092 | 0.432 ± 0.095 | 0.368 ± 0.104 |
| succinate | 0.426 ± 0.082 | 0.408 ± 0.078 | 0.361 ± 0.083 |
| vitamin E | 0.514 ± 0.101 | 0.430 ± 0.081 | 0.367 ± 0.075 |
| 5 mg•L-1 VES | 0.550 ± 0.115 | 0.621 ± 0.086b | 0.584 ± 0.097b |
| 10 mg•L-1 VES | 0.471 ± 0.086 | 0.584 ± 0.101a | 0.421 ± 0.077 |
| 20 mg•L-1 VES | 1.663 ± 0.109b | 0.905 ± 0.099b | 0.411 ± 0.094 |
P < 0.05,
P < 0.01, vs UT control.
Effect of VES on the expression of c-Jun protein in SGC-7901 cells
Western blot analysis showed that the level of c-Jun protein was obviously elevated in VES-treated SGC-7901 cells at 20 mg•L-1 for 3 h in a significant dose-dependent manner (Figure 2A, 2B). Meanwhile, compared with the cells in UT control group, the VES-treated cells at 20 mg•L-1 exhibited 1.8-, 2.0-, 2.3- and 2.8-fold increases in the expression of c-jun protein for 3, 6, 12 and 24 h, respectively, with an evident time-effect relationship (Figure 3A, 3B).
Figure 2.
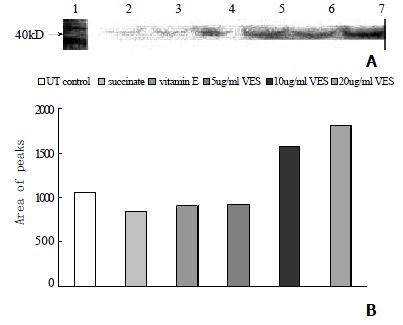
The expression of c-jun protein in SGC-7901 cells following treatment of VES for 3 h. Lane1: Molecular weight marker; Lane2: UT control; Lane3: succinate; Lane4: vitamin E; Lane5: VES at 5 mg•L-1; Lane6: VES at 10 mg•L-1; Lane7:VES at 20 mg•L-1.
Figure 3.
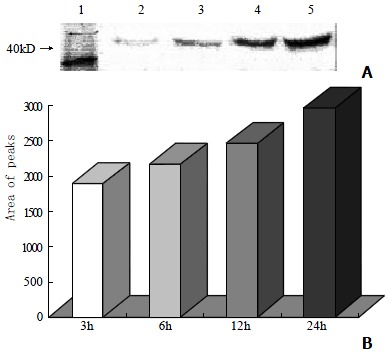
The expression of c-jun protein in SGC-7901 cells following treatment of VES at 20 mg•L-1 for different time poits. Lane1: Molecular weight marker; Lane2: 3 h; Lane3: 6 h; Lane4: 12 h; Lane5: 24 h.
DISCUSSION
The oncogene, c-jun, belongs to an immediate early gene and can be rapidly and transiently induced in response to multiple extracellular stimuli[36-38]. The product of c-jun gene is a nuclear transcription factor, an important composition of activation protein 1 (AP-1) dimmers, involved in signal transduction and regulation of many kinds of genes[39-41].
Transcription of c-jun mRNA rises after exposure of cells to a number of treatment including ultraviolate, irradition, heat shock, H2O2, TNF-α and other apoptosis-associated factors[42-46]. In addition to this transcriptional mode of regulation, c-jun activity can also be modulated directly at the protein level. In certain cell types, induction of c-jun is observed during apoptosis. There is some evidence that prolonged expression of c-jun in selected vulnerable cells suggests neuronal cell death[47].
Apoptosis is an innate program of cell suicide that is required for removal of unnecessary or damaged cells from bodily structures. Apoptosis is complex and regulated by a variety of factors[48-58]. Previous studies showed that the induction of apoptosis in tumor cells is one of the important mechanisms of VES-induced cell growth inhibition[59-61]. In the present study, the expression of c-jun mRNA and protein was measured in human gastric cancer SGC-7901 cells treated with VES at different doses for different time points. We found that the expression of c-jun mRNA was evidently promoted after 3 h of VES treatment at 20 mg•L-1 and reduced to the normal level after 24 h of treatment; whereas in the case of VES treatment at 5 mg•L-1, that was also increased after 3 h and remained a high level after 24 h. The data above showed that the c-jun activation was enhanced and prolonged by VES, therefore indicating that c-jun is involved in VES-triggered apoptosis in SGC-7901 cells. The results from western blot ananlysis showed that the level of c-jun protein was elevated following SGC-7901 cells were treated with VES at different doses for 3 h and with VES at 20 mg•L-1 for different time in a dose- and time-dependent manner.
The diversity of signals and signaling pathways that are directed toward c-jun is also reflected in the biological responses, in which the transcription factors have been implicated. It is reported that the mainly biological functions of c-jun are blockage of cell cycle and induction of apoptosis[62-64]. The study presented here demonstrated that VES can obviously increase the expression of c-jun mRNA and protein in human gastric cancer SGC-7901 cells, implicating that c-jun is involved in VES-induced apoptosis.
Footnotes
Edited by Pang LH
Supported by National Natural Science Foundation of China, No.39870662
References
- 1.Fariss MW, Fortuna MB, Everett CK, Smith JD, Trent DF, Djuric Z. The selective antiproliferative effects of alpha-tocopheryl hemisuccinate and cholesteryl hemisuccinate on murine leukemia cells result from the action of the intact compounds. Cancer Res. 1994;54:3346–3351. [PubMed] [Google Scholar]
- 2.Ottino P, Duncan JR. Effect of alpha-tocopherol succinate on free radical and lipid peroxidation levels in BL6 melanoma cells. Free Radic Biol Med. 1997;22:1145–1151. doi: 10.1016/s0891-5849(96)00529-1. [DOI] [PubMed] [Google Scholar]
- 3.Israel K, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits the proliferation of human prostatic tumor cells with defective cell cycle/differentiation pathways. Nutr Cancer. 1995;24:161–169. doi: 10.1080/01635589509514404. [DOI] [PubMed] [Google Scholar]
- 4.Turley JM, Ruscetti FW, Kim SJ, Fu T, Gou FV, Birchenall-Roberts MC. Vitamin E succinate inhibits proliferation of BT-20 human breast cancer cells: increased binding of cyclin A negatively regulates E2F transactivation activity. Cancer Res. 1997;57:2668–2675. [PubMed] [Google Scholar]
- 5.Neuzil J, Weber T, Gellert N, Weber C. Selective cancer cell killing by alpha-tocopheryl succinate. Br J Cancer. 2001;84:87–89. doi: 10.1054/bjoc.2000.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pussinen PJ, Lindner H, Glatter O, Reicher H, Kostner GM, Wintersperger A, Malle E, Sattler W. Lipoprotein-associated alpha-tocopheryl-succinate inhibits cell growth and induces apoptosis in human MCF-7 and HBL-100 breast cancer cells. Biochim Biophys Acta. 2000;1485:129–144. doi: 10.1016/s1388-1981(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 7.Malafa MP, Neitzel LT. Vitamin E succinate promotes breast cancer tumor dormancy. J Surg Res. 2000;93:163–170. doi: 10.1006/jsre.2000.5948. [DOI] [PubMed] [Google Scholar]
- 8.Kline K, Sanders BG. RRR-alpha-tocopheryl succinate inhibition of lectin-induced T cell proliferation. Nutr Cancer. 1993;19:241–252. doi: 10.1080/01635589309514255. [DOI] [PubMed] [Google Scholar]
- 9.Yu W, Sanders BG, Kline K. Modulation of murine EL-4 thymic lymphoma cell proliferation and cytokine production by vitamin E succinate. Nutr Cancer. 1996;25:137–149. doi: 10.1080/01635589609514436. [DOI] [PubMed] [Google Scholar]
- 10.Kline K, Yu W, Sanders BG, Vitamin E. Mechanisms of Action as Tumor Cell Growth Inhibitors. Cancer and Nutrition. K.N. Prasad and W.C. Cole (Eds) IOS Press. 1998:37–53. doi: 10.1093/jn/131.1.161S. [DOI] [PubMed] [Google Scholar]
- 11.Kline K, Yu W, Zhao B, Turley JM, Sanders BG. Vitamin E Succinate: Mechanisms of action as tumor cell growth inhibitor. In: Nutrients in Cancer Prevention and Treatment., editor. Prasad KN. Santamaria L and Williams RM (eds). Totowa. NY: Humana; 1995. pp. 39–56. [Google Scholar]
- 12.Kim SJ, Bang OS, Lee YS, Kang SS. Production of inducible nitric oxide is required for monocytic differentiation of U937 cells induced by vitamin E-succinate. J Cell Sci. 1998;111(Pt 4):435–441. doi: 10.1242/jcs.111.4.435. [DOI] [PubMed] [Google Scholar]
- 13.Simmons-Menchaca M, Qian M, Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits DNA synthesis and enhances the production and secretion of biologically active transforming growth factor-beta by avian retrovirus-transformed lymphoid cells. Nutr Cancer. 1995;24:171–185. doi: 10.1080/01635589509514405. [DOI] [PubMed] [Google Scholar]
- 14.Ariazi EA, Satomi Y, Ellis MJ, Haag JD, Shi W, Sattler CA, Gould MN. Activation of the transforming growth factor beta signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999;59:1917–1928. [PubMed] [Google Scholar]
- 15.Turley JM, Fu T, Ruscetti FW, Mikovits JA, Bertolette DC, Birchenall-Roberts MC. Vitamin E succinate induces Fas-mediated apoptosis in estrogen receptor-negative human breast cancer cells. Cancer Res. 1997;57:881–890. [PubMed] [Google Scholar]
- 16.Yu W, Israel K, Liao QY, Aldaz CM, Sanders BG, Kline K. Vitamin E succinate (VES) induces Fas sensitivity in human breast cancer cells: role for Mr 43, 000 Fas in VES-triggered apoptosis. Cancer Res. 1999;59:953–961. [PubMed] [Google Scholar]
- 17.Yu W, Liao QY, Hantash FM, Sanders BG, Kline K. Activation of extracellular signal-regulated kinase and c-Jun-NH (2)-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer Res. 2001;61:6569–6576. [PubMed] [Google Scholar]
- 18.Neuzil J, Weber T, Schröder A, Lu M, Ostermann G, Gellert N, Mayne GC, Olejnicka B, Nègre-Salvayre A, Stícha M, et al. Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. FASEB J. 2001;15:403–415. doi: 10.1096/fj.00-0251com. [DOI] [PubMed] [Google Scholar]
- 19.Hua JS. Effect of Hp: cell proliferation and apoptosis on stomach cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:647–648. [Google Scholar]
- 20.Zhu ZH, Xia ZS, He SG. The effects of ATRA and 5 Fu on telomerase activity and cell growth of gastric cancer cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:669–673. [Google Scholar]
- 21.Xia JZ, Zhu ZG, Liu BY, Yan M, Yin HR. Significance of immunohistoche mically demonstrated micrometastases to lymph nodes in gastric carcinomas. Shijie Huaren Xiaohua Zazhi. 2000;8:1113–1116. [Google Scholar]
- 22.Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532–539. doi: 10.3748/wjg.v6.i4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County, Fujian Province, China. World J Gastroenterol. 2000;6:374–376. doi: 10.3748/wjg.v6.i3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao XX, Yin L, Zhang JY, Bai WY, Li YM, Sun ZC. hTERT expression and cellular immunity in gastric cancer and precancerosis. Shijie Huaren Xiaohua Zazhi. 2001;9:508–512. [Google Scholar]
- 25.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB, et al. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500–505. doi: 10.3748/wjg.v7.i4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR, et al. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54–59. doi: 10.3748/wjg.v8.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao WX, Ou JM, Fei XF, Zhu ZG, Yin HR, Yan M, Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol. 2002;8:230–232. doi: 10.3748/wjg.v8.i2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu K, Ren Y, Guo J. The effects of vitamin E succinate on the cyclic regulation protein of human gastric cancer cells. Weisheng Dulixue Zazhi. 1998;12:203–207. [Google Scholar]
- 30.Liu B, Wu K, Zhao D. [Inhibition of human gastric carcinoma cell growth by vitamin E succinate] Wei Sheng Yan Jiu. 2000;29:172–174. [PubMed] [Google Scholar]
- 31.Wu K, Guo J, Shan YJ, Liu BH. The effects of vitamin E succinate on apoptosis in human gastric cancer. Weisheng Dulixue Zazhi. 1999;13:84–90. [Google Scholar]
- 32.Liu BH, Wu K. Study on the growth inhibition of Vitamin E Succinate in human gastric cancer cell. Aibian Jibian Tubian. 2000;12:79–81. [Google Scholar]
- 33.Wu K, Shan YJ, Zhao Y, Yu JW, Liu BH. Inhibitory effects of RRR-alpha-tocopheryl succinate on benzo (a) pyrene (B (a) P)-induced forestomach carcinogenesis in female mice. World J Gastroenterol. 2001;7:60–65. doi: 10.3748/wjg.v7.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu K, Liu BH, Zhao DY, Zhao Y. Effect of vitamin E succinate on expression of TGF-beta1, c-Jun and JNK1 in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2001;7:83–87. doi: 10.3748/wjg.v7.i1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tetens F, Kliem A, Tscheudschilsuren G, Navarrete Santos A, Fischer B. Expression of proto-oncogenes in bovine preimplantation blastocysts. Anat Embryol (Berl) 2000;201:349–355. doi: 10.1007/s004290050324. [DOI] [PubMed] [Google Scholar]
- 36.Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33–36. doi: 10.3748/wjg.v7.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leppä S, Saffrich R, Ansorge W, Bohmann D. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 1998;17:4404–4413. doi: 10.1093/emboj/17.15.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu YH, Hu DR, Nie QH, Liu GD, Tan ZX. Study on activation and c-fos, c-jun expression of in vitro cultured human hepatic stellate cells. Shijie Huaren Xiaohua Zazhi. 2000;8:299–302. [Google Scholar]
- 39.Guo YS, Hellmich MR, Wen XD, Townsend CM. Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. J Biol Chem. 2001;276:22941–22947. doi: 10.1074/jbc.M101801200. [DOI] [PubMed] [Google Scholar]
- 40.Herdegen T, Waetzig V. AP-1 proteins in the adult brain: facts and fiction about effectors of neuroprotection and neurodegeneration. Oncogene. 2001;20:2424–2437. doi: 10.1038/sj.onc.1204387. [DOI] [PubMed] [Google Scholar]
- 41.Yuen MF, Wu PC, Lai VC, Lau JY, Lai CL. Expression of c-Myc, c-Fos, and c-jun in hepatocellular carcinoma. Cancer. 2001;91:106–112. doi: 10.1002/1097-0142(20010101)91:1<106::aid-cncr14>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 42.Schroeter H, Spencer JP, Rice-Evans C, Williams RJ. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J. 2001;358:547–557. doi: 10.1042/0264-6021:3580547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang LX, Fu XB, Sun TZ, Yang YH, Gu XM. Relationship between oncogene c-jun activation and fibroblast growth factor receptor expression of ischemia reperfusion intestine in rats. Shijie Huaren Xiaohua Zazhi. 1999;7:498–500. [Google Scholar]
- 44.Fan M, Goodwin ME, Birrer MJ, Chambers TC. The c-Jun NH (2)-terminal protein kinase/AP-1 pathway is required for efficient apoptosis induced by vinblastine. Cancer Res. 2001;61:4450–4458. [PubMed] [Google Scholar]
- 45.Kondo T, Matsuda T, Kitano T, Takahashi A, Tashima M, Ishikura H, Umehara H, Domae N, Uchiyama T, Okazaki T. Role of c-jun expression increased by heat shock- and ceramide-activated caspase-3 in HL-60 cell apoptosis. Possible involvement of ceramide in heat shock-induced apoptosis. J Biol Chem. 2000;275:7668–7676. doi: 10.1074/jbc.275.11.7668. [DOI] [PubMed] [Google Scholar]
- 46.Potapova O, Basu S, Mercola D, Holbrook NJ. Protective role for c-Jun in the cellular response to DNA damage. J Biol Chem. 2001;276:28546–28553. doi: 10.1074/jbc.M102075200. [DOI] [PubMed] [Google Scholar]
- 47.Behrens A, Sabapathy K, Graef I, Cleary M, Crabtree GR, Wagner EF. Jun N-terminal kinase 2 modulates thymocyte apoptosis and T cell activation through c-Jun and nuclear factor of activated T cell (NF-AT) Proc Natl Acad Sci USA. 2001;98:1769–1774. doi: 10.1073/pnas.98.4.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 49.Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 50.Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223–227. doi: 10.3748/wjg.v6.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu K, Guo J, Shan YJ. Inhibitory effects of VES on the growth of human squamous gastric carcinoma cells. In: Johnson IT and Fenwick GR (eds), Dietary anticarcinogens and antimutagens-Chemical and biological aspects., editors. RS·C, UK: Athenaeum Press; 2000. pp. 304–307. [Google Scholar]
- 52.Zhao Y, Wu K. Cell death molecule Fas/CD95 and apoptosis. Aibian Jibian Tubian. 2001;13:55–58. [Google Scholar]
- 53.Wei XC, Wang XJ, Chen K, Zhang L, Liang Y, Lin XL. Killing effect of TNF-related apoptosis inducing ligand regulated by tetracycline on gastric cancer cell line NCI-N87. World J Gastroenterol. 2001;7:559–562. doi: 10.3748/wjg.v7.i4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 55.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-κB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431–435. doi: 10.3748/wjg.v8.i3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tao HQ, Zou SC. Effect of preoperative regional artery chemotherapy on proliferation and apoptosis of gastric carcinoma cells. World J Gastroenterol. 2002;8:451–454. doi: 10.3748/wjg.v8.i3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483–487. doi: 10.3748/wjg.v8.i3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu W, Simmons-Menchaca M, You H, Brown P, Birrer MJ, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate induction of prolonged activation of c-jun amino-terminal kinase and c-jun during induction of apoptosis in human MDA-MB-435 breast cancer cells. Mol Carcinog. 1998;22:247–257. [PubMed] [Google Scholar]
- 60.Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits EL4 thymic lymphoma cell growth by inducing apoptosis and DNA synthesis arrest. Nutr Cancer. 1997;27:92–101. doi: 10.1080/01635589709514508. [DOI] [PubMed] [Google Scholar]
- 61.Kogure K, Morita M, Nakashima S, Hama S, Tokumura A, Fukuzawa K. Superoxide is responsible for apoptosis in rat vascular smooth muscle cells induced by alpha-tocopheryl hemisuccinate. Biochim Biophys Acta. 2001;1528:25–30. doi: 10.1016/s0304-4165(01)00168-4. [DOI] [PubMed] [Google Scholar]
- 62.Leppä S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- 63.Jiang LX, Fu XB, Sun TZ, Yang YH, Gu XM. Relationship between oncogene c-jun activation and fibroblast growth factor receptor expression of ischemia reperfusion intestine in rats. Shijie Huaren Xiaohua Zazhi. 1999;7:498–500. [Google Scholar]
- 64.Teng CS. Differential expression of c-Jun proteins during müllerian duct growth and apoptosis: caspase-related tissue death blocked by diethylstilbestrol. Cell Tissue Res. 2000;302:377–385. doi: 10.1007/s004410000288. [DOI] [PubMed] [Google Scholar]


