Abstract
AIM: To investigate the possible roles of p53 and C-myc genes in the primary hepatocellular carcinogenesis and the relationship between the liver hyperplastic nodule (LHN) and hepatocellular carcinoma (HCC).
METHODS: The expression of p53 and C-myc genes was detected immunohist-ochemically in 73 and 60 cases of HCC and pericarcinomatous tissues, respectively.
RESULTS: The positive expression of p53 in HCC was significantly higher than that in pericarcinomatous tissues (P < 0.05). In pericarcinomatous tissues, the p53 expression was observed only in LHN, but not in liver cirrhosis (LC) and normal liver tissues. The positive expression rate of C-myc in HCC or LHN was significantly higher than that in LC or normal liver tissues (P < 0.05 and P < 0.01), however, no significant difference was found between HCC and LHN (P > 0.05). The positive expression rate of p53 and C-myc in HCC was correlated with the histological differentiation, that in the poorly differentiated was significantly higher than that in well differentiated samples (P < 0.05).
CONCLUSION: The overexpression of p53 and C-myc genes might play a role in the carcinogenesis of HCC; And LHN seems a preneoplastic lesion related to hepatocarcinogenesis; No evidence supports that LC contribute directly to the hepatocarcinogenesis.
INTRODUCTION
Primary hepatocellular carcinoma (HCC) is one of the most common malignant tumors in China[1-11], and the incidence of HCC reported has apparently increased in recent years. Despite a variety of therapeutic strategies, HCC remains a significant cause of cancer death. Therefore, to study the HCC pathogenesis is of the utmost importance to the prevention and treatment of this disease. With the advancement of HCC study, it becomes clear that the biologic behavior of HCC is closely related with the overactivation of the oncogenes and the inactivation of the tumor suppressor genes[12,13].
In the present study, the immunohistochemical LSAB (labelled streptavidin biotin) method was used to detect the expression of p53 and C-myc genes in HCC and pericarcinomatous tissues, in order to investigate the possible roles of these genes played in the HCC carcinogenesis, and to find out the relationship between the liver hyperplastic nodule and HCC. In addition, the relationship between the expression of p53 and C-myc genes and clinicopathological parameters of HCC was preliminarily investigated.
MATERIALS AND METHODS
Materials
HCC specimens of 100 cases obtained from surgical resections or biopsies performed at the Affiliated Hospital of Medical College of Qingdao University, China. Of these patients, 76 were male and 24 female with an average of 50.4 years. None of the patients had received chemo- or radio-therapy before resection. We randomly selected 73 and 60 cases of HCC to detect the expression of p53 and C-myc genes respectively owing to the limitation of antibodies. The specimens for detecting p53 were classified into 4 grades according to Edmondson's grading criteria, 4 specimens were in grade I, 24 in grade II, 39 in grade III, and 6 in grade IV. All 73 specimens contained pericarcinomatous tissues, in which including 39 liver hyperplastic nodules (LHN), 35 liver cirrhosis (LC) and 10 normal liver tissues. Among the specimens for detecting C-myc gene, 4 were in grade I, 17 in grade II, 30 in grade III, and 9 in grade IV. All 60 specimens contained pericarcinomatous tissues, in which including 37 LHN, 30 LC and 12 normal liver tissues.
Methods
All specimens were routinely processed, alcohol-fixed and paraffin-embedded. Serial paraffin sections of 4 um thickness were cut and used for hematoxylin and eosin (HE) and immunohistochemical stains. Immunohistochemical LSAB method was used to detect p53 and C-myc genes. Anti-p53 monoclonal antibody DO-7, anti-C-myc monoclonal antibody and LSAB kits were purchased from Dako Co. Before staining, the sections were heated with microwave in 0.05 mol·L-1 citric acid solution for antigen retrieval. In each staining, a known p53 or C-myc positive section was added as the positive control, and PBS was used as the substitute of the first antibody for the negative control.
Analysis of immunohistochemical staining
Cells with brown granules under microscope were regarded as positive. The criteria for the evaluation of the p53 expression in the present study were as follows: the positive nuclei number was semiquantitatively evaluated by counting that in 8-10 randomly-chosen medium power (× 100 magnification), and the four degrees of the p53 expression were considered as: negative (-), no positive cells; weak positive (+), the positive cells < 10%; moderately positive (++), the positive cells between 10%-50%; strong positive (+++), the positive cells > 50%. For the evaluation of C-myc expression, the percentage of positively stained cells was employed as an index, which was obtained from counting 500 cells at more than 5 high power fields for each section, and classified into 4 grades: grade I, the positive cells between 1%-25%; grade II, the positive cells 26%-50%; grade III, the positive cells 51%-75%; grade IV, the positive cells 76%-100%. No positive cells were scored negative (-).
Statistical analysis
Results were analysed by χ² test. Differences at P < 0.05 were considered to be statistically significant.
RESULTS
Expression of p53 gene in HCC and its pericarcinomatous tissue
The positive staining for p53 gene expressed as brown granules, which was mainly located in the cell nuclei of tumor cells (Figure 1). The staining intensity and extent varied among tumors, different tumor regions and individual tumor cells. Immunostaining of p53 protein was negative in all tumor stroma, bile duct epithelia, LC and normal liver tissues. A few p53 weakly positive cells were found in LHN (Figure 2). The significant difference existed between HCC and LHN (χ² value 10.57, P < 0.01), and so did between LHN and LC (χ² value 6.94, P < 0.01) (Table 1).
Figure 1.
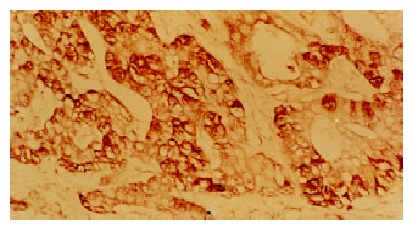
The positive expression of p53 gene in HCC. LSAB × 200
Figure 2.
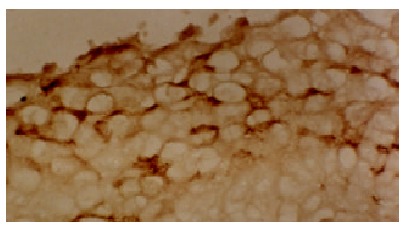
The positive expression of p53 gene in LHN. LSAB × 200
Table 1.
Expression of p53 gene in HCC and its pericarcinomatous tissue
| Histological type | n |
Expression of p53 gene |
Positive rate (%) | |||
| - | + | ++ | +++ | |||
| HCC | 73 | 37 | 12 | 19 | 5 | 49.3 |
| LHN | 39 | 32 | 7 | 0 | 0 | 17.9 |
| LC | 35 | 35 | 0 | 0 | 0 | 0 |
| Normal liver tissues | 17 | 17 | 0 | 0 | 0 | 0 |
Expression of C-myc gene in HCC and its pericarcinomatous tissue
The positive staining for C-myc gene was also expressed as brown granules, which was distributed mainly in cell nuclei, partly in cytoplasms. Although the expression rate of C-myc was higher in LHN than that in HCC, the statistical significance did not reached (χ² value 0.05, P > 0.05). The expression of C-myc gene in HCC and LHN was significantly higher than that in LC (χ² values 4.38, 4.51, P < 0.05). In HCC (Figure 3) and LHN (Figure 4) showing strong expression of C-myc, the positive-staining cells were distributed dominantly in a diffused pattern; whereas in LC (Figure 5) showing weak expression of C-myc, they preferred in a focalized pattern. The expression of C-myc was negative in normal liver tissues (Table 2).
Figure 3.
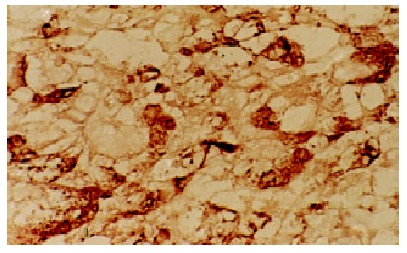
The positive expression of C-myc gene in HCC. LSAB × 200
Figure 4.
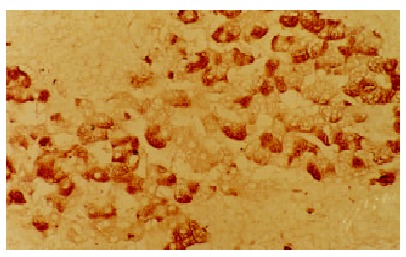
The positive expression of C-myc gene in LHN. LSAB × 200
Figure 5.
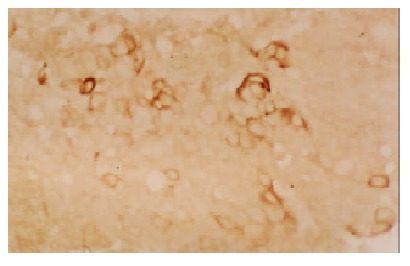
The positive expression of C-myc gene in LC. LSAB × 200
Table 2.
Expression of C-myc gene in HCC and its pericarcinomatous tissue
| Histological type | n |
Expression of C-myc gene |
Positive rate (%) | ||||
| - | grade I | grade II | grade III | grade IV | |||
| HCC | 60 | 37 | 0 | 2 | 9 | 12 | 38.3 |
| LHN | 37 | 22 | 1 | 3 | 3 | 8 | 40.5 |
| LC | 30 | 25 | 4 | 1 | 0 | 0 | 16.7 |
| Normal liver tissues | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
The relationship between the expression of p53 and C-myc genes and histological grade of HCC
There were varieties of positive rates of p53 and C-myc genes in different HCC histological grades of HCC, with a close relationship between the genes expression and the tumor differentiation. The positive rates of p53 expression in Edmondson'sgrading III and IV and Edmondson's grading I and II were 60% (27/45), 32.1% (9/28), respectively, which manifested a significant difference (χ² value 5.36, P < 0.05); The positive rates of C-myc expression in Edmondson’s grading III and IV and Edmondson's grading I and II were 48.7% (19/39), 19% (4/21), respectively, their differences were also significant (χ² value 5.08, P < 0.05) (Table 3).
Table 3.
The relationship between the expression of p53 and C-myc genes and histological grade of HCC
| Edmondson's grading |
Expression of p53 gene |
Expression of C-myc gene |
||
| Negative | Positive | Negative | Positive | |
| I | 4 | 0 | 3 | 1 |
| II | 15 | 9 | 14 | 3 |
| III | 18 | 21 | 18 | 12 |
| IV | 0 | 6 | 2 | 7 |
Correlation between p53 and C-myc protein expressions and HCC clinicopathological parameters
No significant relation was found between p53 or C-myc gene expression and patient age, sex and tumor size (P > 0.05).
DISCUSSION
The p53 gene is one of the most important tumor suppressor genes determined so far[14-19]. In recent years, it has been found that the p53 protein seems not express in the benign and preneoplastic lesions, and that there was no obvious relation between the p53 protein and carcinogenesis[20]. However, in the present study, the positive rate of p53 protein staining in LHN was 17.9%, which coincided basically with some results in literature[21-25]. The facts that the p53 protein expressed low in LHN and high in HCC, indicate that the high expression of p53 protein is probably associated with the cancerous transformation of hepatocytes, the early event in the carcinogenesis of HCC. It has been proved that wild type p53 protein can induce cell apoptosis whereas the mutant p53 protein can inhibit cell apoptosis and promote cell transformation and proliferation, resulting in carcinogenesis[26-30]; The p53 protein confirmed by immunohistochemical staining was considered as the mutation type[31]; Therefore, we speculate that p53 protein may exert its carcinogenic effect in the early stage of carcinogenesis on hepatocytes by two ways: (1) As described above, the mutant p53 protein might be associated with cell rapid proliferation and cell transformation, which ultimately results in hepatocellular carcinogenesis; (2) with the increase of the mutant p53 expression, and its inhibiting effect on apoptosis there will be an abnormal in cell numbers, which may eventually initiate the hepatocellular carcinogenesis. In addition, our results indicated that there existed a close relationship between the p53 gene expression and tumor cell differentiation in HCC, which suggests that the expression of p53 gene might serve as an index for the judgement of HCC malignant degree and its clinical prognosis.
It has been reported that the inept expression of C-myc gene correlated with carcinogenesis[23,32,33]. However, some authors held that the overexpression of C-myc gene could not be observed until the hepatocytes had thoroughly transformed into malignancy in the late stage of HCC, which reflected the continuous proliferation of tumor cells[34-36]. On the contrary, most experiments demonstrated the expression of C-myc in HCC and its pericarcinomatous tissue[24,37]. In the present study, the expression of C-myc gene was observed both in LHN and LC with varied degree and LHN was similar to HCC in the expression of C-myc, which were coincident with the results of others and suggests that the overexpression of C-myc gene occurs in the early phase of HCC formation, and correlates with preneoplastic transformation and proliferation. Our results also indicated that the expression of C-myc gene in HCC was related to the cell differentiation, which suggests that C-myc gene expression may exist in the sequential process of hepatocarcinogenesis, and be related to the phase of hepatocarcinogenesis.
In the present study, the expression of p53 gene in LHN was similar to that in HCC to some extent and the overexpression of C-myc gene was seen in both samples. The mutation of p53 gene that may lead to cell malignization, thus may reflect the alterations in different biologic state of LHN, and suggest that LHN is probably in the process of malignant transformation and in relation to hepatocarcinogenesis. In the present study the expression of C-myc gene in LHN had no significant difference from that in HCC, which reveals that parts of LHN were actually in the preneoplastic state or might be cancerous though they seemed normal in histology. Some study indicated that the increased oncogene expression brought cells into a state of active proliferation that resulted in an increased frequency of mutation[38]. This suggests that the overexpression of C-myc gene may make LHN be transformed malignantly. Another study showed that not all altered hepatocyte foci manifested abnormal expression of C-myc in the early stage of experimental HCC and the high expression of C-myc was only seen in the poorly differentiated foci[39]. It shows that the overexpression of C-myc gene may relate to the tumor's differentiation. Therefore, the overexpression of C-myc gene may be responsible for the low differentiation of LHN. It has been postulated that C-myc products might serve as a valid index for identifying preneoplastic lesion of HCC, the foci overexpressed C-myc were in danger of carcinogenesis[24,33]. However, few research reports till now have been found on the relationship between LHN and HCC. The present study thus provides a new possible way to diagnose HCC at earliest possible stage, which is of great importance in improving the prognosis because early diagnosis usually means high curability.
For many years, it has been generally considered that LC was closely associated with HCC and hepatocarcinogenesis. According to carcinogenic hypothesis on oncogene, at least two activated oncogenes are required-namely, the ras gene which was representative of transforming gene and the C-myc representative of immortalizing gene. Only when the two sorts of oncogenes function coordinately can stock-cultured cells be transformed malignantly. In the present study, we found that the expression rate of C-myc protein in LC was 26.7%, but, the expression intensity of C-myc in LC was significantly less than that in LHN. Our previous studies have indicated that there was no mutation of the ras gene in LC, and that the expression of c-erbB-2 oncogene was negative in LC, indicating that there are no mutation and activation of c-erbB-2 in LC, that is, it is impossible in this situation for malignant transformation. In addition, according to the present study, there was no mutation of p53 in LC, suggesting that LC does not necessarily link with hepatocarcinogenesis. Alcohol is the major cause of cirrhosis in European countries and the United States, responsible for 60 to 70 percent of all cases of cirrhosis, but it only infrequently leads to HCC; carbon tetrachloride can lead to LC rather than HCC. However, the reason why the ratio of HCC accompanied by LC in China is obviously higher than that in European countries and the United States perhaps lies in HBV infections, there is not a cause and effect but a accompanying relationship between LC and HCC. The significance of C-myc expression in LC remains to be investigated.
What requires a special explanation is that the LHN used in the present study is totally different from the nodules in LC. We noticed that though the majority of the LHN developed from LC, it is a cell population that differs from LC in properties and proliferative patterns. Although the liver cell cords in LC are in disarray, the hepatocytes are primarily arranged in a single line, most of which are normal in morphology; However, the hepatocytes in LHN grow by expansion, one nodule primarily contains a sort of cells, and in mixed cell nodules there exists a clear margin between the cellgroups, suggesting the nodules are of clone origin.
As to the relationship between the expression of p53 and C-myc genes and clinicopathological parameters of HCC, we found that there was no link of p53 or C-myc gene expression with patient age, sex and tumor size, which was in accordance with the previous reports[40]. Some studies indicated that the overexpression of p53 or C-myc was closely related to the prognosis of HCC[18,33,41-50], which was not our results have not yet confirmed that p53 or C-myc gene expression is directly associated with the prognosis of HCC. However, in the present study, the low expression of p53 gene and the overexpression of C-myc gene were found in LHN. It can be deduced that although normal in histology, LHN is surely abnormal in gene expression. Whether the phenomenon has a tie with the recurrence of HCC after resection needs to be further studied.
Footnotes
Edited by Zhu L
Supported by the scientific research fundation of Shandong Provincial Education Committee (J94, K26)
References
- 1.Sithinamsuwan P, Piratvisuth T, Tanomkiat W, Apakupakul N, Tongyoo S. Review of 336 patients with hepatocellular carcinoma at Songklanagarind Hospital. World J Gastroenterol. 2000;6:339–343. doi: 10.3748/wjg.v6.i3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun BH, Zhang J, Wang BJ, Zhao XP, Wang YK, Yu ZQ, Yang DL, Hao LJ. Analysis of in vivo patterns of caspase 3 gene expression in primary hepatocellular carcinoma and its relationship to p21 (WAF1) expression and hepatic apoptosis. World J Gastroenterol. 2000;6:356–360. doi: 10.3748/wjg.v6.i3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu HY, Yang YL, Guan XL, Song G, Jiang AM, Shi LJ. Expression of regulating apoptosis gene and apoptosis index in primary liver cancer. World J Gastroenterol. 2000;6:721–724. doi: 10.3748/wjg.v6.i5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33–36. doi: 10.3748/wjg.v7.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui J, Yang DH, Bi XJ, Fan ZR. Methylation status of c-fms oncogene in HCC and its relationship with clinical pathology. World J Gastroenterol. 2001;7:136–139. doi: 10.3748/wjg.v7.i1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan ZR, Yang DH, Cui J, Qin HR, Huang CC. Expression of insulin like growth factor II and its receptor in hepatocellular carcinogenesis. World J Gastroenterol. 2001;7:285–288. doi: 10.3748/wjg.v7.i2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q, Lin ZY, Feng XL. Alterations in metastatic properties of hepatocellular carcinoma cell following H-ras oncogene transfection. World J Gastroenterol. 2001;7:335–339. doi: 10.3748/wjg.v7.i3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao XY, Liu J, Lian ZR, Clayton M, Hu JL, Zhu MH, Fan DM, Feitelson M. Differentially expressed genes in hepatocellular carcinoma induced by woodchuck hepatitis B virus in mice. World J Gastroenterol. 2001;7:575–578. doi: 10.3748/wjg.v7.i4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC, Ye SL, Wu ZQ, Fan J, Qin LX, Zheng BH. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91:1479–1486. doi: 10.1002/1097-0142(20010415)91:8<1479::aid-cncr1155>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Cheung ST, So S, Fan ST, Barry C, Higgins J, Lai KM, Ji J, Dudoit S, Ng IO, et al. Gene expression patterns in human liver cancers. Mol Biol Cell. 2002;13:1929–1939. doi: 10.1091/mbc.02-02-0023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JM, Wang RQ, Bu BG, Zhou ZC, Fang DC, Luo YH. Effect of HCV infection on expression of several cancer-associated gene products in HCC. World J Gastroenterol. 1999;5:25–27. doi: 10.3748/wjg.v5.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bian JC, Shen FM, Shen L, Wang TR, Wang XH, Chen GC, Wang JB. Susceptibility to hepatocellular carcinoma associated with null genotypes of GSTM1 and GSTT1. World J Gastroenterol. 2000;6:228–230. doi: 10.3748/wjg.v6.i2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234–238. doi: 10.3748/wjg.v6.i2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang XJ, Yuan SL, Li CP, Iida N, Oda H, Aiso S, Ishikawa T. Infrequent p53 gene mutation and expression of the cardia adenocarcinomas from a high-incidence area of Southwest China. World J Gastroenterol. 2000;6:750–753. doi: 10.3748/wjg.v6.i5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fei SJ, Chen YL, Lin IF, Chen SM, Liu GZ. Expression of ras, p21, and p53 in gastric cancer and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2001;9:465–466. [Google Scholar]
- 18.Steele RJ, Thompson AM, Hall PA, Lane DP. The p53 tumour suppressor gene. Br J Surg. 1998;85:1460–1467. doi: 10.1046/j.1365-2168.1998.00910.x. [DOI] [PubMed] [Google Scholar]
- 19.Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Qin LL, Su JJ, Li Y, Yang C, Ban KC, Yian RQ. Expression of IGF- II, p53, p21 and HBxAg in precancerous events of hepatocarcinogenesis induced by AFB1 and/or HBV in tree shrews. World J Gastroenterol. 2000;6:138–139. doi: 10.3748/wjg.v6.i1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang JM, Han DW, Liang QC, Zhao JL, Hao SY, Ma XH, Zhao YC. Effects of endotoxin on expression of ras, p53 and bcl-2 oncoprotein in hepatocarcinogenesis induced by thioacetamide in rats. China Natl J New Gastroenterol. 1997;3:213–217. doi: 10.3748/wjg.v3.i4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins C, Kedda MA, Kew MC. Characterization of six tumor suppressor genes and microsatellite instability in hepatocellular carcinoma in southern African blacks. World J Gastroenterol. 1999;5:470–476. doi: 10.3748/wjg.v5.i6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai DW, Gao CZ, Wang NJ. [c-myc gene and p53 protein expression in human primary liver carcinoma] Zhonghua Binglixue Zazhi. 1994;23:100–103. [PubMed] [Google Scholar]
- 24.Yang S, Wang M, You W. [Overexpression of c-myc and p53 gene in human hepato-cellular carcinoma--a study with immunohistochemistry and in situ hybridization] Zhonghua Zhongliu Zazhi. 1995;17:415–417. [PubMed] [Google Scholar]
- 25.Kang YK, Kim CJ, Kim WH, Kim HO, Kang GH, Kim YI. p53 mutation and overexpression in hepatocellular carcinoma and dysplastic nodules in the liver. Virchows Arch. 1998;432:27–32. doi: 10.1007/s004280050130. [DOI] [PubMed] [Google Scholar]
- 26.Lane DP, Lu X, Hupp T, Hall PA. The role of the p53 protein in the apoptotic response. Philos Trans R Soc Lond B Biol Sci. 1994;345:277–280. doi: 10.1098/rstb.1994.0106. [DOI] [PubMed] [Google Scholar]
- 27.Terada T, Nakanuma Y. Expression of apoptosis, proliferating cell nuclear antigen, and apoptosis-related antigens (bcl-2, c-myc, Fas, Lewis (y) and p53) in human cholangiocarcinomas and hepatocellular carcinomas. Pathol Int. 1996;46:764–770. doi: 10.1111/j.1440-1827.1996.tb03546.x. [DOI] [PubMed] [Google Scholar]
- 28.Hall PA. p53: The Challenge of Linking Basic Science and Patient Management. Oncologist. 1998;3:218–224. [PubMed] [Google Scholar]
- 29.Yuen MF, Wu PC, Lai VC, Lau JY, Lai CL. Expression of c-Myc, c-Fos, and c-jun in hepatocellular carcinoma. Cancer. 2001;91:106–112. doi: 10.1002/1097-0142(20010101)91:1<106::aid-cncr14>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Feng D, Zheng H, Shen M, Cheng R, Yan Y. [Regulation of p53 and bcl-2 proteins to apoptosis and cell proliferation in liver cirrhosis and hepatocellular carcinoma] Hunan Yike Daxue Xuebao. 1999;24:325–328. [PubMed] [Google Scholar]
- 31.Hall PA, Lane DP. p53 in tumour pathology: can we trust immunohistochemistry?--Revisited! J Pathol. 1994;172:1–4. doi: 10.1002/path.1711720103. [DOI] [PubMed] [Google Scholar]
- 32.Ninomiya I, Yonemura Y, Matsumoto H, Sugiyama K, Kamata T, Miwa K, Miyazaki I, Shiku H. Expression of c-myc gene product in gastric carcinoma. Oncology. 1991;48:149–153. doi: 10.1159/000226915. [DOI] [PubMed] [Google Scholar]
- 33.Kawate S, Fukusato T, Ohwada S, Watanuki A, Morishita Y. Amplification of c-myc in hepatocellular carcinoma: correlation with clinicopathologic features, proliferative activity and p53 overexpression. Oncology. 1999;57:157–163. doi: 10.1159/000012024. [DOI] [PubMed] [Google Scholar]
- 34.Li DC. [Expression of cellular oncogenes in human primary liver cell carcinoma] Zhonghua Binglixue Zazhi. 1990;19:116–118. [PubMed] [Google Scholar]
- 35.Su TS, Lin LH, Lui WY, Chang CM, Chou CK, Ting LP, Hu CP, Han SH, P'eng FK. Expression of c-myc gene in human hepatoma. Biochem Biophys Res Commun. 1985;132:264–268. doi: 10.1016/0006-291x(85)91017-4. [DOI] [PubMed] [Google Scholar]
- 36.Beer DG, Schwarz M, Sawada N, Pitot HC. Expression of H-ras and c-myc protooncogenes in isolated gamma-glutamyl transpeptidase-positive rat hepatocytes and in hepatocellular carcinomas induced by diethylnitrosamine. Cancer Res. 1986;46:2435–2441. [PubMed] [Google Scholar]
- 37.Ling CQ, Qian Y, Zhao JA, Jin Y. Expression of C-myc IGF- II gene and cyclinD1 in experimental hepatocarcinogenesis. Shijie Huaren Xiaohua Zazhi. 2001;9:1452–1453. [Google Scholar]
- 38.Lian ZR. [HBV status and expression of ets-2, IGF-II, C-myc and N-ras in human hepatocellular carcinoma and adjacent nontumorous tissues--a comparative study] Zhonghua Zhongliu Zazhi. 1991;13:5–8. [PubMed] [Google Scholar]
- 39.Lin YZ. [The expression of C-myc, N-ras mRNA and its relations to the differentiation of preneoplastic altered hepatocytes] Zhonghua Zhongliu Zazhi. 1993;15:97–100. [PubMed] [Google Scholar]
- 40.Ng IO, Chung LP, Tsang SW, Lam CL, Lai EC, Fan ST, Ng M. p53 gene mutation spectrum in hepatocellular carcinomas in Hong Kong Chinese. Oncogene. 1994;9:985–990. [PubMed] [Google Scholar]
- 41.Wang D, Shi JQ. Overexpression and mutations of tumor suppressor gene p53 in hepatocellular carcinoma. China Natl J New Gastoenterol. 1996;15:161–164. [Google Scholar]
- 42.Li JQ, Zhang CQ, Feng KT. PCNA, p53 protein and prognosis in primary liver cancer. China Natl J New Gastoenterol. 1996;2:220–222. [Google Scholar]
- 43.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin LX, Tang ZY, Ma ZC, Wu ZQ, Zhou XD, Ye QH, Ji Y, Huang LW, Jia HL, Sun HC, et al. P53 immunohistochemical scoring: an independent prognostic marker for patients after hepatocellular carcinoma resection. World J Gastroenterol. 2002;8:459–463. doi: 10.3748/wjg.v8.i3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng IO, Lai EC, Chan AS, So MK. Overexpression of p53 in hepatocellular carcinomas: a clinicopathological and prognostic correlation. J Gastroenterol Hepatol. 1995;10:250–255. doi: 10.1111/j.1440-1746.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 46.Yano M, Asahara T, Dohi K, Mizuno T, Iwamoto KS, Seyama T. Close correlation between a p53 or hMSH2 gene mutation in the tumor and survival of hepatocellular carcinoma patients. Int J Oncol. 1999;14:447–451. doi: 10.3892/ijo.14.3.447. [DOI] [PubMed] [Google Scholar]
- 47.Sugo H, Takamori S, Kojima K, Beppu T, Futagawa S. The significance of p53 mutations as an indicator of the biological behavior of recurrent hepatocellular carcinomas. Surg Today. 1999;29:849–855. doi: 10.1007/BF02482774. [DOI] [PubMed] [Google Scholar]
- 48.Shen L, Qui D, Fang J. [Correlation between hypomethylation of c-myc and c-N-ras oncogenes and pathological changes in human hepatocellular carcinoma] Zhonghua Zhongliu Zazhi. 1997;19:173–176. [PubMed] [Google Scholar]
- 49.Jiang W, Lu Q, Pan G. [p53 gene mutation in hepatocellular carcinoma] Zhonghua Waike Zazhi. 1998;36:531–532. [PubMed] [Google Scholar]
- 50.Fang Y, Huang B, Liang Q, Li H, Huang C. [Clinical significance of c-myc oncogene amplification in primary hepatocellular carcinoma by interphase fluorescence in situ hybridization] Zhonghua Binglixue Zazhi. 2001;30:180–182. [PubMed] [Google Scholar]


