Abstract
AIM: To investigate the role of cytokine gene expression in organ damage at different tissue sites during sepsis.
METHODS: Male NIH mice were subjected to cecal ligation and puncture (CLP) or sham operation (Sham). Pro-inflammatory cytokine (TNFα, IL-1β and IL-6) and anti-inflammatory cytokine (IL-4) gene expression in the liver and lung tissue were assessed by RT-PCR. The permeability of microvascular and water content in the lungs and liver were also examined.
RESULTS: Significant increase in TNFα, IL-1β and IL-6 gene expression was observed at 3 and 12 h after CLP both in the liver and lungs (P < 0.01).The level of IL-4 gene expression was not changed after CLP in the lungs, but increased at 12 h after CLP (P < 0.01) in the liver tissue. Both the liver and lungs showed a significant increase in microcirculatory permeability at 12 h after CLP(P < 0.01), and the increase in the lungs was higher than that in the liver. The water mass fractions in the liver (P < 0.05) and lungs (P < 0.01) were increased after CLP, and the increase in the lungs happened earlier and more severely than that in the liver.
CONCLUSION: The inflammatory response in the liver and lungs was different during sepsis. At the early stage of sepsis, pro-inflammatory reaction dominates both in the liver and lungs. But at the later stage of sepsis, induction of compensatory anti-inflammatory response was seen in the liver but not in the lungs. This difference in situ activity may contribute to the different vulnerability of organ damage during sepsis. The strategy of systemic administration of anti-inflammatory drugs to sepsis should be reconsidered.
INTRODUCTION
Despite modern techniques of resuscitation and intensive care and an ever-increasing number of powerful and effective antibiotics, sepsis remainsa major cause of death in the critically ill[1], including severe trauma, burns, hemorrhage, major operations[2], etc. Sepsis-related mortality frequently results from multiple organ dysfunction syndrome, which is characterized by impaired pulmonary function, hepatic failure, cardiac dysfunction, acute renal failure, and disseminated intravascular coagulation[3,4]. The physiologic derangements of MODS are believed to result from the excessive activation of the systemic inflammatory response by infection, ischemia, injury, or immunologic activation[5,6].
Post-sepsis MODS is now believed to be caused by an overexpression of host defenses[7]. Mediators such as TNFα, IL-β and other cytokines are acknowledged to be pivotal early effectors. There is evidence that the development of tissue damage in sepsis is closely related to the release of an ever-increasing number of cytokines and accumulation of neutrophils at the sites of infection or injury[8,9]. Though there were many studies on the role of cytokines in sepsis. Most studies detect only circulating mediators, not mediators bound to cells or receptors[10,11]. Analyses of serum levels are troublesome to interpret. They may underestimate the effective amount of mediator acting at a cellular level. Bioassays, which measure the functional activity of cytokines, often lack specificity and may over-report amounts.
Polymicrobial sepsis induced by cecal ligation and puncture (CLP) is a model of sepsis which reproduces many of the inflammatory and pathological sequelae that are observed clinically[12]. Following CLP, animals develop bacteremia, hypothermia, hypotension, and damage to multiple organ systems[13]. The present study was designed to observe the gene expression of cytokines in hepatic and pulmonary tissues with CLP model, in order to investigate the role of cytokines in organ damage during sepsis.
MATERIALS AND METHODS
Animal model and experimental groups
NIH mice were obtained from the animal center of the general hospital of Chinese PLA. The mice were randomly divided into two groups: CLP group and sham group. Sepsis was induced by cecal ligation and puncture (CLP). The mice were anesthetized, and the cecum was ligated below the ileocecal junction; and intestinal continuity was maintained. The cecum was punctured twice with a 20-gauge needle and a small amount of cecal contents was expressed through the punctures. The incision was closed and 1ml of normal saline was administered subcutaneously. Sham-operated mice underwent the same surgical procedure, but without CLP. The mice were sacrificed at 3 or 12 h after the procedure.
Preparation of RNA
Total RNA was extracted from liver and lung tissues according to the guanidinium isothiocyanate single-step methods. The RNA concentration of each sample was estimated by measuring the absorbance at 260 nm.
Reverse transcription and PCR
Total RNA from experimental samples was used to synthesize cDNA using AMV reverse transcriptase. β-actin and β2-MG were used as internal control primers. The primers for the cytokines and control were as follows: β-actin (478 bp): 5’AGGGAAATCGTGCGTGACATCAAA3’, 5’ACTCATCGTACTCCTGCTTGCTGA3’; β2-MG (300 bp): 5’GGCTCGCTCGGTGACCCTAGTCTTT3’, 5’TCTGCAGGCGTATGTATCAGTCTCA3’; TNF-α (349 bp): 5’TTCTGTCCCTTTCACTCACTGG3’, 5’TTGGTGGTTTGCTACGACGTGG3’; IL-1β (441 bp): 5’ATTAGACAGCTGCACTACAGGCTC3’, 5’AGATTCCATGGTGAAGTCAATTAT3’; IL-6 (156 bp): 5’TGGAGTCACAGAAGGAGTGGCTAAG3’, 5’TCTGACCACAGTGAGGAATGTCCAC3’; IL-4 (181 bp): 5’CGAAGAACACCACAGAGAGTGAGCT3’, 5’GACTCATTCATGGTGCAGCTTATCG3’. PCR was performed in a 25 μL reaction volume. A hot start was applied for 5 min at 95 °C. The amplification cycle (denaturation step at 94 °C for 30 s, an annealing step at 55 °C for 30 s and an extension step at 72 °C for 90 s ) was repeated 30 times and followed by a final extension for 10 min at 72 °C. Amplified products were separated by electrophoresis in ethidium bromide-stained 15 g·L-1 agarose gel and visualized with UV illumination. The bands representing reaction product on the film were scanned by densitometry. A normalization quotient (Q) was calculated between the integrated optical density values (IOD) for the adhesion molecules and the β-actin or β2-MG bands (Q = IOD adhesion molecules band/internal control band). The level of adhesion molecules mRNA were expressed as the quotient of the integrated values for the adhesion molecules and the β- actin or β2-MG bands.
Microvascular permeability in the liver and lungs
Evans blue ( 15 g·L-1) was injected intravenously in concentrations of 10 μL·g-1 0.1 mL/10 g. The liver and lung tissues were excised and weighed. To each sample of tissues, 4.0 mL formamide was added and incubated at 56 °C for 72 h. If necessary, the incubation time was prolonged, until the blue color of the samples completely disappeared. After filtration with glass filter, the absorbance of the filtrate was measured at 620 nm in a Beckman spectrophotometer. The total amount of dye can be calculated by means of a standard calibration curve. The permeability of microvascular in the liver and lungs was shown as the μg of Evans blue in every mg tissues.
Water mass fractions in the liver and lungs
Water mass fractions were used as a parameter of organ water accumulation after CLP. The liver and lung tissues were removed in a humidity chamber and the wet mass was measured immediately. The tissues were dried at 70 °C to a constant mass for the determination of dry mass. Organ edema was determined by calculating tissue water content according to the following formula: Water mass fractions (%) = (1-dry mass/wet mass) × 100%.
Statistics
All data were reported as -x ± s. Data were analyzed by t test for comparisons between two groups.P < 0.05 was accepted as the level of significance.
RESULTS
Cytokine mRNA expression in the liver and lungs
Significant increase in TNFα, IL-1β and IL-6 gene expression was observed at 3 and 12 h after CLP both in the liver and lungs. But the level of IL-4 gene expression was not changed after CLP in the lungs, while it was increased at 12 h after CLP (Figures 1 and 2) in the liver tissue.
Figure 1.
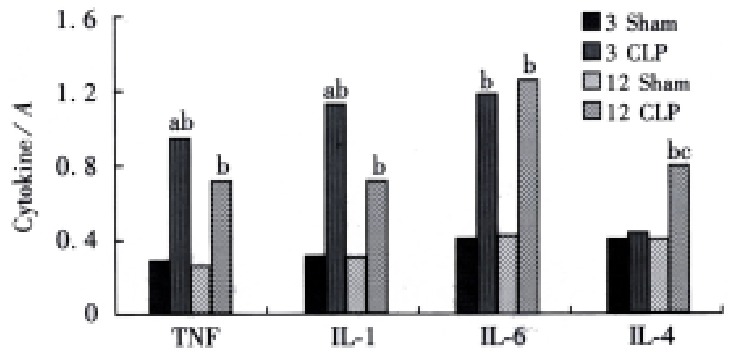
The cytokines gene expression in the river aP < 0.01, vs CLP 12 h group, bP < 0.01, vs sham group, cP < 0.01, vs CLP 3 h group
Figure 2.
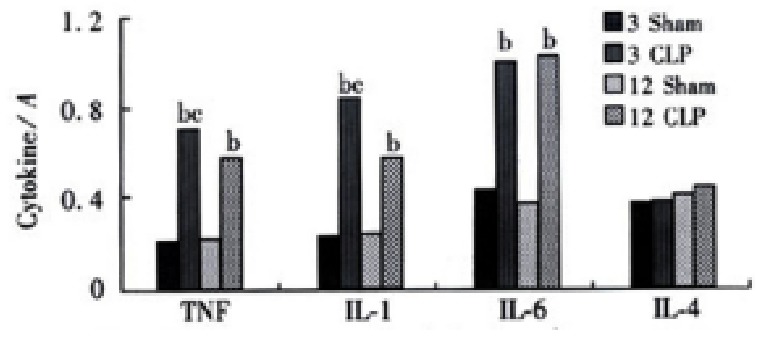
The cytokines gene expression in the lungs bP < 0.01, vs sham group, cP < 0.05, vs CLP 12 h group
Microvascular permeability in the liver and lungs
Both the liver and lungs showed a significant increase in microcirculatory permeability at 12 h after CLP, and the increase in the lungs was higher than that in the liver (Figure 3).
Figure 3.
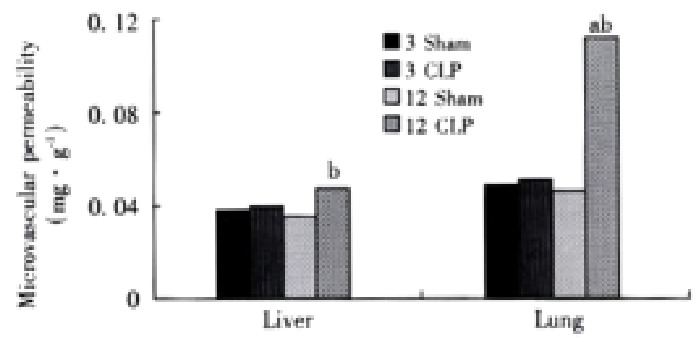
Microvascular permeability in the liver and lungs aP < 0.01, vs CLP 3h group, bP < 0.01, vs sham group.
Water mass fractions in the liver and lungs
The water mass fractions in the liver and lungs were increased after CLP, and the increase in the lungs happened earlier and more severely than that in the liver (Figure 4).
Figure 4.
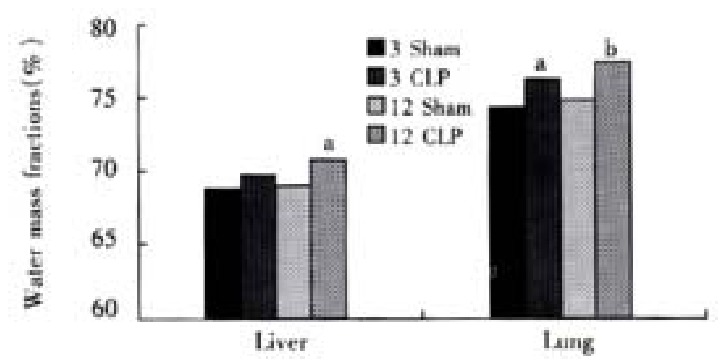
Water mass fractions in the liver and lungs aP < 0.05, vs sham group, bP < 0.01, vs sham group
DISCUSSION
SIRS affects all aspects of systemic homeostasis. It is arbitrary, but convenient, to consider its effects from the perspective of the dysfunction of certain key organ systems. It must be recognized, however, that there is no defining combination of systems or temporal sequence of physiologic deterioration that characterizes the syndrome[14]. A systematic review of 30 descriptions of MODS published between 1969 and 1993 revealed that respiratory system is the most commonly cited organ system[15,16]. The liver with its rich supply of blood and sinusoid is directly exposed to bacteria and endotoxins drained from the GI tract[17-19]. Previous works have proclaimed that liver is the most susceptible and vulnerable organ during sepsis and multiple organ failure[20]. So in this study, we chose the liver and lungs as the samples to study the role of local cytokine gene expression in organ damage.
Cytokines are polypeptides or glycoproteins of low molecular weight. Most cytokines are not stored as preformed molecules, hence their production requires new gene transcription and translation. Unlike mediators derived from the classical endocrine system, cytokines are produced. The production of cytokines at various tissue sites depends, in part, on the proximity of the site to the injurious stimulus. A lot of cells such as macrophage[21] and endothelial cells[22] can produce cytokines. Cytokines act predominantly as paracrine and autocrine messengers, not endocrine mediators. Thus, cytokines may exert their major effects locally, within organs and tissues. Assays of circulating cytokine concentrations may be misleading because they do not detect cytokines bound to soluble receptors[23]. These assays may also fail to detect cytokines when inhibitors or receptor antagonists are present[24]. Therefore investigations in this area require more precise determination of the temporal sequencing and tissue-specific expression of cytokines.
In this study we assessed the gene expression of pro-inflammatory and anti-inflammatory cytokines in the hepatic and pulmonary tissues. Pro-inflammatory cytokines such as TNFα, and IL-1β are known to play predominant roles in the normal inflammatory response. But exaggerated endogenous production is likely responsible for the complications associated with sepsis as tissue injury and ultimate organ failure[25]. These pro-inflammatory cytokines result in the production of secondary mediators (including nitric oxide, arachidonic acid metabolites, bradykinin, and histamine) which in turn activate neutrophils and endothelial cells to perpetuate tissue injury[26,27]. But attempts to modulate the biological activity of cytokines with monoclonal antibodies against endotoxin, TNFα, IL-1 have not been successful in improving outcome in several clinical series. Recently, evidence is accumulating that in response to the original inciting event (the inflammatory response), the body also mounts an anti-inflammatory response. Agents that have been identified so far as participating in this anti-inflammatory response include interleukins-4, -10, -11 and -13; transforming growth factor-α; colony-stimulating factors; soluble receptors to tumor necrosis factor and receptor antagonists to IL-1[28]. Because most of these mediators have been discovered recently, we know very little about their actions, and even less about their effects on the pro-inflammatory cascade. However, it had already been shown that some of these mediators, especially some of these interleukins, have a profound effect on monocyte function, including antigen presenting activity[29]. They also inhibit T- and B-lymphocyte activity, including antigen-specific T-lymphocyte proliferation. The result is immune suppression, which can sometimes be profound. Of great interest is the fact that these mediators can down-regulate their own synthesis, thereby providing a mechanism through which homeostasis can be restored. Results in this study showed that the conditions of inflammatory reaction in the liver and lungs were different during sepsis. At the early stage of sepsis, the power of pro-inflammatory reaction was stronger than that of anti-inflammatory reaction both in the liver and lungs. But at the later stage of sepsis, compensatory anti-inflammatory response was found in the liver but not in the lungs.
The consensus of the concept of systemic inflammatory responses has brought about a promising approach in the treatment of SIRS/MODS[30] - anti-inflammatory instead of anti-infection[31,32]. Various approaches aimed to interrupt the cascade of host inflammatory responses have been tested[33]. These include interventions targeted at the inflammation effector cells as monoclonal antibodies or receptor antagonist to pro-inflammatory cytokines[34,35]. However, many of these seemingly effective measures in experimental study failed when moved from the laboratory bench to clinical ward. As results showed in this study that the conditions of inflammatory reaction in the liver and lungs were different during sepsis, it is postulated that in the strategy of anti-inflammatory interventions such as monoclonal antibodies against endotoxin and TNFα, in addition to timing, organ targeting may also be considered.
Footnotes
Supported by the National Natural Science Foundation of China, No. 39870796
Edited by Ma JY
References
- 1.Memmini G, Buggiani B, Ciulli L, Cavallini MR, Turini M, Pierini A, Bianchi F, Moggi C. Study on 480 hospitalized febrile children: evaluation of the septic risk and results of the antibiotic and corticosteroid combined therapy. Pediatr Med Chir. 1999;21:119–123. [PubMed] [Google Scholar]
- 2.D'Amico D, Cillo U. Impact of severe infections on the outcome of major liver surgery: a pathophysiologic and clinical analysis. J Chemother. 1999;11:513–517. doi: 10.1179/joc.1999.11.6.513. [DOI] [PubMed] [Google Scholar]
- 3.Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches (Review) Int J Mol Med. 1999;4:575–583. doi: 10.3892/ijmm.4.6.575. [DOI] [PubMed] [Google Scholar]
- 4.Crouser ED, Julian MW, Weinstein DM, Fahy RJ, Bauer JA. Endotoxin-induced ileal mucosal injury and nitric oxide dysregulation are temporally dissociated. Am J Respir Crit Care Med. 2000;161:1705–1712. doi: 10.1164/ajrccm.161.5.9907043. [DOI] [PubMed] [Google Scholar]
- 5.Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26 Suppl 1:S64–S74. doi: 10.1007/s001340051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin RY, Zou SQ, Wu ZD, Qiu FZ. Influence of splanchnic vascular infusion on the content of endotoxins in plasma and the translocation of intestinal bacteria in rats with acute hemorrhage necrosis pancreatitis. World J Gastroenterol. 2000;6:577–580. doi: 10.3748/wjg.v6.i4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent JL. Update on sepsis: pathophysiology and treatment. Acta Clin Belg. 2000;55:79–87. doi: 10.1080/17843286.2000.11754275. [DOI] [PubMed] [Google Scholar]
- 8.Terregino CA, Lopez BL, Karras DJ, Killian AJ, Arnold GK. Endogenous mediators in emergency department patients with presumed sepsis: are levels associated with progression to severe sepsis and death. Ann Emerg Med. 2000;35:26–34. doi: 10.1016/s0196-0644(00)70101-6. [DOI] [PubMed] [Google Scholar]
- 9.Todoroki H, Nakamura S, Higure A, Okamoto K, Takeda S, Nagata N, Itoh H, Ohsato K. Neutrophils express tissue factor in a monkey model of sepsis. Surgery. 2000;127:209–216. doi: 10.1067/msy.2000.103027. [DOI] [PubMed] [Google Scholar]
- 10.Presneill JJ, Waring PM, Layton JE, Maher DW, Cebon J, Harley NS, Wilson JW, Cade JF. Plasma granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor levels in critical illness including sepsis and septic shock: relation to disease severity, multiple organ dysfunction, and mortality. Crit Care Med. 2000;28:2344–2354. doi: 10.1097/00003246-200007000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Paterson RL, Webster NR. Sepsis and the systemic inflammatory response syndrome. J R Coll Surg Edinb. 2000;45:178–182. [PubMed] [Google Scholar]
- 12.Salkowski CA, Detore G, Franks A, Falk MC, Vogel SN. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66:3569–3578. doi: 10.1128/iai.66.8.3569-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Kunkel SL. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response. J Immunol. 2000;164:5362–5368. doi: 10.4049/jimmunol.164.10.5362. [DOI] [PubMed] [Google Scholar]
- 14.Zhang WZ, Han TQ, Tang YQ, Zhang SD. Rapid detection of sepsis complicating acute necrotizing pancreatitis using polymerase chain reaction. World J Gastroenterol. 2001;7:289–292. doi: 10.3748/wjg.v7.i2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somogyi-Zalud E, Zhong Z, Lynn J, Dawson NV, Hamel MB, Desbiens NA. Dying with acute respiratory failure or multiple organ system failure with sepsis. J Am Geriatr Soc. 2000;48:S140–S145. doi: 10.1111/j.1532-5415.2000.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 16.Medoff BD, Harris RS, Kesselman H, Venegas J, Amato MB, Hess D. Use of recruitment maneuvers and high-positive end-expiratory pressure in a patient with acute respiratory distress syndrome. Crit Care Med. 2000;28:1210–1216. doi: 10.1097/00003246-200004000-00051. [DOI] [PubMed] [Google Scholar]
- 17.Fu WL, Xiao GX, Yue XL, Hua C, Lei MP. Tracing method study of bacterial translocation in vivo. World J Gastroenterol. 2000;6:153–155. doi: 10.3748/wjg.v6.i1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo DJ, Chaudry IH, Wang P. Mechanism of hepatocellular dysfunction during sepsis: the role of gut-derived norepinephrine (review) Int J Mol Med. 2000;5:457–465. doi: 10.3892/ijmm.5.5.457. [DOI] [PubMed] [Google Scholar]
- 19.Gong JP, Han BL. Effect of CD14 in LPS mediating inativation of Kupffer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:875–877. [Google Scholar]
- 20.Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J Gastroenterol. 2001;7:128–130. doi: 10.3748/wjg.v7.i1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun. 1999;67:6603–6610. doi: 10.1128/iai.67.12.6603-6610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marie C, Muret J, Fitting C, Payen D, Cavaillon JM. Interleukin-1 receptor antagonist production during infectious and noninfectious systemic inflammatory response syndrome. Crit Care Med. 2000;28:2277–2282. doi: 10.1097/00003246-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Zhang GQ, Yu H, Zhou XQ, Liao D, Xie Q, Wang B. TNF-α induced apoptosis and necrosis of mice hepatocytes. Shijie Huaren Xiaohua Zazhi. 2000;8:303–306. [Google Scholar]
- 24.Bulger EM, Maier RV. Lipid mediators in the pathophysiology of critical illness. Crit Care Med. 2000;28:N27–N36. doi: 10.1097/00003246-200004001-00004. [DOI] [PubMed] [Google Scholar]
- 25.Yu PW, Xiao GX, Fu WL, Yuan JC, Zhou LX, Qin XJ. Pathogenetic effects of platelet activating factor on enterogenic endotoxemia after burn. World J Gastroenterol. 2000;6:451–453. doi: 10.3748/wjg.v6.i3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi Y, Katayose D, Shindoh C. Interleukin-13 prevents diaphragm muscle deterioration in a septic animal model. Tohoku J Exp Med. 1999;189:191–202. doi: 10.1620/tjem.189.191. [DOI] [PubMed] [Google Scholar]
- 27.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Strieter RM, Kunkel SL. Expression and contribution of endogenous IL-13 in an experimental model of sepsis. J Immunol. 2000;164:2738–2744. doi: 10.4049/jimmunol.164.5.2738. [DOI] [PubMed] [Google Scholar]
- 28.Bernard GR. Research in sepsis and acute respiratory distress syndrome: are we changing course. Crit Care Med. 1999;27:434–436. doi: 10.1097/00003246-199902000-00057. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa K, Tanaka H, Nakamori Y, Hosotsubo H, Ogura H, Nishino M, Shimazu T, Sugimoto H. Difference in the responses after administration of granulocyte colony-stimulating factor in septic patients with relative neutropenia. J Trauma. 2000;48:814–824; discussion 824-825. doi: 10.1097/00005373-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Faist E, Kim C. Therapeutic immunomodulatory approaches for the control of systemic inflammatory response syndrome and the prevention of sepsis. New Horiz. 1998;6:S97–102. [PubMed] [Google Scholar]
- 31.Suputtamongkol Y, Intaranongpai S, Smith MD, Angus B, Chaowagul W, Permpikul C, Simpson JA, Leelarasamee A, Curtis L, White NJ. A double-blind placebo-controlled study of an infusion of lexipafant (Platelet-activating factor receptor antagonist) in patients with severe sepsis. Antimicrob Agents Chemother. 2000;44:693–696. doi: 10.1128/aac.44.3.693-696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham E. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med. 1999;25:556–566. doi: 10.1007/s001340050903. [DOI] [PubMed] [Google Scholar]
- 33.Deitch EA, Goodman ER. Prevention of multiple organ failure. Surg Clin North Am. 1999;79:1471–1488. doi: 10.1016/s0039-6109(05)70088-8. [DOI] [PubMed] [Google Scholar]
- 34.Lazaron V, Barke RA. Gram-negative bacterial sepsis and the sepsis syndrome. Urol Clin North Am. 1999;26:687–699. doi: 10.1016/s0094-0143(05)70211-1. [DOI] [PubMed] [Google Scholar]
- 35.Nasraway SA. Sepsis research: we must change course. Crit Care Med. 1999;27:427–430. doi: 10.1097/00003246-199902000-00054. [DOI] [PubMed] [Google Scholar]


