Abstract
AIM: To study the effect of angiogenesis inhibitor TNP-470 on peritoneal dissemination of colon cancer in nude mice.
METHODS: The MTT assay was used to evaluate the inhibitory effect of TNP-470 on human colon cancer cell line Lovo. Lovo cells were injected into the peritoneal cavity of BABL/C nu/nu mice and the models of peritoneal dissemination were developed. Thirty nude mice were randomly divided into control and TNP-470-treated group. In TNP-470-treated group, TNP-470 was injected subcutaneously every other day from day 1 until sacrifice or death (30 mg•kg⁻¹). The control group received a sham injection of the same volume saline solution.
RESULTS: In vitro, TNP-470 inhibited the growth of Lovo cells, with its IC50 at 2.14 × 102 μg•L-1. In vivo, TNP-470 demonstrated growth inhibition of tumors. Mice body weight and abdominal circumferences were significantly different between TNP-470-treated group (24.5 ± 3.2 g, 7.0 ± 1.1 cm) and control group (29.5 ± 2.1 g, 10.3 ± 1.5 cm), P = 0.005 and P = 0.001. The number of disseminated foci was significantly different between the control group (92.1 ± 20.6) and the TNP-470-treated group (40.3 ± 12.3), P < 0.001. The maximal size of foci was significantly smaller in TNP-470-treated group (3.3 ± 0.7 mm) than that of control (7.3 ± 2.3 mm), P = 0.004. Mean survival time was significantly longer in TNP-470-treated group (98.00 ± 12.06 d) than that in control group (41.86 ± 9.51 d), P < 0.001.
CONCLUSION: Angiogenesis inhibitor TNP-470 might be effective in treating peritoneal dissemination of colon cancer and improve the survival rate of nude mice.
INTRODUCTION
Colorectal cancer still remains the most frequent malignancy in Japan, Unite States of American and China. Although combined therapies, including chemotherapy, radiation therapy and immunotherapy are performed in addition to surgical radical resection, nearly 50% of patients still die of recurrence and a major form of recurrence was peritoneal dissemination[1]. Therefore, new therapeutic programs are needed to raise the survival rate of colorectal cancer patients. The importance of tumor angiogenesis is widely accepted in cases of blood-born metastases[2,3]. Although the form of blood supply is markedly different between metastases in solid organs and those at the peritoneum, it has been generally accepted that any foci larger than 0.2 mm require new tumor vessels for their growth[1-4]. Thus, inhibition of angiogenesis would prevent the tumor growth and their peritoneal dissemination.
TNP-470 is a semisynthetic analogue of fumagillin isolated from Aspergillus fumigatus. TNP-470 has been reported to inhibit neovascularization by preventing endothelial cells growth and proliferation[5-9]. Recently, the therapeutic effects of TNP-470 on various human and rodent tumors have been reported and this agent shows a marked inhibitory effect on tumor growth and metastasis in vivo[10-11]. However, the importance of angiogenesis in the establishment and growth of peritoneal dissemination remains unknown and there has been no report that evaluates the effect of TNP-470 on establishment and growth of peritoneal dissemination and ascites production of human colon cancer.
In this study, we investigated the inhibitory effects of TNP-470 on an establishment and growth of intraperitoneally inoculated human colon cancer cell line, Lovo, and survival of nude mice with this tumor in vivo. We also examined the inhibitory effect of TNP-470 on cell growth in vitro.
MATERIALS AND METHODS
Drug and reagents
TNP-470 was a generous gift from Takeda Chemical Industries (Osaka, Japan). 3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyl- tetrazolium bromide (MTT), gum arabic and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO); RPMI 1640 and heat-inactivated fetal calf serum (FCS) were purchased from Gibco (Grand Island, NY).
TNP-470 was stored dry at -20 °C. In vitro experiments, TNP-470 was dissolved in DMSO and RPMI 1640 medium supplemented with 10% FCS. The final concentration of DMSO was 0.1%, while in vivo experiments, TNP-470 was suspended in a vehicle of 3% ethanol and 5% gum arabic in saline.
Cell line
Human colon adenocarcinoma cell line, Lovo was kindly provided by the Department of Pathology, Cancer Center, First Military Medical University (FIMMU). Cells were cultured in RPMI 1640 supplemented with 10% FCS, and were maintained at 37 °C in 5% CO. All experiments were performed using cells harvested at the 80%-90% subconfluent stage.
Animals
Female BALB/c nude mice were obtained from the Experimental Animal Center, FIMMU, and reared under specific pathogene-free condition. Four-week-old mice weighing 17-22 g were used in the experiments.
In vitro experiments
The MTT assays were made to evaluate the sensitivity of TNP-470 to Lovo cells[8]. Lovo cells were plated in 96-well mocrotiter plates at a concentration of 5 × 104 cells in 50 μL of RPMI 1640 medium. After 24 h incubation, the medium was changed to RPMI 1640 medium with various concentrations of TNP-470 (5 × 10-4 μg•L-1 - 5 × 105 μg•L-1), and the medium was incubated for 48 h, 20 μg MTT (5 g•L-1) solution was then added to each well. After the plates were incubated for 3 h, 150 μL DMSO was added. The absorbance at 570 nm was determined using a microplate reader (Bio-Rad Model 3550, Hercules, CA). Dose-response curves were plotted, and the 50% inhibitory concentration (IC50) was extrapolated as the drug dose causing a 50% reduction in absorbance as compared with control values. The experiments were repeated in three independent experiments.
In vivo experiments
Lovo cells were harvested after being cultured for 48 h and the model of peritoneal dissemination in nude mice was developed as follows. Approximately 5 × 107 cells in 0.2 mL saline solution were injected into the peritoneal cavity of an nude mouse (day 0). Thirty nude mice were randomly divided into a control group (n = 16) and a TNP-470-treated group (n = 14). In the TNP-470-treated group, TNP-470 of 30 mg•kg⁻¹ was injected subcutaneously every other day from day 1 until sacrifice. The control group received a sham injection of the same volume of saline. On day 10, two mice in control group were sacrificed and disseminated nodules on the peritoneum were evaluated. Seven mice each were sacrificed on day 30. Body weight and abdominal circumferences (substitute abdominal circumferences for ascites) of each mouse in two groups were measured. The number and maximum size of disseminated nodules on the peritoneum and mesentery were evaluated. The remaining 14 mice, 7 in each group, were followed for the survival experiment.
Statistical analysis
Data were expressed as mean ± standard deviation. Comparison between two groups was made by the independent samples t test. The survival curve was calculated by the Kaplan-meier method and compared by the Log-rank test. P < 0.05 was considered statistically significant. All statistics were carried out using SPSS10.0 statistics software.
RESULTS
Effects of TNP-470 on cell growth in vitro
In the colorimetric MTT assay, significant growth inhibition was observed in a dose-dependent manner. The IC50 value was 2.14 × 102 μg•L-1 extrapolated from the dose-response curve following 48 h exposure (Figure 1).
Figure 1.
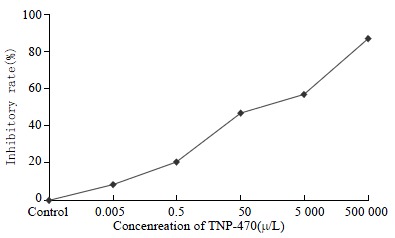
Inhibition curve of Lovo cells after TNP-470 treated 48 h.
Effects of TNP-470 on establishment and growth of peritoneal dissemination in vivo
Two mice in control group sacrificed on day 10 developed disseminated nodules, suggesting that small nodules at the peritoneum developed within 10 d after inoculation in this model. Body weight and abdominal circumferences were gained in two groups when mice sacrificed on day 30. Body weight and abdominal circumferences in the control group and the TNP-470-treated group are summarized in Table 1. The difference of body weight and abdominal circumferences were statistically significant (P = 0.005 and P = 0.001). The number and maximum size of disseminated foci of the control group and the TNP-470-treated group are shown in Table 2. The difference of foci number and maximal size of disseminated foci were also statistically significant (P < 0.001 and P = 0.004).
Table 1.
Body weight and abdominal circumference of two groups (¯x ± s)
| Groups | Mice | Body weight (g) | Abdominal circumference (cm) |
| Control | 7 | 29.5 ± 2.1 | 10.3 ± 1.5 |
| TNP-470 | 7 | 24.5 ± 3.2a | 7.0 ± 1.1b |
t = 3.394, P = 0.005,
t = 4.624, P = 0.001, vs control
Table 2.
Numbers and Maximum size of disseminated foci of two groups (¯x ± s)
| Groups | Mice | Number of foci | Maximum size of foci (mm) |
| Control | 7 | 92.1 ± 20.6 | 7.3 ± 2.3 |
| TNP-470 | 7 | 40.3 ± 12.3a | 3.3 ± 0.7b |
t = 5.715, P < 0.001,
t = 4.319, P = 0.004, vs control
In the control group, mice died from day 31 to day 57. In a TNP-470-treated group, 5 mice were died from day 74 to day 110. Two mice survived more than 120 d and TNP-470 treatment was continued. These 2 mice did not have any disseminated foci and were sacrificed on day 120 and day 130, respectively. The median survival time in the control group and the TNP-470-treated group were 40 and 92 d, the mean survival time being 41.86 ± 9.51 d and 98.00 ± 12.06 d, respectively (P < 0.001). The survival rate was significantly smaller in those in the control group than those of TNP-470-treated group (P < 0.001) (Figure 2).
Figure 2.
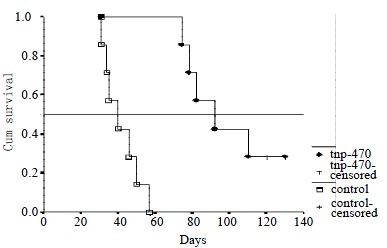
Survival curves in the control and TNP-470-treated groups
DISCUSSION
About 33% patients with colorectal cancer have recurrence after operation and 50% patients died of tumor metastasis[13,14], and the peritoneal dissemination or liver metastasis represents the most common form of recurrence. When the tumor has extended through the serosa or been resected, tumor cells are carried to distant points of the peritoneal cavity and "seeding" on peritoneum. Supported by peritoneal permeability and growth of neovascularization, these tumor cells would develop into micro-metastatic nodules, eventually producing generalized peritoneal dissemination[15,16]. With tumor recurrence as peritoneal dissemination, patient prognoses are extremely poor. Although combined therapies, including chemotherapy, radiation therapy and immunotherapy are performed in addition to surgical radical resection, no effective treatment can prevent the recurrence. New therapeutic strategies have to be invented to overcome the poor prognosis.
Angiogenesis, has been shown to be essential for tumor growth not only at the primary but also at the site of metastases, and the peritoneum would not be an exception[17-20]. Inhibition of angiogenesis is emerging as a promising strategy for cancer treatment[21-24]. Anti-angiogenic agents have demonstrated a remarkable inhibition effect on tumor growth and metastasis, and anti-angiogenic therapy may prevent the tumor recurrence[25].
Among the most potent inhibitors of angiogenesis is the fumagillin family of natural products. An analog of fumagillin, known as TNP-470 or AGM-1470, has the anti-angiogenic activity by inhibiting endothelial cell growth with high potency both invitro and in vivo[26,27]. Studies have shown that the molecular mechanism of TNP-470 inhibiting endothelial cell proliferation is associated with the two type methionine aminopeptidase (MetAp-2). TNP-470 was found to bind MetAp-2 covalently, leading to specific inhibition of its activity, and strong correlation has been found between inhibition of MetAp-2 enzymatic activity and inhibition of endothelial cell proliferation[28-33]. Investigators for TNP-470 have demonstrated suppression of neovascularization, tumor growth, and distant metastases in vivo[34-44], and the proliferation of various cancer cell lines in vitro[45-49]. There are very few studies on peritoneal dissemination[50-52], and no data are available about preventing peritoneal dissemination or survival benefit of colon cancer treated by TNP-470.
Tsujimoto et al[53] reported that angiogenesis occurred in peritoneal foci 1 week after intraperitoneal inoculation of tumor cells in a mouse model. Our study indicated that TNP-470 inhibied the proliferation of Lovo cells in vitro, with its IC50 at 2.14 × 102 μg/L, and markedly suppressed the growth of peritoneal dissemination in vivo. The mice survival time was siginificantly longer in TNP-470 treated group than that of control. Our results suggest that these effects are exerted not only by inhibiting neovascularization necessary for tumor growth, but also by directly inhibiting the proliferantion of Lovo cells.
Body weight loss was known to be the major side effect of TNP-470. There was a slight body weight gain when mice were sacrificed on day 30, and the increase was associated with the production of malignant ascites. In the survival experiments, body weight loss was observed in the control group and 3 tumor-bearing mice became cachectic with the progression of tumor. No body weight loss was observed in the treatment group, suggesting that suppression of the growth of peritoneal dissemination foci by TNP-470 resulted in a preservation of body weight and prevention of cachexia in this model.
In conclusion, angiogenesis inhibitor TNP-470 might be effective in treating peritoneal dissemination of colon cancer by inhibiting the growth of the seeded tumor cells on the peritoneum.
Footnotes
Edited by Ma JY
Supported by the Natural Science Foundation of Guangdong Province, No.013072
References
- 1.Fan YF, Huang ZH. Progress in the studies of gene therapy for colorectal cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:427–430. [Google Scholar]
- 2.Liu H, Wu JS, Li LH, Yao X. The expression of Platelet-derived growth factor and angiogenesis in human colorectal carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:661–664. [Google Scholar]
- 3.Wu J, Fan DM. Neoplastic vascularization and vascular inhibitory treatment. Shijie Huaren Xiaohua Zazhi. 2001;9:316–321. [Google Scholar]
- 4.Blood CH, Zetter BR. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990;1032:89–118. doi: 10.1016/0304-419x(90)90014-r. [DOI] [PubMed] [Google Scholar]
- 5.Kusaka M, Sudo K, Matsutani E, Kozai Y, Marui S, Fujita T, Ingber D, Folkman J. Cytostatic inhibition of endothelial cell growth by the angiogenesis inhibitor TNP-470 (AGM-1470) Br J Cancer. 1994;69:212–216. doi: 10.1038/bjc.1994.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoine N, Greimers R, De Roanne C, Kusaka M, Heinen E, Simar LJ, Castronovo V. AGM-1470, a potent angiogenesis inhibitor, prevents the entry of normal but not transformed endothelial cells into the G1 phase of the cell cycle. Cancer Res. 1994;54:2073–2076. [PubMed] [Google Scholar]
- 7.Abe J, Zhou W, Takuwa N, Taguchi J, Kurokawa K, Kumada M, Takuwa Y. A fumagillin derivative angiogenesis inhibitor, AGM-1470, inhibits activation of cyclin-dependent kinases and phosphorylation of retinoblastoma gene product but not protein tyrosyl phosphorylation or protooncogene expression in vascular endothelial cells. Cancer Res. 1994;54:3407–3412. [PubMed] [Google Scholar]
- 8.Farinelle S, Malonne H, Chaboteaux C, Decaestecker C, Dedecker R, Gras T, Darro F, Fontaine J, Atassi G, Kiss R. Characterization of TNP-470-induced modifications to cell functions in HUVEC and cancer cells. J Pharmacol Toxicol Methods. 2000;43:15–24. doi: 10.1016/s1056-8719(00)00080-0. [DOI] [PubMed] [Google Scholar]
- 9.Hotz HG, Reber HA, Hotz B, Sanghavi PC, Yu T, Foitzik T, Buhr HJ, Hines OJ. Angiogenesis inhibitor TNP-470 reduces human pancreatic cancer growth. J Gastrointest Surg. 2001;5:131–138. doi: 10.1016/s1091-255x(01)80024-x. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa H, Sato Y, Kondo M, Takahashi N, Oshima T, Sasaki F, Une Y, Nishihira J, Todo S. Combined treatment with TNP-470 and 5-fluorouracil effectively inhibits growth of murine colon cancer cells in vitro and liver metastasis in vivo. Oncol Rep. 2000;7:467–472. doi: 10.3892/or.7.3.467. [DOI] [PubMed] [Google Scholar]
- 11.Yoshizawa J, Mizuno R, Yoshida T, Hara A, Ashizuka S, Kanai M, Kuwashima N, Kurobe M, Yamazaki Y. Inhibitory effect of TNP-470 on hepatic metastasis of mouse neuroblastoma. J Surg Res. 2000;93:82–87. doi: 10.1006/jsre.2000.5956. [DOI] [PubMed] [Google Scholar]
- 12.Verma VN, Shenoy CN, Nadkarni JJ. Augmentation of cisplatin cytotoxicity using cytokines on cervical carcinoma cell lines. Cancer Biother Radiopharm. 1996;11:349–354. doi: 10.1089/cbr.1996.11.349. [DOI] [PubMed] [Google Scholar]
- 13.Zaniboni A. Adjuvant chemotherapy in colorectal cancer with high-dose leucovorin and fluorouracil: impact on disease-free survival and overall survival. J Clin Oncol. 1997;15:2432–2441. doi: 10.1200/JCO.1997.15.6.2432. [DOI] [PubMed] [Google Scholar]
- 14.Cady B, Stone MD. The role of surgical resection of liver metastases in colorectal carcinoma. Semin Oncol. 1991;18:399–406. [PubMed] [Google Scholar]
- 15.Sayag AC, Gilly FN, Carry PY, Perdrix JP, Panteix G, Brachet A, Banssillon V, Braillon G. Intraoperative chemohyperthermia in the management of digestive cancers. A general review of literature. Oncology. 1993;50:333–337. [PubMed] [Google Scholar]
- 16.Loggie BW, Perini M, Fleming RA, Russell GB, Geisinger K. Treatment and prevention of malignant ascites associated with disseminated intraperitoneal malignancies by aggressive combined-modality therapy. Am Surg. 1997;63:137–143. [PubMed] [Google Scholar]
- 17.Folkman J. Proceedings: Tumor angiogenesis factor. Cancer Res. 1974;34:2109–2113. [PubMed] [Google Scholar]
- 18.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 19.Gengrinovitch S, Greenberg SM, Cohen T, Gitay-Goren H, Rockwell P, Maione TE, Levi BZ, Neufeld G. Platelet factor-4 inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms. J Biol Chem. 1995;270:15059–15065. doi: 10.1074/jbc.270.25.15059. [DOI] [PubMed] [Google Scholar]
- 20.Jia L, Chen TX, Sun JW, Na ZM, Zhang HH. Relationship between microvessel density and proliferating cell nuclear antigen and prognosis in colorectal cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:74–76. [Google Scholar]
- 21.Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ. Interferons alpha and beta down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc Natl Acad Sci USA. 1995;92:4562–4566. doi: 10.1073/pnas.92.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 24.Wojtowicz-Praga S, Low J, Marshall J, Ness E, Dickson R, Barter J, Sale M, McCann P, Moore J, Cole A, et al. Phase I trial of a novel matrix metalloproteinase inhibitor batimastat (BB-94) in patients with advanced cancer. Invest New Drugs. 1996;14:193–202. doi: 10.1007/BF00210790. [DOI] [PubMed] [Google Scholar]
- 25.Kanai T, Konno H, Tanaka T, Matsumoto K, Baba M, Nakamura S, Baba S. Effect of angiogenesis inhibitor TNP-470 on the progression of human gastric cancer xenotransplanted into nude mice. Int J Cancer. 1997;71:838–841. doi: 10.1002/(sici)1097-0215(19970529)71:5<838::aid-ijc23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Griffith EC, Sage J, Jacks T, Liu JO. Cell cycle inhibition by the anti-angiogenic agent TNP-470 is mediated by p53 and p21WAF1/CIP1. Proc Natl Acad Sci USA. 2000;97:6427–6432. doi: 10.1073/pnas.97.12.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sin N, Meng L, Wang MQ, Wen JJ, Bornmann WG, Crews CM. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc Natl Acad Sci USA. 1997;94:6099–6103. doi: 10.1073/pnas.94.12.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klinkenberg M, Ling C, Chang YH. A dominant negative mutation in Saccharomyces cerevisiae methionine aminopeptidase-1 affects catalysis and interferes with the function of methionine aminopeptidase-2. Arch Biochem Biophys. 1997;347:193–200. doi: 10.1006/abbi.1997.0345. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Lou P, Henkin J. Selective inhibition of endothelial cell proliferation by fumagillin is not due to differential expression of methionine aminopeptidases. J Cell Biochem. 2000;77:465–473. [PubMed] [Google Scholar]
- 31.Griffith EC, Su Z, Turk BE, Chen S, Chang YH, Wu Z, Biemann K, Liu JO. Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem Biol. 1997;4:461–471. doi: 10.1016/s1074-5521(97)90198-8. [DOI] [PubMed] [Google Scholar]
- 32.Griffith EC, Su Z, Niwayama S, Ramsay CA, Chang YH, Liu JO. Molecular recognition of angiogenesis inhibitors fumagillin and ovalicin by methionine aminopeptidase 2. Proc Natl Acad Sci USA. 1998;95:15183–15188. doi: 10.1073/pnas.95.26.15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turk BE, Griffith EC, Wolf S, Biemann K, Chang YH, Liu JO. Selective inhibition of amino-terminal methionine processing by TNP-470 and ovalicin in endothelial cells. Chem Biol. 1999;6:823–833. doi: 10.1016/s1074-5521(99)80129-x. [DOI] [PubMed] [Google Scholar]
- 34.Yamaoka M, Yamamoto T, Masaki T, Ikeyama S, Sudo K, Fujita T. Inhibition of tumor growth and metastasis of rodent tumors by the angiogenesis inhibitor O- (chloroacetyl-carbamoyl) fumagillol (TNP-470; AGM-1470) Cancer Res. 1993;53:4262–4267. [PubMed] [Google Scholar]
- 35.Qian CN, Min HQ, Lin HL, Hong MH, Ye YL. Primary study in experimental antiangiogenic therapy of nasopharyngeal carcinoma with AGM-1470 (TNP-470) J Laryngol Otol. 1998;112:849–853. doi: 10.1017/s0022215100141878. [DOI] [PubMed] [Google Scholar]
- 36.Katzenstein HM, Rademaker AW, Senger C, Salwen HR, Nguyen NN, Thorner PS, Litsas L, Cohn SL. Effectiveness of the angiogenesis inhibitor TNP-470 in reducing the growth of human neuroblastoma in nude mice inversely correlates with tumor burden. Clin Cancer Res. 1999;5:4273–4278. [PubMed] [Google Scholar]
- 37.Konno H. Antitumor effect of the angiogenesis inhibitor TNP-470 on human digestive organ malignancy. Cancer Chemother Pharmacol. 1999;43 Suppl:S85–S89. doi: 10.1007/s002800051104. [DOI] [PubMed] [Google Scholar]
- 38.Kanai T, Konno H, Tanaka T, Matsumoto K, Baba M, Nakamura S, Baba S. Effect of angiogenesis inhibitor TNP-470 on the progression of human gastric cancer xenotransplanted into nude mice. Int J Cancer. 1997;71:838–841. doi: 10.1002/(sici)1097-0215(19970529)71:5<838::aid-ijc23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Watson JC, Sutanto-Ward E, Osaku M, Weinstein JK, Babb JS, Sigurdson ER. Importance of timing and length of administration of angiogenesis inhibitor TNP-470 in the treatment of K12/TRb colorectal hepatic metastases in BD-IX rats. Surgery. 1999;126:358–363. [PubMed] [Google Scholar]
- 40.Konno H, Tanaka T, Kanai T, Baba S. [Therapeutic effect of angiogenesis inhibitors on liver metastases of human colorectal carcinoma] Nihon Geka Gakkai Zasshi. 1998;99:441–445. [PubMed] [Google Scholar]
- 41.Tanaka T, Konno H, Matsuda I, Nakamura S, Baba S. Prevention of hepatic metastasis of human colon cancer by angiogenesis inhibitor TNP-470. Cancer Res. 1995;55:836–839. [PubMed] [Google Scholar]
- 42.Konno H, Tanaka T, Matsuda I, Kanai T, Maruo Y, Nishino N, Nakamura S, Baba S. Comparison of the inhibitory effect of the angiogenesis inhibitor, TNP-470, and mitomycin C on the growth and liver metastasis of human colon cancer. Int J Cancer. 1995;61:268–271. doi: 10.1002/ijc.2910610221. [DOI] [PubMed] [Google Scholar]
- 43.Hori K, Li HC, Saito S, Sato Y. Increased growth and incidence of lymph node metastases due to the angiogenesis inhibitor AGM-1470. Br J Cancer. 1997;75:1730–1734. doi: 10.1038/bjc.1997.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gervaz P, Scholl B, Padrun V, Gillet M. Growth inhibition of liver metastases by the anti-angiogenic drug TNP-470. Liver. 2000;20:108–113. doi: 10.1034/j.1600-0676.2000.020002108.x. [DOI] [PubMed] [Google Scholar]
- 45.Emoto M, Ishiguro M, Iwasaki H, Kikuchi M, Kawarabayashi T. TNP-470 inhibits growth and the production of vascular endothelial growth factor of uterine carcinosarcoma cells in vitro. Anticancer Res. 2000;20:601–604. [PubMed] [Google Scholar]
- 46.Yamamoto D, Kiyozuka Y, Adachi Y, Takada H, Hioki K, Tsubura A. Synergistic action of apoptosis induced by eicosapentaenoic acid and TNP-470 on human breast cancer cells. Breast Cancer Res Treat. 1999;55:149–160. doi: 10.1023/a:1006283131240. [DOI] [PubMed] [Google Scholar]
- 47.Singh Y, Shikata N, Kiyozuka Y, Nambu H, Morimoto J, Kurebayashi J, Hioki K, Tsubura A. Inhibition of tumor growth and metastasis by angiogenesis inhibitor TNP-470 on breast cancer cell lines in vitro and in vivo. Breast Cancer Res Treat. 1997;45:15–27. doi: 10.1023/a:1005826129756. [DOI] [PubMed] [Google Scholar]
- 48.Satoh H, Ishikawa H, Fujimoto M, Fujiwara M, Yamashita YT, Yazawa T, Ohtsuka M, Hasegawa S, Kamma H. Angiocytotoxic therapy in human non-small cell lung cancer cell lines--advantage of combined effects of TNP-470 and SN-38. Acta Oncol. 1998;37:85–90. doi: 10.1080/028418698423221. [DOI] [PubMed] [Google Scholar]
- 49.Satoh H, Ishikawa H, Fujimoto M, Fujiwara M, Yamashita YT, Yazawa T, Ohtsuka M, Hasegawa S, Kamma H. Combined effects of TNP-470 and taxol in human non-small cell lung cancer cell lines. Anticancer Res. 1998;18:1027–1030. [PubMed] [Google Scholar]
- 50.Yoshikawa T, Yanoma S, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Angiogenesis inhibitor, TNP-470, suppresses growth of peritoneal disseminating foci. Hepatogastroenterology. 2000;47:298–302. [PubMed] [Google Scholar]
- 51.Kato H, Ishikura H, Kawarada Y, Furuya M, Kondo S, Kato H, Yoshiki T. Anti-angiogenic treatment for peritoneal dissemination of pancreas adenocarcinoma: a study using TNP-470. Jpn J Cancer Res. 2001;92:67–73. doi: 10.1111/j.1349-7006.2001.tb01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu B, Lin Y, Yin H. [Experimental study of the effect of angiogenesis inhibitor TNP-470 on the growth and metastasis of gastric cancer in vivo] Zhonghua Zhongliu Zazhi. 1998;20:34–36. [PubMed] [Google Scholar]
- 53.Tsujimoto H, Hagiwara A, Shimotsuma M, Sakakura C, Osaki K, Sasaki S, Ohyama T, Ohgaki M, Imanishi T, Yamazaki J, et al. Role of milky spots as selective implantation sites for malignant cells in peritoneal dissemination in mice. J Cancer Res Clin Oncol. 1996;122:590–595. doi: 10.1007/BF01221190. [DOI] [PMC free article] [PubMed] [Google Scholar]


