Abstract
AIM: To study the apoptosis of hepatoma cells SMMC-7721 induced by polysaccharide isolated from Ginkgo biloba seed.
METHODS: Ginkgo biloba seed polysaccharide (GBSP) was isolated by ethanol fractionation of Ginkgo biloba seed and purified by Sephadex G-200 chromatography. The purity of GBSP was verified by reaction with iodine-potassium iodide and ninhydrin and confirmed by UV spectrophotometer, cellulose acetate membrane electrophoresis and Sepharose 4B gel filtration chromatography. The Scanning Electron Microscope (SEM) and Flow Cytometry (FCM) were used to examine the SMMC-7721 cells with and without GBSP treatment at 500 mg/mL for 36 h.
RESULTS: GBSP product obtained was of high purity with the average molecular weight of 1.86 × 105. Quantitative analysis of SMMC-7721 cells in vitro with FCM showed that the percentages of G2-M cells without and with GBSP treatment were 17.01% ± 1.28% and 11.77% ± 1.50% (P < 0.05), the debris ratio of the cells were 0.46% ± 0.12% and 0.06% ± 0.06% (P < 0.01), and the apoptosis ratio of cells was 3.84% ± 0.55% and 9.13% ± 1.48% (P < 0.01) respectively. Following GBSP treatment, microvilli of SMMC-7721 cells appeared thinner and the number of spherical cells increased markedly. Most significantly, the apoptosis bodies were formed on and around the spherical cells treated with GBSP.
CONCLUSION: GBSP could potentially induce the apoptosis of SMMC-7721 cells.
INTRODUCTION
Ginkgo biloba L., also named after white-seed tree and gongsun tree, is one of the immemorial gymnosperm of the mesozoic era. It is regarded as a living fossil and is also best known for its pharmaceutical value. According to Pen-ts'so Kan-mu (i.e. Compendium of Materia Medica), Ginkgo biloba L. can help cure about 20 different diseases. More recently, it has been widely accepted that flavonoid and terpeneand are the effective components of leaves of Ginkgo biloba[1-5] for treating cardiovascular and nervous system diseases, scavenging free radicals and antioxidating, etc.[6-11]. Water-soluble polysaccharides of Ginkgo biloba leaves, endocarp, seeds and cultured cells were isolated and purified and their structures and some biological activities such as immunoregulation, antineoplastic action, scavenging free radicals and antioxidating were identified[12-16]. Furthermore, in vivo and in vitro induction of apoptosis of cancer cells by polysaccharides has been reported lately[17-22]. However, there was no report about the effects on apoptosis of tumor cells by polysaccharides isolated from Ginkgo biloba L. except from Ginkgo biloba endocarp[23]. In this study, high-purity polysaccharide was extracted from Ginkgo biloba seeds and the apoptotic effect of Ginkgo biloba seed polysaccharides (GBSP) on hepatoma cell line SMMC-7721 was investigated by scanning electron microscope (SEM) and flow cytometry (FCM).
MATERIALS AND METHODS
Materials
High-quality Ginkgo biloba seeds (i.e. milk white color, equal weight, and smooth surface without mildew) were purchased from Jianlian Chinese Traditional Medicine Store in Jinan, Shandong Province. Hepatoma cell line SMMC-7721 was obtained from Shanghai Cell Institute, China Academy of Sciences. Culture medium RPMI1640 was obtained from Gibco Co. (USA). Sephadex G-200, Sepharose 4B, monose as standard, calf serum and propidium iodide (PI) were obtained from Sigma Co. (USA).
Isolation and purification of GBSP
Ginkgo biloba seeds of 200 g were crushed into fine particles and extracted with 3000 mL of distilled water for 8 h at 75 °C for 3 times. The extracts were pooled, concentrated to 30% of the original volume in a rotary evaporator at 45 °C and then centrifuged at 3000 rpm for 15 min. The supernatant was collected and added with 3 volumes of 95% ethanol to precipitate the polysaccharide. Following centrifugation at 4000 rpm for 15 min, the polysaccharide pellet was dissolved in appropriate volume of distilled water completely, dialyzed with distilled water and decontaminated by means of Sevag to remove protein. The polysaccharide was then freeze-dried, re-dissolved in salt solution and purified further by Sephadex G-200 chromatography. The purity of the resulting GBSP was analyzed by Sepharose 4B gel filtration chromatography and cellulose acetate membrane electrophoresis[24].
Culture of SMMC-7721 cells and treatment with GBSP[25-28]
The SMMC-7721 cells were grown to logarithmic phase of proliferation, washed 3 times with culture medium RPMI1640 and collected at a concentration of 106 cells/mL. This cell suspension was then aliquoted into 6 culture bottles and cultured at 37 °C and 5% CO2 (CO2 incubator, MCO-17AC, SANYO, Japan) for 24 h. For cultures that were prepared for SCM test, cover slips were placed into bottles in advance. After the cells stuck on the walls of culture bottles, GBSP solution made up with culture medium was added into 3 of 6 culture bottles at the final concentration of 500 mg/mL. The other 3 culture bottles were added with equal volume of culture medium. The cells were cultured for further 36 h under the same conditions.
Flow Cytometry[29-31]
Supernatants of the cultures were discarded and SMMC-7721 cells with and without GBSP treatment were collected by digestion with pancreatin followed by centrifugation. PI was added to the cells for 15 min to label DNA. FCM (FACS/420, Becton Dickinson, USA) was used to analyze cell cycles and apoptosis ratios.
Scanning Electron Microscopy[32-33]
Supernatants of the cultures were discarded and SMMC-7721 cells stuck on the cover slips with and without GBSP treatment were examined by SEM (S-570, Hitachi, Japan).
RESULTS
Characterization of GBSP
One of the objectives of this work was to obtain high-purity GBSP product from the Ginkgo biloba seeds. The purity of GBSP was first tested by reactions with iodine-potassium iodide and ninhydrin respectively. The results of these two reactions were negative, indicating absence of starch and protein in the GBSP product obtained. The reaction of GBSP with Molish reagent was positive, indicating that the GBSP product was composed of monose. The GBSP solution was then analyzed by UV absorption (UV-1200, The Second Beijing Optical Instrument Manufactory, China). As shown in Figure 1, there were no peaks at wavelengths of 260 nm and 280 nm on the UV spectrum, indicating that the GBSP product was not contaminated with nucleic acid and protein. The purity of GBSP was further analyzed by Sepharose 4B gel filtration chromatography and cellulose acetate membrane electrophoresis. The elution profile of Sepharose 4B was a single symmetrical peak (Figure 2). The cellulose acetate membrane electrophoresis displayed a single stripe of GBSP on the membrane. These indicated that the present GBSP was of high purity. The average molecular weight of the GBSP was 1.86 × 105 based on the linear calibration curve derived from Sephadex G-200 chromatography, as shown in Figure 3.
Figure 1.
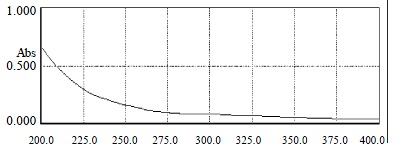
The UV spectrum adsorption curve of GBSP from 200 nm to 400 nm
Figure 2.
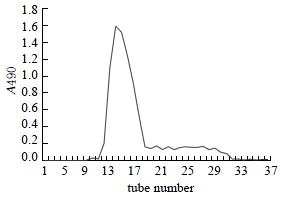
The profile of Sepharose 4B gel filtration chromatography
Figure 3.
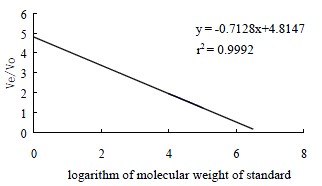
Standard linear calibration curve of Sephadex G-200 chromatography
G2-M cell ratio with and without GBSP treatment
The percentages of G2-M cells without and with GBSP treatment were 17.01% ± 1.28% and 11.77% ± 1.50% (P < 0.05) respectively (Figure 4, Figure 5 and Table 1). This indicated that GBSP inhibited the proliferation of SMMC-7721 cells.
Figure 4.
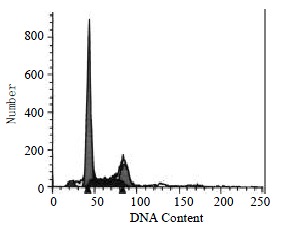
In the absence of GBSP treatment, the apoptosis ratio of SMMC-7721 cells was 3.84% ± 0.55%, the cells debris was 0.46% ± 0.12% and the percentage of G2-M cells was 17.01% ± 1.28%.
Figure 5.
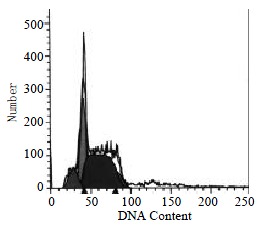
In the presence of GBSP treatment, the apoptosis ratio of SMMC-7721 cells was 9.13% ± 1.48%, the cells debris was 0.06% ± 0.06%, and the percentage of G2-M cells was 11.77% ± 1.50%.
Table 1.
The cycles and apoptosis ratios of SMMC-7721 cell with and without GBSP treatment
| GBSP treatment (mg/mL) | Number of culture bottles tested | G2-M cell ratio (%) | Apoptosis cell ratio (%) | Debris ratio (%) |
| 0 | 3 | 17.01 ± 1.28 | 3.84 ± 0.55 | 0.46 ± 0.12 |
| 500 | 3 | 11.77 ± 1.50a | 9.13 ± 1.48b | 0.06 ± 0.06b |
P < 0.05,
P < 0.01 vs control group (t test)
Apoptosis ratio of the SMMC-7721 cell with and without GBSP treatment
The apoptosis ratio of SMMC-7721 cells without GBSP treatment was 3.84% ± 0.55% (Figure 4 and Table 1). After GBSP treatment, the apoptosis ratio increased to 9.13% ± 1.48% (P < 0.01 vs control group) (Figure 5 and Table 1). The debris ratio of the cells without and with GBSP treatment was 0.46% ± 0.12% and 0.06% ± 0.06%, respectively (P < 0.01) (Figure 4, Figure 5 and Table 1). These results showed that GBSP could induce and promote the apoptosis of SMMC-7721 cells, rather than kill SMMC-7721 cells directly.
Morphology of the SMMC-7721 cell with and without GBSP treatment
The morphology of SMMC-7721 cells with and without GBSP treatment was studied by SEM. The majority of SMMC-7721 cells without GBSP treatment was of shuttle shape (Figure 6) and small proportion of cells was of spherical shape. Close examination revealed dense microvilli on the surface of the cells, and occasionally, 2 to 3 protuberances were also observed on the cell surface. After the treatment of GBSP, these microvilli became thinner, protuberances disappeared (Figure 7) and number of spherical cells increased markedly. These spherical cells shrunk so that their volume decreased and wrinkles were formed on their surface. Most significantly, apoptosis bodies were formed on and around the spherical cells (Figure 7). These observations suggest that GBSP could induce apoptosis in hepatoma cell line SMMC-7721, consistent with the results of FCM analysis.
Figure 6.
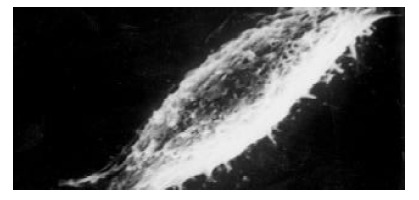
The majority of SMMC-7721 cells were of shuttle shape without GBSP treatment (× 2500)
Figure 7.
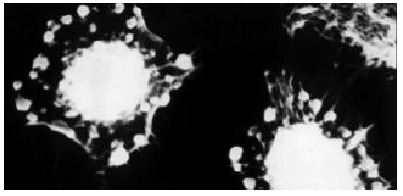
There were more spherical SMMC-7721 cells after GBSP treatment (× 2200). These cells shrunk and apoptosis bodies were formed on and around them.
DISCUSSION
The expansion of neoplasma is associated with inhibition of apoptosis of tumor cells. There is evidence that high expression of the inhibition gene bcl-2 led to inactivation of the tumor suppression gene p53[34,45]. When apoptosis was inhibited, tumour cells would not be eliminated in time in vivo and, therefore, would proliferate and diffuse more rapidly. At present, drugs such as VP-16, ADR, MTX, and Hydroxyl urea etc. are commonly used in clinical chemotherapy. These agents interfere with growth, metabolism and proliferation processes of the cells so that the apoptosis of tumour cells can be induced[46-48]. However, like most of chemotherapical medicines, they have cytotoxic side-effects which cause damage to normal cells while killing neoplasma cells.
Experiments showed that ingredients of Chinese herbal medicine could induce apoptosis in tumour cells. For example, β-elemi-olefin, arabinose-cytidine and etoposide could induce apoptosis in leukemia cell line[49-55]. It was also reported that polysaccharides isolated from Chinese herbs could inhibit the growth of tumour by activating immune system in vivo rather than killing tumour cells directly[56,57]. In this in vitro study, we found that GBSP could effectively inhibit division of the SMMC-7721 cells and, meanwhile, induced apoptosis in hepatoma cell line SMMC-7721. Although Ginkgo biloba seed has been mainly used for treatment of asthm for over hundred years, it has not been known to have anti-tumour activity. The results of our present study provided valuable data for exploring the clinical application of GBSP for cancer therapy.
Footnotes
Edited by Liu HX
References
- 1.Liebgott T, Miollan M, Berchadsky Y, Drieu K, Culcasi M, Pietri S. Complementary cardioprotective effects of flavonoid metabolites and terpenoid constituents of Ginkgo biloba extract (EGb 761) during ischemia and reperfusion. Basic Res Cardiol. 2000;95:368–377. doi: 10.1007/s003950070035. [DOI] [PubMed] [Google Scholar]
- 2.Bastianetto S, Zheng WH, Quirion R. The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: involvement of its flavonoid constituents and protein kinase C. J Neurochem. 2000;74:2268–2277. doi: 10.1046/j.1471-4159.2000.0742268.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim SJ, Lim MH, Chun IK, Won YH. Effects of flavonoids of Ginkgo biloba on proliferation of human skin fibroblast. Skin Pharmacol. 1997;10:200–205. doi: 10.1159/000211505. [DOI] [PubMed] [Google Scholar]
- 4.Wójcicki J, Gawrońska-Szklarz B, Bieganowski W, Patalan M, Smulski HK, Samochowiec L, Zakrzewski J. Comparative pharmacokinetics and bioavailability of flavonoid glycosides of Ginkgo biloba after a single oral administration of three formulations to healthy volunteers. Mater Med Pol. 1995;27:141–146. [PubMed] [Google Scholar]
- 5.Oyama Y, Fuchs PA, Katayama N, Noda K. Myricetin and quercetin, the flavonoid constituents of Ginkgo biloba extract, greatly reduce oxidative metabolism in both resting and Ca (2+)-loaded brain neurons. Brain Res. 1994;635:125–129. doi: 10.1016/0006-8993(94)91431-1. [DOI] [PubMed] [Google Scholar]
- 6.Shen L, Cui Y. [Effects of the leaf of Ginkgo biloba L. extract on blood rheology in animals] Zhongguo Zhongyao Zazhi. 1998;23:622–63, 640- inside back cover. [PubMed] [Google Scholar]
- 7.Logani S, Chen MC, Tran T, Le T, Raffa RB. Actions of Ginkgo Biloba related to potential utility for the treatment of conditions involving cerebral hypoxia. Life Sci. 2000;67:1389–1396. doi: 10.1016/s0024-3205(00)00741-4. [DOI] [PubMed] [Google Scholar]
- 8.Tadano T, Nakagawasai O, Tan-no K, Morikawa Y, Takahashi N, Kisara K. Effects of ginkgo biloba extract on impairment of learning induced by cerebral ischemia in mice. Am J Chin Med. 1998;26:127–132. doi: 10.1142/S0192415X98000178. [DOI] [PubMed] [Google Scholar]
- 9.McKenna DJ, Jones K, Hughes K. Efficacy, safety, and use of ginkgo biloba in clinical and preclinical applications. Altern Ther Health Med. 2001;7:70–86, 88-90. [PubMed] [Google Scholar]
- 10.Yao Z, Drieu K, Papadopoulos V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from beta-amyloid-induced cell death by inhibiting the formation of beta-amyloid-derived diffusible neurotoxic ligands. Brain Res. 2001;889:181–190. doi: 10.1016/s0006-8993(00)03131-0. [DOI] [PubMed] [Google Scholar]
- 11.Smith PF, Maclennan K, Darlington CL. The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF) J Ethnopharmacol. 1996;50:131–139. doi: 10.1016/0378-8741(96)01379-7. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Yang GW, An LG. Isolation, Purification and Analysis of a Polysaccharide from the Ginkgo biloba Seed. Zhongguo Yaoxue Zazhi. 2002;37:331–333. [Google Scholar]
- 13.Kraus J. Water-soluble polysaccharides from Ginkgo biloba leaves. Phytochemistry. 1991;30:3017–3020. doi: 10.1016/s0031-9422(00)98243-8. [DOI] [PubMed] [Google Scholar]
- 14.Jin JQ, Ding DN, Bian XL, Dong HY, Ge G. The study of chemical and scavenging action to hydroxyl free radical of polysaccharides of Ginkgo biloba leaf. Xian Yike Daxue Xuebao. 2000;21:417–419. [Google Scholar]
- 15.Song LY, Ma WX, Yu RM, Kan QM, Yao XS. Studies on the biological activities of polysaccharides from the cell cultures and the leaves of Ginkgo biloba. Zhongguo Shenghua Yaowu Zazhi. 1999;20:278–280. [Google Scholar]
- 16.Zhao SQ, Li F, Sun YM. Research progress of the exopleura of Ginkgo biloba L. Wuhan Zhiwuxue Yanjiu. 2000;18:515–518. [Google Scholar]
- 17.Lin X, Cai YJ, Li ZX, Liu ZL, Yin SF, Zhao JC. Cladonia furcata polysaccharide induced apoptosis in human leukemia K562 cells. Acta Pharmacol Sin. 2001;22:716–720. [PubMed] [Google Scholar]
- 18.Fullerton SA, Samadi AA, Tortorelis DG, Choudhury MS, Mallouh C, Tazaki H, Konno S. Induction of apoptosis in human prostatic cancer cells with beta-glucan (Maitake mushroom polysaccharide) Mol Urol. 2000;4:7–13. [PubMed] [Google Scholar]
- 19.Sogawa K, Yamada T, Sumida T, Hamakawa H, Kuwabara H, Matsuda M, Muramatsu Y, Kose H, Matsumoto K, Sasaki Y, et al. Induction of apoptosis and inhibition of DNA topoisomerase-I in K-562 cells by a marine microalgal polysaccharide. Life Sci. 2000;66:PL227–PL231. doi: 10.1016/s0024-3205(00)00473-2. [DOI] [PubMed] [Google Scholar]
- 20.Sogawa K, Matsuda M, Okutani K. Induction of apoptosis by a marine microalgal polysaccharide in a human leukemic cell line. J Mar Biotechnol. 1998;6:241–243. [PubMed] [Google Scholar]
- 21.Sogawa K, Yamada T, Muramatsu Y, Sumida T, Hamakawa H, Oda H, Miyake H, Tashiro S, Matsuda M, Matsumoto K, et al. Decrease of nuclear protein phosphatase 1 activity and induction of mitotic arrest and apoptosis by a marine microalgal polysaccharide in human myeloid leukemia U937 cells. Res Commun Mol Pathol Pharmacol. 1998;99:267–282. [PubMed] [Google Scholar]
- 22.Kim HS, Kacew S, Lee BM. In vitro chemopreventive effects of plant polysaccharides (Aloe barbadensis miller, Lentinus edodes, Ganoderma lucidum and Coriolus versicolor) Carcinogenesis. 1999;20:1637–1640. doi: 10.1093/carcin/20.8.1637. [DOI] [PubMed] [Google Scholar]
- 23.Xu AH, Jia SQ, Chen HS, Zhou ZY, Zhu YQ. Studies of Ginkgo biloba endocarp polysaccharides inhibiting liver cancer and inducing apoptosis of liver cancer cells in mice. Zhongyao Xinyao Yu Linchuang Yaoli. 2001;12:340–341. [Google Scholar]
- 24.Cao PR, Wu ZD, Wang RC. Isolation, purification and analysis of a polysaccharide PA3DE from the fruit bodies of Flammulina velutipes (Curt. ex Fr.) Sing. Acta Biochimica et Biophysica Sinica. 1989;21:152–156. [Google Scholar]
- 25.Jiang SM, Xiao ZM, Xu ZH. Inhibitory activity of polysaccharide extracts from three kinds of edible fungi on proliferation of human hepatoma SMMC-7721 cell and mouse implanted S180 tumor. World J Gastroenterol. 1999;5:404–407. doi: 10.3748/wjg.v5.i5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483–487. doi: 10.3748/wjg.v8.i3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui M, Zhang HJ, An LG. Tumor growth Inhibition by polysaccharide from Coprinus comatus. Shijie Huaren Xiaohua Zazhi. 2002;10:287–290. [Google Scholar]
- 28.Yang JQ, Yang LY, Zhu HC. Mitomycin C-induced apoptosis of human hepatoma cell. Shijie Huaren Xiaohua Zazhi. 2001;9:268–272. [Google Scholar]
- 29.Xia PY, Zheng J, Zhou H, Pan WD, Qin XJ, Xiao GX. Relationship between lymphocyte apoptosis and endotoxin translocation after thermal injury in rats. World J Gastroenterol. 2002;8:546–550. doi: 10.3748/wjg.v8.i3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao XX, Tang YW, Yao DM, Xiu HM. Effects of Yigan Decoction on proliferation and apoptosis of hepatic stellate cells. World J Gastroenterol. 2002;8:511–514. doi: 10.3748/wjg.v8.i3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao HQ, Zou SC. Effect of preoperative regional artery chemotherapy on proliferation and apoptosis of gastric carcinoma cells. World J Gastroenterol. 2002;8:451–454. doi: 10.3748/wjg.v8.i3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang XL, Liu L, Jiang HQ. Salvia miltiorrhiza monomer IH764-3 induces hepatic stellate cell apoptosis via caspase-3 activation. World J Gastroenterol. 2002;8:515–519. doi: 10.3748/wjg.v8.i3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen XJ, Ai ZL, Liu ZS. Apoptosis: a study on mechanism of injuries in human hepatocyte induced by mitomycin. Shijie Huaren Xiaohua Zazhi. 2000;8:746–750. [Google Scholar]
- 34.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-κB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431–435. doi: 10.3748/wjg.v8.i3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang LD, Zhou Q, Wei JP, Yang WC, Zhao X, Wang LX, Zou JX, Gao SS, Li YX, Yang C. Apoptosis and its relationship with cell proliferation, p53, Waf1p21, bcl-2 and c-myc in esophageal carcinogenesis studied with a high-risk population in northern China. World J Gastroenterol. 1998;4:287–293. doi: 10.3748/wjg.v4.i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu HY, Yang YL, Guan XL, Song G, Jiang AM, Shi LJ. Expression of regulating apoptosis gene and apoptosis index in primary liver cancer. World J Gastroenterol. 2000;6:721–724. doi: 10.3748/wjg.v6.i5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keith FJ, Bradbury DA, Zhu YM, Russell NH. Inhibition of bcl-2 with antisense oligonucleotides induces apoptosis and increases the sensitivity of AML blasts to Ara-C. Leukemia. 1995;9:131–138. [PubMed] [Google Scholar]
- 39.Ibrado AM, Huang Y, Fang G, Liu L, Bhalla K. Overexpression of Bcl-2 or Bcl-xL inhibits Ara-C-induced CPP32/Yama protease activity and apoptosis of human acute myelogenous leukemia HL-60 cells. Cancer Res. 1996;56:4743–4748. [PubMed] [Google Scholar]
- 40.Bullock G, Ray S, Reed JC, Krajewski S, Ibrado AM, Huang Y, Bhalla K. Intracellular metabolism of Ara-C and resulting DNA fragmentation and apoptosis of human AML HL-60 cells possessing disparate levels of Bcl-2 protein. Leukemia. 1996;10:1731–1740. [PubMed] [Google Scholar]
- 41.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang LX, Zhang L. Apoptosis, apoptotic regulation genes and their relationship with Atrophic gastritis. Shijie Huaren Xiaohua Zazhi. 2002;10:581–583. [Google Scholar]
- 43.Lu P, Luo HS, Yu BP. Hepatocellular apoptosis and its apoptosis-regulating gene in rat liver fibrosis model. Shijie Huaren Xiaohua Zazhi. 2001;9:165–169. [Google Scholar]
- 44.Wang JM, Zou Q, Zou SQ. Bcl-2 and Bax expressions and apoptosis in rat liver with obstructive jaundice. Shijie Huaren Xiaohua Zazhi. 2001;9:911–914. [Google Scholar]
- 45.Liu HF, Liu WW, Fang DC, Gao JH, Wang ZH. Apoptosis and proliferation in duced by Helicobacter pylori and its association with p53 protein expres si on in gastric epithelial cells. Shijie Huaren Xiaohua Zazhi. 2001;9:1265–1268. [Google Scholar]
- 46.Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, Su WJ. Induction of apoptosis by TPA and VP-16 is through translocation of TR3. World J Gastroenterol. 2002;8:446–450. doi: 10.3748/wjg.v8.i3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JU, Hosotani R, Wada M, Doi R, Kosiba T, Fujimoto K, Miyamoto Y, Mori C, Nakamura N, Shiota K, et al. Mechanism of apoptosis induced by cisplatin and VP-16 in PANC-1 cells. Anticancer Res. 1997;17:3445–3450. [PubMed] [Google Scholar]
- 48.Ogretmen B, Safa AR. Down-regulation of apoptosis-related bcl-2 but not bcl-xL or bax proteins in multidrug-resistant MCF-7/Adr human breast cancer cells. Int J Cancer. 1996;67:608–614. doi: 10.1002/(SICI)1097-0215(19960904)67:5<608::AID-IJC3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 49.Zou L, Liu W, Yu L. [beta-elemene induces apoptosis of K562 leukemia cells] Zhonghua Zhongliu Zazhi. 2001;23:196–198. [PubMed] [Google Scholar]
- 50.Yu C, Wang Z, Dent P, Grant S. MEK1/2 inhibitors promote Ara-C-induced apoptosis but not loss of Deltapsi (m) in HL-60 cells. Biochem Biophys Res Commun. 2001;286:1011–1018. doi: 10.1006/bbrc.2001.5513. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J, Chen Y, Li C. [Ara-c induced apoptosis in human myeloid leukemia cell line HL-60] Zhonghua Zhongliu Zazhi. 1997;19:107–110. [PubMed] [Google Scholar]
- 52.Chen Y, Zhou J, Li C, Wang B, Li H. Effect of concurrent use of rh-IL-3 and rh-GM-CSF on apoptosis of HL-60 cells induced by Ara-C. J Tongji Med Univ. 1997;17:13–17. doi: 10.1007/BF02887994. [DOI] [PubMed] [Google Scholar]
- 53.Yang H, Wang X, Yu L. [The antitumor activity of elemene is associated with apoptosis] Zhonghua Zhongliu Zazhi. 1996;18:169–172. [PubMed] [Google Scholar]
- 54.Ren X, Wang D, Li H. [Study on electrochemical behavior of HL-60 cells during the etoposide-inducing apoptosis] Zhonghua Xueyexue Zazhi. 1999;20:82–84. [PubMed] [Google Scholar]
- 55.Jin ML, Zhang P, Ding MX, Yun JP, Chen PF, Chen YH, Chew YQ. Altered expression of nuclear matrix proteins in etoposide induced apoptosis in HL-60 cells. Cell Res. 2001;11:125–134. doi: 10.1038/sj.cr.7290077. [DOI] [PubMed] [Google Scholar]
- 56.Wang JZ, Tsumura H, Shimura K, Ito H. Antitumor activity of polysaccharide from a Chinese medicinal herb, Acanthopanax giraldii Harms. Cancer Lett. 1992;65:79–84. doi: 10.1016/0304-3835(92)90216-i. [DOI] [PubMed] [Google Scholar]
- 57.Wang JC, Hu SH, Su CH, Lee TM. Antitumor and immunoenhancing activities of polysaccharide from culture broth of Hericium spp. Kaohsiung J Med Sci. 2001;17:461–467. [PubMed] [Google Scholar]


