Abstract
AIM: To estimate the effect of a therapeutic vaccine against pancreatic carcinoma based on dendritic cell (DC) vaccine modified with tumor lysate and Interleukin-18 gene.
METHODS: The BALB/C mice model of pancreatic carcinoma was induced with DMBA. DC vaccine was constructed through pulsed with tumor lysate and transfected by the recombinant adenoviral vector encoding IL-18 gene. The immnotherapeutic effects of DC vaccine on mice with pancreatic carcinoma were assessed (divided into DC-IL18-Lysate group, DC-Lysate group, DC-IL18 group, DC group, PBS group).
RESULTS: After vaccination of the DC vaccine, the concentration of IL-18 and IFN-γ were 2161 ± 439 ng·L-1 and 435 ± 72 ng·L-1 in DC-IL18-Lysate group and there was significant difference compared with other groups (P < 0.01). After vaccination of the DC vaccine, the transplanted tumors were observed on 30 d in DC-Lysate groups, on 16 d in DC-IL18 groups, on 3 d in control group, but mice remained tumor-free for at least 50 d in DC-IL18-Lysate group and there was significant difference between DC-IL18-Lysate group and other groups (P < 0.01). The median survival exceeds 62 d in DC-IL18-Lysate group. But the median survival was 48.6 d in DC-Lysate group, 33 d in DC-IL18 group, 17 d in PBS group. The survival period was obviously prolonged in DC-IL18-Lysate group than in other groups (P < 0.05, P < 0.01). The weight of pancreatic tumor was 0.22 ± 0.083 g in DC-IL18-Lysate group, 1.45 ± 0.74 g in DC-Lysate group, 1.89 ± 1.34 g in DC-IL18 group, 3.0 ± 1.6 g in DC group, 2.9 ± 2.0 g in PBS group and the weight of tumor obviously reduced in DC-IL18-Lysate group than in other groups (P < 0.05, P < 0.01).
CONCLUSION: DC vaccine modified with tumor lysate and Interleukin-18 gene can induce a specific and effective immune response against pancreatic carcinoma cell.
INTRODUCTION
Because of the lack of methods for early diagnosis and the limited effects of surgical treatment, chemotherapy and radiation therapy, about 98% of the patients with pancreatic carcinoma don't live more than 5 years[1-4]. So we clearly need the new therapies to improve the prognosis of the patients with pancreatic carcinoma. Immunotherapy is moving close to become a promising approach of anticancer therapy as it has fewer side effects and, more importantly, the opportunity to generate long-term immunity[5-7]. Dendritic cell (DC) is highly effective antigen presenting cell (APC) with the unique capability of inducing primary immune response against tumor-associated antigens. Animal studies have shown that the DC vaccine pulsed with tumor antigen could elicit specific T-cell response against tumor[8-12]. Recently, Geiger et al[13] reported that they have completed the first phase trial of tumor lysate-pulsed DC vaccine in the therapy of pediatric solid tumor, including osteosarcoma and fibrosarcoma, which were partial or complete regression.
Interleukin-18 (IL-18), originally termed IFN-inducing factor, induces IFN-γ production in both T cells and NK cells[14-15]. In addition, IL-18 induces T cells to produce GM-CSF, and enhances the cytolytic activity of T cells and NK cells[16]. In some animal model systems, IL-18 gene transfected into tumor cells should enhance both specific and nonspecific antitumor immune responses, which indicate if IL-18 gene were transfered into DC, it should induce highly effective antitumor immune responses[17-20].
In this study, an in vivo model to estimate the effect of a therapeutic vaccine against pancreatic carcinoma based on DC vaccine modified with tumor lysate and Interleukin-18 gene was designed. We hope those results should provided a scientific basis for our next step, clinical trials, in the future.
MATERIALS AND METHODS
Materials
BALB/c mice (6-8 week old, male) were purchased from the experimental animal center of the TongJi Medical Collegy. 3H-TdR, 51Gr was from Beijing Institute of Atomic Energy, 7, 12-Dimethylbenzanthracene (DMBA) from Aldrich Co. Germany. IL-18, IFN-γ ELISA Kits from Zhongke Biotech Co. Wuhan. A recombinant adenoviral vector encoding IL-18 gene termed pCR3.1-IL-18 was kind gift from Dr. Chenwen Ye (Department of Immunology, Institut Pasteur de Lille, Paris, France). The mouse dendritic cell line MTSC4 derived from the thymic of BALB/c mice (4 week old) were obtained from the department of immunology of Beijing medical university, which were maintained in CM.
Methods
The mice model of pancreatic carcinoma The membrance and partial parenchyma of BALB/c mice's pancreas were opened about 1 mm depth and the DMBA (7 mg) was put into there, as previously described[21]. After 3-4 mo, the mice developed pancreatic ductal adenocarcinomas with glandular duct-like distribution of cancer cells. The tumors from mice's pancrease were removed, carefully detached with a cell scraper, washed twice in PBS, and resuspended at a density of 2 × 107/mL in serum-free medium.0.5 mL (2 × 107/mL) viable tumor cells were injected into the left flank of BALB/c to develop the mice model of pancreatic carcinoma.
DC pulsed with tumor lysate Pancreatic carcinoma cells from the fresh solid tumor of mice were incubated with 0.01% EDTA-solution for 10 min, washed twice in PBS, and resuspended at a density of 5 × 106/mL in serum-free medium. The cell suspensions were frozen at -80 °C (2 min) and thawed in 37 °C water (4 min), which were disrupted by four freeze-thaw cycles. For the removal of crude debris, the lysate was centrifuged for 10 min at 300 × g. The supernatant was collected and passed through a 0.2-um filter for later use. Dendritic cells (DCs) were incubated with tumor lysates at a ratio of three tumor cells equivalents to one DC (i.e., 3:1) in CM. After 18 hr of incubation, DCs were harvested, washed twice in HBSS, and resuspended in HBSS for further study.
Cell transfection A recombinant adenoviral vector encoding IL-18 gene termed pCR3.1-IL-18 was kind gift from Dr. Chemen Ye (Immunology, Paris, France). For the transfection, DC and DC pulsed with tumor lysates were washed twice in HBSS, and incubated at 37 °C with the adenoviral vectors, respectively. Virus was used at a dose of 100 multiplicity of infection (MOI). Under these conditions, more than 80% of DCs were infected.
Induction of tumor-specific CTL in vivo 30 BALB/c mice were at random divided into five groups. Every group included 6 mice, which were immunized s.c. in the right flank, (1) 0.2 mL DC-IL18-Lysate (2 × 104 DCs modified with tumor lysate and IL-18, DC-IL18-Lysate group); (2) 0.2 mL DC-Lysate (2 × 104 DCs modified with tumor lysate, DC-lysate group); (3)0.2 mL DC-IL18 (2 × 104 DCs modified with IL-18, DC-IL18 group); (4) 0.2 mL DC (2 × 104 DCs, DC group) and (5) 0.2 mL PBS (control group), respectively, twice at 7 d. After 7 d, spleen-derived T cells were isolated from mice by Nylon wool-separated and were cocultured in vitro with tumor cells for 5 d. After 5 d, T cells were tested for cytolytic activity in a standard 4-h 51Cr-release assay. Effector-to target (E/T) ratio were from 20:1 to 100:1. Each assay was performed in triplicate and triplicate wells were averaged and percentage of special CTL was calculated by the formula [(sample-spontaneous release)/(maximum release-spontaneous release) × 100%][22].
Immunological protection of DCs vaccine 30 BALB/c mice were at random divided into five groups and were immunized s.c. in the right flank with DC-IL18-Lysate, DC-Lysate, DC-IL18, DC, PBS, respectively, twice at 7 d (described above). These mice were challenged 7 d after the last immunization with 0.5 mL (1 × 107/mL) viable tumor cells from mice pancreas by s.c. in the left flank. The development of pancreatic carcinoma was observed in every mice.
Immunotherapeutic effect of DCs vaccine 30 mice from the mice model of pancreatic carcinoma with average tumor size 0.3 × 0.5 cm2 were at random divided into five groups (described above), which were respectively injected in the right flank with 2 × 104 DC-IL18-Lysate, DC-Lysate, DC-IL18, DC, PBS, respectively, twice at 7 d. The size of the tumors was recorded twice weekly by measuring the largest perpendicular diameters (PD) and transverse diameter (TD) with calipers. The weight of the tumor was calculated by the formula[(PD × TD2)/2].
Cytokine analysis After 7 d of the mice with pancreatic carcinoma immunized (described above), serum was obtained from the carotid artery of mice. The concentration of IL-18 and IFN-γ in the serum were measured by ELISA.
Statistical analysis
Data were expressed as the mean ± SEM and were analyzed by t test or ANOVA. Differation were considered significant when P was < 0.05. Tests were performed using SAS (Statistical Analysis Software).
RESULTS
Tumor-specific CTL in vivo
Spleen cells obtained from mice at 7 d after the final immunization were cocultured with pancreatic carcinoma cells for 5 d. T cells of DC-IL18-Lysate group, DC-Lysate group and DC-IL18 groups were able to efficiently lyse the pancreatic carcinoma cells. The lytic efficiency increases following the rise of E/T ratio. The lytic efficiency of DC-IL18-Lysate group was the best, DC-Lysate group was the subsequence, and DC-IL18 group was the final. But T cells from additional groups obviously lack this ability (P < 0.01, Figure 1).
Figure 1.
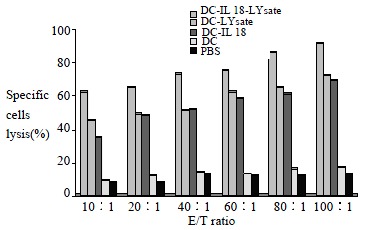
T cells were tested for cytolytic activity in a standard 4-h 51Cr-release assay. Effector-to target (E/T) ratio were from 20:1 to 100:1.
Cytokine analysis
After 7 d of the mice immunized, the concentration of IL-18 and IFN-γ in the serum were measured by ELISA. The concentration of IL-18 and IFN-γ were 2161 ± 439 ng·L-1 and 435 ± 72 ng·L-1 in DC-IL18-Lysate group. There were significant differences in DC-IL18-Lysate group versus other groups (P < 0.05, P < 0.01, Figure 2A, Figure 2B).
Figure 2.
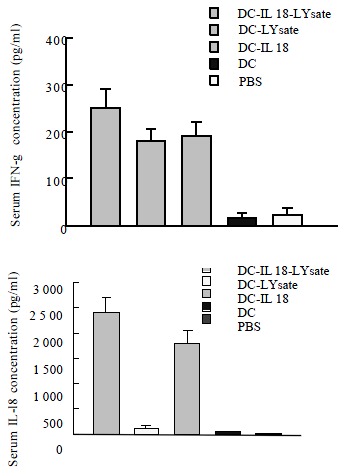
A, B The concentration of IL-18 and IFN-γ in the serum were measured by ELISA
Immunological protection of DCs vaccine
The transplanted tumors were not observed in DC-IL18-Lysate group after the viable tumor cells from mice pancreas injected into the left flank 50 d. After 30 d, the transplanted tumors were observed in DC-Lysate groups. The first time was 16 d the transplanted tumor observed in DC-IL18 groups after the tumor cells injected into mice. Additional groups all were observed the development of transplanted tumors from 3 to 9 d (P < 0.01, Figure 3).
Figure 3.
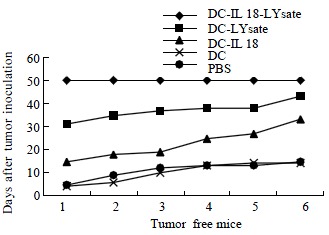
The development of the transplanted tumors was observed in every day
TImmunotherapeutic effect of DCs vaccine
The growth velocity of tumor was observed obviously slowing in the mice immunized with DC-IL18-Lysate, DC-Lysate and DC-IL18. There were 3 cases in DC-IL18-Lysate group and 1 case in DC-Lysate group observed almost complete regression of the tumors. In contrast, the tumor cells displayed infiltrating style and adherent with nearby tissue in the mice immunized with PBS and DC. There was significant difference in the weight of tumor between the DC-IL18-Lysate group and the other groups (P < 0.01, versus PBS group, DC group and DC-IL18 group; P < 0.05, versus DC-Lysate group). The median survival exceeds 62 d in DC-IL18-Lysate group. But the median survival was only 48.6 d in DC-Lysate group, 33 d in DC-IL18 group, 17 d in PBS group (Table 1).
Table 1.
Immunotherapeutic Effect of DCs Vaccine in Mice with Pancreatic Carcinoma
| DC-IL18-Lysate I group | DC-Lysate II group | DC-IL18 III group | DC IV group | PBS V group | |
| Tumor Weight (g) | 0.22 ± 0.083a | 1.45 ± 0.74bc | 1.89 ± 1.34d | 3.0 ± 1.6 | 2.9 ± 2.0 |
| Median Survival (d) | > 62a | 48.6 ± 9.4bc | 33 ± 12.3d | 17 ± 3.2 | 19.6 ± 5.4 |
P < 0.01, vs the other groups;
P < 0.05, vs III group;
P < 0.01, vs IV and V groups;
P < 0.05, vs IV and V group
DISCUSSION
Dendritic cells (DCs) play an important role as primary antigen-presenting cell to initiate and maintain T-cell responses[23-25]. In our study, we designed an in vivo model to estimate the effect of a therapeutic vaccine against pancreatic carcinoma based on DCs modified with tumor lysate and Interleukin-18 gene. It was observed that DCs were pulsed with tumor lysate could obviously increase the efficiency against the pancreatic carcinoma cells.
At present, potential targets for the immunotherapy of pancreatic carcinoma are antigens such as carcinoembryonic antigen[26], HER-2/neu[27], mutant ras[28], p53[29]. However, vaccinating a single antigen has disadvantages, because it is unknown which of the identified antigens was the potential to induce an effective antitumor immune response. Furthermore, immunity against a single antigen maybe ineffective in tumors with pancreatic carcinoma and carries the risk of inducing tumor antigen escape variant[30,31]. In addition, this strategy is restricted to those patients with a specific HLA type.
In our research, we selected the tumor lysate as the target antigent because these unfractionated tumor-derived antigens could circumvent these disadvantages. Tumor lysates contain multiple known as well as unknown antigens that could be presented to T cells by MHC class I- and class II-pathways[32-35]. Therefore, lysate-load DCs are more likely to induce a polyclonal expansion of T cells, including MHC class II restricted T-helper cells. These have been recognized to play an important role in the activation of CTLs, which were probably the most important cells in antitumor immune response. The generation of CTL clones with multiple specificities may be an advantage in heterogeneous tumors and could also reduce the risk of tumor escape variants. Furthermore, lysate from the autologous tumor can be used independently of the HLA type the patient. A major drawback of unfractionated tumor antigens is the possibility of inducing an autoimmune reactivity to epitopes that are shared by normal tissues[36]. However, in clinical trials using lysate as the source of antigen, no clinically relevant autoimmune responses were detected[37-44].
IL-18 is a recently discovered cytokine cloned from mice with fluminant hepatitis induced by challenge with propionibacterium acnes and subsequent administration of LPS[14]. IL-18 lacks a signal sequence and is processed into the mature form by an IL-1β-converting enzyme (ICE)[15]. IL-18 is produced by cells of monocyte lineage, augments NK cytolytic activity, and enhances proliferation of T cells. IL-18 also promotes NK and T cells to secrete IFN-γ and GM-CSF. Based on those findings, IL-18 was demonstrated to confer a superior antitumor activity in some murine tumor systems. However, the systemic administration of recombinant IL-18 proteins, though effectively inhibited the tumor growth, resulted in death of all animals because of toxicity[45-47]. Systemic administration of IL-18 also was associated with severe dose-dependent toxicity in patient during the first human trial. The transfer of cytokine genes may circumvent the toxicity of systemic IL-18, at same time, may delivery and provide adequate local cytokine levels for immune cell activation.
In our study, the IL-18 gene was transfected into DCs by recombinant adenoviral vector and we observed cytokine releases in serum of the mice were immunized with DCs modified with IL-18 and tumor lysate by ELISA. After 7 d of the mice immunized, the concentration of IL-18 and IFN-γ was obviously increased in DC-IL18-Lysate group. This indicated DCs transfected with the cytokines gene exhibited significant levels of IL-18 production, At same time, augmented the production of IFN-γ.
In our research, the effect of immunological protection and immunotherapy of DCs vaccines was obviously increased when DCs were modified with IL-18 gene and tumor lysate than IL-18 gene alone, tumor lysate alone. Especially, from the results of our study we found DCs modified with IL-18 gene alone induced a limited antitumor immunologic reaction. These findings strongly suggest that IL-18, tumor lysate and DC interacted and there were congenerous effect against pancreatic carcinoma. At first, IL-18 enhanced NK cytolytic activity to induce more frequent and effective tumor cell death and promoted NK and T cells to secrete IFN-γ. Zitvoget et al[48] reported the antitumor effect of DC-based vaccination was dependent on production of Th1-associated cytokines such as IFN-γ. Therefore, IL-18 may play an important role in antitumor activity of DCs through enhancing the production of IFN-γ[49]. In turn, DCs sever as effective antigen-presenting cells to induce potent and specific immunologic reaction. At same time, DCs expressed more ICE that was required when the preprotein of IL-18 was processed into the biological activity form. As shown in a recent study, the functions of DCs were affected by contact with tumor cells. Dynamic changes in chemokine receptor expression (up-regulation of CCR7) were identified on DCs following contact apoptotic tumor cells[50]. Fumiaki et al[51] also demonstrated that direct contact with DCs and tumor cell could be important for generating CTLs. Thus, the implications are that IL-18/Tumor cell/DC plays a critical inductive and interactive role in promoting the efficiency of immunotherpy against pancreatic carinoma.
In summary, DC vaccines modified with tumor lysate and Interleukin-18 gene can induce a specific and effective immune response against pancreatic carcinoma cells. However, the feasibility and security of this DCs vaccine still need to be observed in additional experiments.
Footnotes
Edited by Qi QH
References
- 1.Motoi F, Sunamura M, Ding L, Duda DG, Yoshida Y, Zhang W, Matsuno S, Hamada H. Effective gene therapy for pancreatic cancer by cytokines mediated by restricted replication-competent adenovirus. Hum Gene Ther. 2000;11:223–235. doi: 10.1089/10430340050015978. [DOI] [PubMed] [Google Scholar]
- 2.Zhou ZH, Song MZ. Current therapies of pancreatic cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:214–215. [Google Scholar]
- 3.Liu JW, Li KZ. Pancreatic cancer, oncogene and anti oncogene. Shijie Huaren Xiaohua Zazhi. 2001;9:72–73. [Google Scholar]
- 4.Zhang SN, Yuan SZ. Gene therapy for pancreatic carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:269–270. [Google Scholar]
- 5.Liu MP, Zhou JC, Guo XZ, Chen W, Dai B, An TY, Ma SY. Purification and characterization of antigen SC6 for pancreatic cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:593–595. [Google Scholar]
- 6.Leng JJ, Chen YQ, Leng XS. Genetic therapy for pancreatic neoplasms. Shijie Huaren Xiaohua Zazhi. 2000;8:916–918. [Google Scholar]
- 7.Jia L, Yuan SZ. Progress of treatment of advanced pancreatic carcinoma with gemcitabine. Shijie Huaren Xiaohua Zazhi. 1999;7:985–986. [Google Scholar]
- 8.Lambert LA, Gibson GR, Maloney M, Durell B, Noelle RJ, Barth RJ. Intranodal immunization with tumor lysate-pulsed dendritic cells enhances protective antitumor immunity. Cancer Res. 2001;61:641–646. [PubMed] [Google Scholar]
- 9.Shimizu K, Thomas EK, Giedlin M, Mulé JJ. Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res. 2001;61:2618–2624. [PubMed] [Google Scholar]
- 10.Li MS, Yuan AL, Zhang WD. Low immune function of peripheral blood dendritic cells in hepatocarcinoma patients. Shijie Huaren Xiaohua Zazhi. 1998;6:240–241. [Google Scholar]
- 11.Li MS, Yuan AL, Zhang WD, Liu SD, Lu AM, Zhou DY. Dendritic cells in vitro induce efficient and special anti-tumor immune response. Shijie Huaren Xiaohua Zazhi. 1999;7:161–163. [Google Scholar]
- 12.Chen HB, Zhang JK, Huang ZL, Sun JL, Zhou YQ. Effects of cytokines on dendritic cells against human hepatoma cell line. Shijie Huaren Xiaohua Zazhi. 1999;7:191–193. [Google Scholar]
- 13.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mulé JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- 14.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 15.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 16.Wu HG, Zhou LB, Pan YY, Huang C, Chen HP, Shi Z, Hua XG. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol. 1999;5:515–517. doi: 10.3748/wjg.v5.i6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akita K, Ushio S, Ohtsuki T, Tustsui H, Adarki O, Yoshida N, Tanabe F. Comparison between the biological and biochemical aspects of IL-18 (IFN-gamma-inducing factor) and IL-1β. Proc Am Assoc Cancer Res. 1997;38:357–362. [Google Scholar]
- 18.Hanlon L, Argyle D, Bain D, Nicolson L, Dunham S, Golder MC, McDonald M, McGillivray C, Jarrett O, Neil JC, et al. Feline leukemia virus DNA vaccine efficacy is enhanced by coadministration with interleukin-12 (IL-12) and IL-18 expression vectors. J Virol. 2001;75:8424–8433. doi: 10.1128/JVI.75.18.8424-8433.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimm CF, Ortmann D, Mohr L, Michalak S, Krohne TU, Meckel S, Eisele S, Encke J, Blum HE, Geissler M. Mouse alpha-fetoprotein-specific DNA-based immunotherapy of hepatocellular carcinoma leads to tumor regression in mice. Gastroenterology. 2000;119:1104–1112. doi: 10.1053/gast.2000.18157. [DOI] [PubMed] [Google Scholar]
- 20.Gołab J. Interleukin 18--interferon gamma inducing factor--a novel player in tumour immunotherapy? Cytokine. 2000;12:332–338. doi: 10.1006/cyto.1999.0563. [DOI] [PubMed] [Google Scholar]
- 21.Qin RY, Ai DI, Zou SQ, Qiu FZ. Development of a new rat model of pancreatic cancer. Zhonghua Shiyian Waike Zazhi. 2000;17:462–463. [Google Scholar]
- 22.Kirk CJ, Hartigan-O'Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, Mule JJ. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61:2062–2070. [PubMed] [Google Scholar]
- 23.Zhang JK, Chen HB, Sun JL, Zhou YQ. Effect of dendritic cells on LPAK cells induced at different times in killing hepatoma cells. Shijie Huaren Xiaohua Zazhi. 1999;7:673–675. [Google Scholar]
- 24.Li MS, Yuan AL, Zhang WD, Chen XQ, Tian XH, Piao YJ. Immune response induced by dendritic cells induce apoptosis and inhibit proliferation of tumor cells. Shijie Huaren Xiaohua Zazhi. 2000;8:56–58. [Google Scholar]
- 25.Luo ZB, Luo YH, Lu R, Jin HY, Zhang BP, Xu CP. Immunohistochemical study on dendritic cells in gastric mucosa of patients with gastric cancer and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:400–402. [Google Scholar]
- 26.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 27.Nukaya I, Yasumoto M, Iwasaki T, Ideno M, Sette A, Celis E, Takesako K, Kato I. Identification of HLA-A24 epitope peptides of carcinoembryonic antigen which induce tumor-reactive cytotoxic T lymphocyte. Int J Cancer. 1999;80:92–97. doi: 10.1002/(sici)1097-0215(19990105)80:1<92::aid-ijc18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Peiper M, Goedegebuure PS, Izbicki JR, Eberlein TJ. Pancreatic cancer associated ascites-derived CTL recognize a nine-amino-acid peptide GP2 derived from HER2/neu. Anticancer Res. 1999;19:2471–2475. [PubMed] [Google Scholar]
- 29.Gjertsen MK, Bjorheim J, Saeterdal I, Myklebust J, Gaudernack G. Cytotoxic CD4+ and CD8+ T lymphocytes, generated by mutant p21-ras (12Val) peptide vaccination of a patient, recognize 12Val-dependent nested epitopes present within the vaccine peptide and kill autologous tumour cells carrying this mutation. Int J Cancer. 1997;72:784–790. doi: 10.1002/(sici)1097-0215(19970904)72:5<784::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Thurner B, Haendle I, Röder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnurr M, Galambos P, Scholz C, Then F, Dauer M, Endres S, Eigler A. Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines. Cancer Res. 2001;61:6445–6450. [PubMed] [Google Scholar]
- 32.Schnurr M, Galambos P, Scholz C, Then F, Dauer M, Endres S, Eigler A. Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines. Cancer Res. 2001;61:6445–6450. [PubMed] [Google Scholar]
- 33.Fields RC, Shimizu K, Mulé JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, Taidi B, Rajapaksa R, Caspar CB, Okada CY, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 35.Jiao X, Lo-Man R, Guermonprez P, Fiette L, Dériaud E, Burgaud S, Gicquel B, Winter N, Leclerc C. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J Immunol. 2002;168:1294–1301. doi: 10.4049/jimmunol.168.3.1294. [DOI] [PubMed] [Google Scholar]
- 36.Ludewig B, Ochsenbein AF, Odermatt B, Paulin D, Hengartner H, Zinkernagel RM. Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J Exp Med. 2000;191:795–804. doi: 10.1084/jem.191.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Höltl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol. 1999;161:777–782. [PubMed] [Google Scholar]
- 38.Li J, Holmes LM, Franek KJ, Burgin KE, Wagner TE, Wei Y. Purified hybrid cells from dendritic cell and tumor cell fusions are superior activators of antitumor immunity. Cancer Immunol Immunother. 2001;50:456–462. doi: 10.1007/s002620100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanigawa K, Takeshita N, Eickhoff GA, Shimizu K, Chang AE. Antitumor reactivity of lymph node cells primed in vivo with dendritic cell-based vaccines. J Immunother. 2001;24:493–501. doi: 10.1097/00002371-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Kammerer R, Stober D, Riedl P, Oehninger C, Schirmbeck R, Reimann J. Noncovalent association with stress protein facilitates cross-priming of CD8+ T cells to tumor cell antigens by dendritic cells. J Immunol. 2002;168:108–117. doi: 10.4049/jimmunol.168.1.108. [DOI] [PubMed] [Google Scholar]
- 41.Orentas RJ, Schauer D, Bin Q, Johnson BD. Electrofusion of a weakly immunogenic neuroblastoma with dendritic cells produces a tumor vaccine. Cell Immunol. 2001;213:4–13. doi: 10.1006/cimm.2001.1864. [DOI] [PubMed] [Google Scholar]
- 42.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459–473. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 43.Foley HD, Otero M, Orenstein JM, Pomerantz RJ, Schnell MJ. Rhabdovirus-based vectors with human immunodeficiency virus type 1 (HIV-1) envelopes display HIV-1-like tropism and target human dendritic cells. J Virol. 2002;76:19–31. doi: 10.1128/JVI.76.1.19-31.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, Klyushnenkova E, Oppenheim JJ, Kwak LW. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 45.Hara I, Nagai H, Miyake H, Yamanaka K, Hara S, Micallef MJ, Kurimoto M, Gohji K, Arakawa S, Ichihashi M, et al. Effectiveness of cancer vaccine therapy using cells transduced with the interleukin-12 gene combined with systemic interleukin-18 administration. Cancer Gene Ther. 2000;7:83–90. doi: 10.1038/sj.cgt.7700083. [DOI] [PubMed] [Google Scholar]
- 46.Heuer JG, Tucker-McClung C, Hock RA. Neuroblastoma cells expressing mature IL-18, but not proIL-18, induce a strong and immediate antitumor immune response. J Immunother. 1999;22:324–335. doi: 10.1097/00002371-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Osaki T, Péron JM, Cai Q, Okamura H, Robbins PD, Kurimoto M, Lotze MT, Tahara H. IFN-gamma-inducing factor/IL-18 administration mediates IFN-gamma- and IL-12-independent antitumor effects. J Immunol. 1998;160:1742–1749. [PubMed] [Google Scholar]
- 48.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohyama M, Saijyo K, Hayasida M, Yasugi T, Kurimoto M, Ohno T. Direct activation of human CD8+ cytotoxic T lymphocytes by interleukin-18. Jpn J Cancer Res. 1998;89:1041–1046. doi: 10.1111/j.1349-7006.1998.tb00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirao M, Onai N, Hiroishi K, Watkins SC, Matsushima K, Robbins PD, Lotze MT, Tahara H. CC chemokine receptor-7 on dendritic cells is induced after interaction with apoptotic tumor cells: critical role in migration from the tumor site to draining lymph nodes. Cancer Res. 2000;60:2209–2217. [PubMed] [Google Scholar]
- 51.Tanaka F, Hashimoto W, Okamura H, Robbins PD, Lotze MT, Tahara H. Rapid generation of potent and tumor-specific cytotoxic T lymphocytes by interleukin 18 using dendritic cells and natural killer cells. Cancer Res. 2000;60:4838–4844. [PubMed] [Google Scholar]


