Abstract
AIM: To study the mechanism and treatment of severe biliary complications arising from hepatic artery embolization (HAE).
METHODS: Of seven cases of intra- and extrahepatic biliary damage resulting from hepatic artery embolization reported since 1987 , 6 patients suffered from hepatic haemangioma, the other case was due to injection of TH compound into the hepatic artery during operation. The hepatic artery was injected with ethanol so as to evaluate the liver damage in experimental rats.
RESULTS: All the cases were found to have destructive damage of intra- and extrahepatic bile duct at the hilum with biliary hepatocirrhosis. Experimental results revealed necrosis of the liver parenchyma, especially around the portal tract and obliteration of intrahepatic bile duct.
CONCLUSIONS: To prevent the severe biliary complications of HAE, the use of HAE for hepatic haemangioma which was widely practiced in China, should be re-evaluated. Hepatic arterial embolization of hepatic haemangioma may resulte in severe destructive biliary damages and its indiscriminate use should be prohibited.
INTRODUCTION
Hepatic artery embolization (HAE) has been used for the treatment of malignant tumors of the liver. At present, in Chinese literatures, HAE has been widely used for liver cancer therapy[1-6]. Recent reports showed that the method has been advocated for the treatment of liver benign tumors especially in hepatic hemangioma[7-15]. However, the value of HAE as well as the pitfalls of this form of treatment in hepatic hemangioma have not been fully evaluated. Some basic differences of hepatic hemodynamics between hepatic hemangioma and hepatic cell carcinoma[16], may in turn affect the result of treatment. Severe complications after HAE for hepatic hemangioma had rarely been mentioned in the literature, therefore, such kind of non-operative treatment may be taken as an “innocuous” procedure and it has been used indiscriminately. Little attention to the biliary complications of HAE has been paid and the treatment of the biliary complication is a very knotty problem[17]. We have treated 7 consecutive cases of severe destructive damages of the bile duct resulting from HAE from February 1987 to September 1999. In addition, damage of bile duct after HAE has been testified by a series of animal experiments. This report reviews our experience in the treatment of severe biliary complications of HAE and the results of animal experiment.
MATERIALS AND METHODS
Animal experiment
Male and female Wistar rats (220 g-280 g) purchased from the Laboratory Animal Unit of the General Hospital of PLA, Beijing. All animals were reared on a standard laboratory diet, and tap water. They were kept in a room where the temperature (20 °C ± 2 °C), humidity (65%-70%), and day : night cycle (12:12 light:dark) were controlled.
Hepatic artery embolization
Ethanol (100%) was selected as the embolizing agent for the study. Hepatic artery embolization was performed under inhalant anesthesia. Branches of the abdominal aorta, and the branches from coeliac artery to spleen, stomach and duodenum were temporarily ligated. Ethanol (100%) with small amount of methylene blue was injected into the abdominal aorta with syringe, 0.2 milliliter ethanol for each rat. After the injection, the ligated arteries were loosened.
The animals lost appetite and 3/20 had obstructive jaundice after the operation. The rats were randomly divided into two groups, ten rats for each group.
Ten rats were killed 3 days (group A) after the embolization, the others were killed after 7 days (group B). Blood samples of Group A, Group B and control (abdominal operation but without ethanol injection) were collected for liver function test including glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), biliruin, alkalin phosphatase (ALP) and total bile acid (TBA). At the same time, the liver was removed and fixed in 10% formalin solution and embedded in paraffin. The specimens were sectioned and stained with hematoxylin-eosin (H&E).
Statistical analysis
The results were expressed as mean ± S.E. ( -x ± S-x).
RESULTS
Liver function changes
Changes of liver function differed among rats with or without jaundice after the embolization. GPT, GOT, ALP and TBA were significantly increased after HAE on the 3rd day and 7th day when compared with the control group, these changes seemed to be recovered on the 7th day (Table 1).
Table 1.
Liver function changes (-x ± S-x)
| GPT(U/L) | GOT(U/L) | TB(μmol/L) | DB(μmol/L) | ALP(U/L) | TBA(μmol/L) | |
| Control | 30 ± 8 | 63 ± 6 | 10 ± 9 | 5 ± 5 | 146 ± 115 | 6 ± 2 |
| Group A | 245 ± 191 | 443 ± 382 | 129 ± 213 | 61 ± 99 | 104 ± 11 | 161 ± 249 |
| Group B | 55 ± 67 | 233 ± 266 | 5 ± 2 | 3 ± 1 | 243 ± 174 | 48 ± 36 |
GPT = glutamic pyruvic transaminase, GOT = glutamic oxaloacetic transaminase, TB = total biliruin, DB = direct bilirubin, ALP = alkalin phosphatase, TBA = total bile acid.
Pathological changes
Small yellowish necrosis patches can be seen by naked eyes in some lobes of the liver of groups A and B.There were small local necrotic areas in the liver parenchyma of groups A and B, the control group showed no liver necrosis. Most of the necrosis was located near the portal triad, the necrotic areas showed eosinophilic staining. The damaged areas presented coagulation necrosis of hepatocytes, where the hepatocytes showed uniformly eosinphilic, the liver cell plate was still visible but the hepatic cell nuclei disappeared. There was a clear borderline around the necrotic areas after the 7th day with infiltration of inflammatory cells. Most of the portal veinules remained normal, but the wall of the surviving artery was thickened and bile duct disappeared from the portal tract. Proliferation of small bile ducts was easily seen outside the necrotic areas (Figure 1). Obliteration of the bile duct with impairment of biliary drainage was responsible for the above findings.
Figure 1.
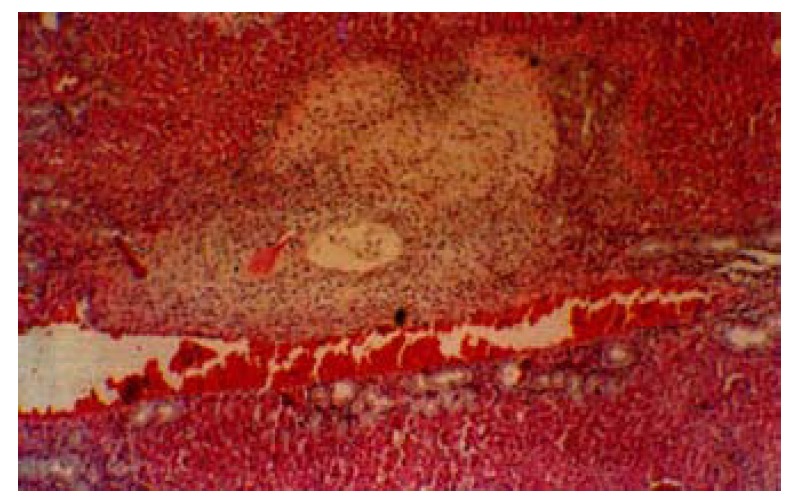
Liver necrosis after HAE in rats. The necrotic area is seen near the portal triad. HE × 100
The above findings showed that the liver damage of HAE could be reproduced in animal experiment. Injecting ethanol through hepatic artery can certainly result in local necrosis of the liver, especially the biliary tract in the portal triads of the liver. Necrosis of portal triads or liver parenchyma will lead to biliary abscess formation and fibrosis of the liver.
CASE REPORTS
Case 1
A 55-year-old male was found to have a 4 cm × 4.5 cm hemangioma in the right lobe of the liver during a routine physial examination in March 1989. He was advised to have his liver thrombosed. HAE with iodized oil 10ml and sodium morrhuate 4ml were injected with Seldinger technique. He felt severe abdominal pain at once after the injection. Pain was not relieved until 5 days after the embolization. Intense vomiting appeared 20 min after the embolization, and persisted for 4 days. Obstructive jaundice appeared after 20 days. Percutaneous transhepatic cholangiogram (PTC) showed changes of the right and left hepatic duct. Occlusion of extrahepatic bile duct was noted in July 1989. Ultrasound showed dilatation of the gallbladder and fluid accumulated around the gallbladder. Gallbladder necrosis with segmental bile duct necrosis were confirmed at operation on July 29, 1989. Cholecystectomy, partial hepatic bile duct excision and choledochocholedochostomy with T-tube stenting were performed. Serum icterus index descended from 90U to 20U with T-tube kept in place for two years. He was admitted to the General Hospital of PLA, Beijing, because of biliary cirrhosis, portal hypertension, enlarged spleen and ascites in 1994. Due to severe hepatocirrhosis, atrophy of the right lobe of the liver and portal hypertension, reconstructive biliary operation was deemed to be unsafe unless the portal pressure has been lowered down. So he was to under go staged operation, the first operation consisted of splenectomy and splenicocaval shunt on June 9, 1994. Hepatocholedocho-jejuostomy was performed 6 months afterwards. The patient remained well without jaundice since the last operation.
Case 2
A 55-year-old female was found to have a large mass (5 cm × 6 cm) in the right lobe of liver in June 1996, but she experienced no remarkable symptoms. Nevertheless, HAE was performed with ethanol (100%) following doctor’s advice. The patient suffered from irregular fever and epigastric pain after the HAE. Ultrasound showed liver abscess formation two months later. She was still febrile and appeared toxic in spite of drainage of bile containing pus by a percutaneous catheter. Laparotomy and liver abscess drainage were performed three months later. But jaundice reappeared 4 months after the operation. Computed tomography scan showed left hepatic duct dilatation, and infective necrotic lesion of unhomogenous density in the right lobe of the liver. Fistulography through the right liver drainage tube showed abnormal communication between the drainage tract and the extrahepatic bile duct, as well as a duodenal fistula. Hepatectomy, hepatocholedocho-jejunostomy and U-tube stenting were performed 4 months later in April 1997. Jaundice disappeared after the operation. The stenting tube was maintained for one and half years. She recovered after withdrawal of the tube.
Case 3
A 62-year-old woman was found to have an asymptomatic hemangioma (10 cm × 9 cm) in the right liver by ultrasound in December 1994. HAE was advised and performed with iodized oil and sodium morrhuate. Persisted epigastric pain followed the procedure. Ultrasound and CT showed cystic lesions (5.6 cm × 6.1 cm) in the left lobe of the liver 2 months later (Figure 2). The patient had had repeated attacks of high fever with chills, and antibiotics administration was not effective. She was admitted with the diagnosis of biliary multi-abscesses of the liver. A transcutancous catheter was placed with drainage of about 180-200 mL bile each day. The last operation was performed in June 1996. A large amount of bile stained necrotic tissue along the portal tract on both sides of the liver was removed. During the operation, the normal intrahepatic ducts were found destroyed. The right and left liver parenchyma was atrophied while the caudate lobe became hypertrophied. A fibrous stricture band was present around the common hepatic duct. The stricture band was removed and U-tube stents were placed during the operation. Jaundice disappeared 2 years after the operation.
Figure 2.
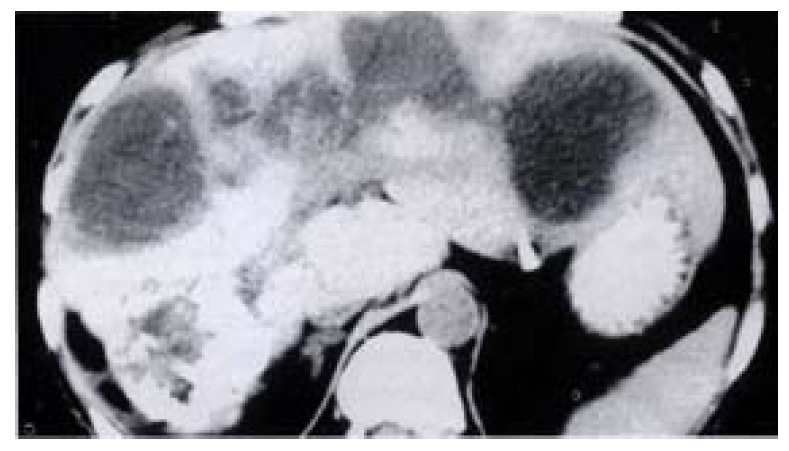
Biliary abscess of liver after HAE. CT shows multi-abscess along portal tract.
Case 4
A 24-year-old female was diagnosed having a space occupying lesion in the right liver. She was operated upon in 1987. Multiple nodular lesions were found in her right liver, which were supposed to be metastatic nodules. A nodule was taken for pathological sections, and the hepatic artery was ligated and methacrylate (TH glue) was injected through distal end of the hepatic artery during operation. The postoperative course was very stormy. She developed continued abdominal pain with high fever and jaundice after the operation. The abdominal X-ray showed that the branches of the left, and right hepatic artery and gastroduodenal artery were embolized. However, the tissues from the right lobe of the liver was inflammotory in nature pathologically. Seven months later, she was admitted to the General Hospital of PLA, and PTC showed that stricture of hilar bile duct and the left hepatic duct with diffuse fibrosis in the perihilar region and necrosis of the right liver and the gallbadder. GI examination revealed an internal fistula between the first portion of duodenum and hepatic hilum. The operation undertaken was very difficult. However, the intestinal fistula was repaired, anastomosis of the dilated segment III bile duct and a long Roux-en-Y jejunal loop was created with a U-tube stent. The tube was removed 11/2 years later. The patient recovered from the operation. She delivered a child two years later, but eventually, the patient died of hepatocellular carcinoma 5 years after the operation.
Case 5
A 60-year-old male was found to have a hepatic hemangioma (5 cm × 5 cm) in 1994. CT examination in 1998 showed an increase in the size of the tumor. HAE was advised and performed with iodized oil, steel wire ring and pingyangmycinum in July 1998. Persistent epigastric pain occurred for 3 days after the procedure, followed by jaundice and fever with gray colored stool 20 days later. This condition was aggravated 4 months later. The patient when seen was suffering from continual high fever and deep jaundice and was admitted to the hospital in January 1999. Diagnoses of hepatic abscesses and gallbadder necrosis after the embolization were made. ERCP showed extensive hepatic bile duct stricture (Figure 3), which was thought to be not amenable to surgery. The patient was treated conservatively.
Figure 3.
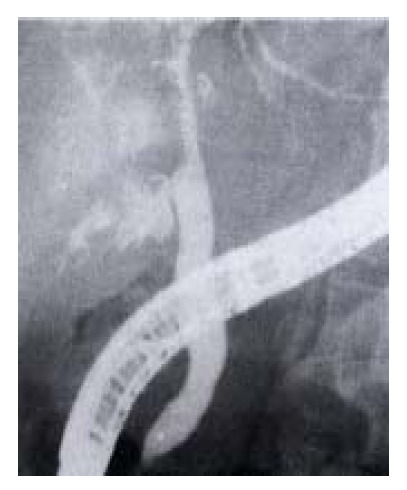
Intrahepatic bile duct stricture after HAE. ERCP shows biliary stricture.
Case 6
A 43-year-old female was found to have a liver hemangioma in October 1998. HAE was performed which was complicated by severe abdominal pain and repeated vomiting for a week, and jaundice occurred 3 months later. Antibiotic therapy was effective. The patient was admitted with the diagnosis of obstructive jaundice in September 1999. MRI showed gallbladder necrosis perforation and with fluid collection around it (Figure 4). Intrahepatic bile ducts were dilated. A hemangioma(3 cm × 3.5 cm) in the right lobe of the liver was still seen. The gallbladder was found to be necrotic, and the abscess cavity communicated with the common bile duct as seen at operation. Cholecystectomy, and T tube stenting were performed. Jaundice disappeared after the operation.
Figure 4.
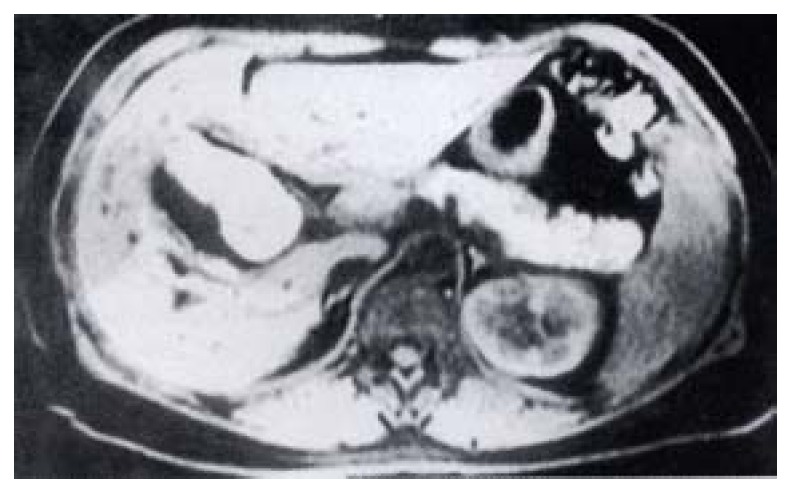
MRI showed fluid around the gallbladder.
Case 7
A 43-year-old man was found to have a hemangioma of the right liver during physical examination in June 1993. HAE was advised and was performed using iodized oil and sodium morrhuate. Serious epigastric pain occurred immediately after the embolization. Jaundice appeared in July 1995. He was then operated upon, three coagulated blood coagula were taken out during choledochostomy. The patient was reoperated in June 1996. Choledochoenterostomy and drainage of the III segmental duct were performed in January 1998. An external bile fistula was formed and biliary drainage of 300 mL was given each day but jaundice did not subside. The patient was admitted for operation in September 1999, marked biliary cirrhosis and atrophy of right lobe were found at operation. Cholangioenterostomy of the dilated III segmental duct and T tube stenting were performed. Jaundice subsided very slowly after the operation.
DISCUSSION
Blood supply of the hepatic duct and mechanism of bile duct injury in HAE
Branches of intrahepatic bile duct, artery and portal vein come together in the same Glisson sheath in the portal tract. Arterioles from the hepatic artery form a dense capillary network around the bile duct, which is the so-called peribiliary plexus. Therefore, only a small portion of hepatic arterial blood directly enters the sinusoids. The blood supply of the bile duct and structure of portal tract comes completely from hepatic artery. Hence, the intrahepatic bile duct receives unique nutrient blood supply from the hepatic artery in contrast to the double blood supply of hepatic cell[18-25]. Therefore, damages of the biliary system are more severe than the liver cell in hepatic arterial embolization. Clinically, continuous hepatic artery infusion of FUDR is expected to cause development of permanent stricture of the biliary system[26]. The complication was thought to be the result of regional drug toxicity and biliary vascular embolism. The end result is sclerosing cholangitis and diffuse fibrosis as well as scarring of biliary tree. Stapleton et al[27] in the study of the blood supply of the right and left hepatic ducts found that the peribiliary plexus of the caudate lobe has bilateral artery blood supply. This may be the reason for atrophy of right and left lobes but accompanied with hypertrophy of the caudate lobe after HAE injury which was consistently found in the cases in this report.
Hemangioma occurs more frequently in the right liver lobe and stricture of hilar hepatic duct was found in the HAE of right lobe lesions as shown in this report. This is explained by the finding that hilar bile duct blood supply chiefly derived from right hepatic and cystic arteries[28-30].
Sodium morrhuate is commonly used as a vascular sclerosing agent[31-33], but it is a strong irritant, it can cause local tissue necrosis and inflammation as well as compete occlusion of large blood vessels in the injecting area. It was used for sclerosing therapy of varicosity vein and it is scarcely used for HAE. Four cases in this report used sodium morrhuate as the sclerosent, resulting in liver necrosis and abscess formation. We are of the opinion that the use of sclerosing drug as an embolizing agent in HAE is very dangerous.
Ethanol caused protein coagulation and damage of vascular endothelium which causes thrombosis and obstruction of blood vessel[34-36]. In this report, one case received ethanol as the embolizing agent. Animal experiment demonstrated that ethanol causes intrahepatic biliary obliteration and acute liver focal necrosis in the rat model.
Surgical treatment
Cases in this report have the following characteristics of: ① 6/7 cases were hepatic hemangiomas and strong destructive embolizing agents were employed; ②clinically, all patients presented persistent abdominal pain following the procedure; ③ all resulted in extensive hepatic necrosis and damage of the biliary tree, hepatic biliary abscesses developed after the embolization; ④ damage of the intra-and extra-hepatic biliary system was destructive, it was difficult to rehabilitate the patients and a prolonged hospitalization is needed.
Treatment of the complications after HAE: Liver parenchyma necrosis was distributed along the portal tract after the HAE. Because of the disruptive effect of sclerosing agent on the biliary tract and the focal necrosis liver cells, some of the liver cells near the foci of necrosis are still secreting bile, the end result is biliary abscess forming in the necrosed area. The bile duct in its entire course may be completely destroyed. The damaged hepatic lobe will eventually be atrophied. Necrosis and fibrous stricture often consequently involve the hepatic duct bifurcation as well as the left hepatic duct, which results in obstructive jaundice in the end. In late cases, when complicated with biliary cirrhosis and portal hypertension, restorative biliary surgery is very difficult. Under such conditions, it is our experience that the treatment needs to be divided into several steps. The first step is the drainage of bile collection and removal of necrotic tissue to control the infection. First step of treatment is to improve patients¡ä general status as well as the local condition by maintaining biliary drainage. Treatment of the second step is hepatotomy and necrotic tissue elimination. If biliary stricture is of perihilar type, operation to relieve hilar bile duct stricture and Roux-en-Y hepatocholedocho-jejunostomy and place U tubes for stenting are neccesary[37-41]. If patients are complicated with biliary cirrhosis and portal hypertension, preliminary operation of portal pressure decompression, for example spleno-renal shunt is often needed before the difficult biliary restoration operation is attempted[42]. If bile ducts were badly damaged, bilateral biliary drainage with U tubes is a better alternative.
To prevent severe biliary complications of HAE, the use of HAE for hepatic hemangioma should be re-evaluated and the indiscriminate use of sclerosing agents in HAE should be prohibited.
Footnotes
Edited by Ma JY
References
- 1.Jia YC, Tian JM, Wang ZT, Chen D, Ye H, Liu Q, Yang JJ, Sun F, Lin L, Lu JP, et al. A retrospective review on interventional treatment of 10000 cases of liver cancer. Huaren Xiaohua Zazhi. 1998;6:2–3. [Google Scholar]
- 2.Ji XL, Liu YX, Wang YH, Zhao H. Histopathological study of hepatocellular carcinoma after transcatheter hepatic arterial embolization. China Natl J New Gastroenterol. 1996;2:79–81. [Google Scholar]
- 3.Zheng CS, Feng GS, Zhou RM, Liang B, Liang HM, Zhen J, Yu JM, Liu H. Hepatic arterial infusion chemotherapy and embolization in the treatment of primary hepatic carcinoma. China Natl J New Gastroenterol. 1997;3:104–107. doi: 10.3748/wjg.v3.i2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan J, Ten GJ, He SC, Guo JH, Yang DP, Wang GY. Arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 1998;4:33–37. doi: 10.3748/wjg.v4.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng XM, Luo PF, Shao PJ, Zhou ZJ, Ma Z. Analysis of the Cause of Death after Chemoembolization for Liver Cancer. Jieru Yixue Zazhi. 1997;2:11–13. [Google Scholar]
- 6.Huang FG, Li Y, Xie XD. Side effects and complcations of hepatic arterial infusion and embolization of liver carcinoma in aged patients and its management. World J Gastroenterol. 1998;4:67–68. [Google Scholar]
- 7.Li GW, Zhao ZR, Li BS, Liu XG, Wang ZL, Liu QF. Source of blood supply of liver cavernous hemangioma and sclerosis and embolization treatment. China Natl J New Gastroenterol. 1997;3:147–149. doi: 10.3748/wjg.v3.i3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie ZG, Wang ZD. Blood supply of hepatic cavernous haemangioma and interventional treatment. Xinxiaohuabingxue Zazhi. 1996;4:46–48. [Google Scholar]
- 9.Li YH. [An experimental study of sodium morrhuate as an agent for arterial embolization] Zhonghua Fangshexue Zazhi. 1987;21:357–60, 66. [PubMed] [Google Scholar]
- 10.Jiang XX. Hepatic artery embolization in treatment of huge cavern-ous hemangioma. Zhonghua Fangshe Zazhi. 1992;26:88–90. [Google Scholar]
- 11.Yan XF, He JG, Song HZ. [Interventional therapy of hepatic cavernous hemangioma] Zhonghua Waike Zazhi. 1994;32:563–564. [PubMed] [Google Scholar]
- 12.Panis Y, Fagniez PL, Cherqui D, Roche A, Schaal JC, Jaeck D. Successful arterial embolisation of giant liver haemangioma. Report of a case with five-year computed tomography follow-up. HPB Surg. 1993;7:141–146. doi: 10.1155/1993/76519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li GW, Zhao ZR, Li BS, Liu XG, Wang ZL, Liu QF. Embolization therapy and its mechanism of cavernous hemangioma of liver. Shanxi Yixue Zaizhi. 1993;22:515–517. [Google Scholar]
- 14.Li IQ, Li JL, Wu S, Liang ST, Liao QH. Fifteen cases lipidol emboliza-tion treatment of hepatic cavernous haemangioma. Zhonghua Shiyanwaike Zazhi. 1994;14:1–3. [Google Scholar]
- 15.Li GW, Liu XG, Li BS, Wang ZL, Le XB, Wang Y, Liu QF, Gao H. Study of sclerosing treatment of liver haemangioma. Zhonghua Shiyanwaike Zazhi. 1992;9:1–3. [Google Scholar]
- 16.Zhou Runsuo, Qiao Hongqing, Deng Jinglan, Huo JP, Ma XR. Analysis of radioimage of hepatic artery perfusion in patients with hepatic space-occupying lesions. Zhongguo Zhongliu Linchuang. 1995;22:381–383. [Google Scholar]
- 17.Huang XQ, Huang ZQ, Duan WD, Zhou NX, Feng YQ. Destructive damage of bile duct of hepatic artery embolization in treatment of hepatic cavernous haemangioma. Junyijingxiu xueyan Xuebao. 2000;21:88–91. [Google Scholar]
- 18.Jing JG, Wang CL, Han MJ, Yang MW. The experimental study of local blood flow and pathologic changes after embolization of portal vein branch with two kinds of embolic materials. Zhongguo Yikedaxue Xuebao. 1995;24:602–604. [Google Scholar]
- 19.Wang X, Zhong YX, Zhang LL, Huang YX, Wen QS, Chu YK, Zhang HX, Wang QL. Effect of IL-8 and ET-1 on secondary liver injury by hepatic arterial embolization in rabbits. Shijie Huaren Xiaohua Zazhi. 2000;8:413–416. [Google Scholar]
- 20.Motta PM. The three-dimensional microanatomy of the liver. Arch Histol Jpn. 1984;47:1–30. doi: 10.1679/aohc.47.1. [DOI] [PubMed] [Google Scholar]
- 21.Huang XQ. Changes of liver microcirculation in cirrhosis and biliary obstruction. Guowaiyixue Waikexue Fence. 1986;6:321–323. [Google Scholar]
- 22.Huang XQ, Yang KZ, Huang ZQ, Ying GQ, Wang BZ. An empriment study of liver microcirculation after ligation bile duct. Zhonghua Shiyanwaike Zazhi. 1987;4:151–154. [Google Scholar]
- 23.Compagno J, Grisham JW. Scanning electron microscopy of extrahepatic biliary obstruction. Arch Pathol. 1974;97:348–351. [PubMed] [Google Scholar]
- 24.Bosch J, Enriquez R, Groszmann RJ, Storer EH. Chronic bile duct ligation in the dog: hemodynamic characterization of a portal hypertensive model. Hepatology. 1983;3:1002–1007. doi: 10.1002/hep.1840030618. [DOI] [PubMed] [Google Scholar]
- 25.Jones AL, Schmucker DL. Current concepts of liver structure as related to function. Gastroenterology. 1977;73:833–851. [PubMed] [Google Scholar]
- 26.Kemeny MM, Battifora H, Blayney DW, Cecchi G, Goldberg DA, Leong LA, Margolin KA, Terz JJ. Sclerosing cholangitis after continuous hepatic artery infusion of FUDR. Ann Surg. 1985;202:176–181. doi: 10.1097/00000658-198508000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stapleton GN, Hickman R, Terblanche J. Blood supply of the right and left hepatic ducts. Br J Surg. 1998;85:202–207. doi: 10.1046/j.1365-2168.1998.00511.x. [DOI] [PubMed] [Google Scholar]
- 28.Zheng JF, Sun FL, Yu XF, Dou YC. Transhepatic arterial chemoembolization using lipiodol and ischemic injury to the gallbladder. Linchuang Waike Zazhi. 1998;6:270–271. [Google Scholar]
- 29.Wang X, Huang YX, Wen QS, Cu YK, Li DY, Zhang HX, Zhang JZ, Wang YD. Experimental study on gastric mucosal injury by hepatic arterial embolization in rabbits. Huaren Xiaohua Zazhi. 1998;6:997–999. [Google Scholar]
- 30.Chen WJ, Ying DJ, Liu ZJ, He ZP. Analysis of the arterial supply of the extrahepatic bile ducts and its clinical significance. Clin Anat. 1999;12:245–249. doi: 10.1002/(SICI)1098-2353(1999)12:4<245::AID-CA2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 31.Zhang TL, Cheng HH, Hou KY, Yan NS. Necrosis of the gastric wall after alpha-cyanoacylate embolization of gastric coronary vein. Beijing Yikedaxue Xuebao. 1996;28:452–453. [Google Scholar]
- 32.Jing YC, Yang ZD, Ding HY, Ma FC. Pathological changes of peri-vascular tissues after gastric and hepatic vascular embolization with medical TH tissue adhesive in rabbits. Linchuang Yushiyan Binglixue Zazhi. 1997;13:51–52. [Google Scholar]
- 33.Lu MD, Chen JW, Xie XY, Liang LJ, Huang JF. Portal vein embolization by fine needle ethanol injection: experimental and clinical studies. World J Gastroenterol. 1999;5:506–510. doi: 10.3748/wjg.v5.i6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu MD, YY W. A study of portal vein embolization with absolute ethanol injection in cirrhotic rats. World J Gastroenterol. 1998;4:415–417. doi: 10.3748/wjg.v4.i5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu MD, Liang LJ, Peng BG, Ren W. Study of portal vein embolization with ethanol injection in cirrhoticrats. Zhonghua Shiyian Waike Zazhi. 1998;15:75–76. [Google Scholar]
- 36.Lu MD, Chen JW, Xie XY, Liang LJ, Huang JF. Portal vein embolization by fine needle ethanol injection: experimental and clinical studies. World J Gastroenterol. 1999;5:506–510. doi: 10.3748/wjg.v5.i6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu YX, Huang ZQ, Zhou YB, Chai ZJ, Qiang GX, Chi YB, Han BL, He ZP, Zhang QZ, Tu JM. Surgical treatment of injury bile duct strictures. Puwai Linchuang. 1986:234–237. [Google Scholar]
- 38.Zhi QH. New development of biliary surgery in China. World J Gastroenterol. 2000;6:187–192. doi: 10.3748/wjg.v6.i2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Liu YU, Huang ZQ, Feng YQ, Zhou NX, Gu WQ, Duan YP, Huang XQ, Zhang WZ. Surgical treatment of 42 cases of traumatic strictures of bile duct. Gandanyipi Waike Zazhi. 1995;1:81–84. [Google Scholar]
- 40.Huang ZQ. Surgical treatment of biliary obstruction in poste-rior segment of the right lobe. Zhongha Waike Zazhi. 1988;26:593–597. [Google Scholar]
- 41.Huang XQ, He ZP, Zhou YB, Zhong JC, Guo ZY, Feng YQ. Repair biliary stricture used mucous segments with blood supply. Zhonghua Waike Zazhi. 1986;24:523–526. [Google Scholar]
- 42.Huang ZQ, Zai JX, Han BL, Qian GX. Surgical treatment of bile duct strictures with portal hypertension. Zhongha Waike Zazhi. 1979;17:351–356. [Google Scholar]


