Abstract
AIM: To investigate the effects of growth inhibition of human gastric cancer SGC-7901 cell with RRR-α-tocopheryl succinate (VES), a derivative of natural Vitamin E, via inducing apoptosis and DNA synthesis arrest.
METHODS: Human gastric cancer SGC-7901 cells were regularly incubated in the presence of VES at 5, 10 and 20 mg·L-1 (VES was dissolved in absolute ethanol and diluted in RPMI 1640 complete condition media correspondingly to a final concentration of VES and 1 mL·L-1 ethanol), succinic acid and ethanol equivalents as vehicle (VEH) control and condition media only as untreated (UT) control. Trypan blue dye exclusion analysis and MTT assay were applied to detect the cell proliferation. 37 kBq of tritiated thymidine was added to cells and [3H] TdR uptake was measured to observe DNA synthesis. Apoptotic morphology was observed by electron microscopy and DAPI staining. Flow cytometry and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay were performed to detect VES-triggered apoptosis.
RESULTS: VES inhibited SGC-7901 cell growth in a dose-dependent manner. The growth curve showed suppression by 24.7%, 49.2% and 68.7% following 24 h of VES treatment at 5, 10 and 20 mg·L-1, respectively, similar to the findings from MTT assay. DNA synthesis was evidently reduced by 35%, 45% and 98% after 24 h VES treatment at 20 mg·L-1 and 48 h at 10 and 20 mg·L-1, respectively. VES induced SGC-7901 cells to undergo apoptosis with typically apoptotic characteristics, including morphological changes of chromatin condensation, chromatin crescent formation/margination, nucleus fragmentation and apoptotic body formation, typical apoptotic sub-G1 peak by flow cytometry and increase of apoptotic cells by TUNEL assay in which 90% of cells underwent apoptosis after 48h of VES treatment at 20 mg·L-1.
CONCLUSION: VES can inhibit human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. Inhibition of SGC-7901 cell growth by VES is dose-and time-dependent. Therefore VES can function as a potent chemotherapeutic agent against human gastric carcinogenesis.
INTRODUCTION
Vitamin E is charactered as a fat-soluble membrane antioxidant[1,2]. Vitamin E succinate (RRR-α -tocopheryl succinate; VES), a derivative of natural vitamin E, however, does not possess antioxidant properties unless the succinate group is removed by a nonspecific esterase. VES has been demonstrated to be a potent growth inhibitor of various cancer cell types in vitro and in vivo[3,4]. For example, VES has been shown to inhibit the growth of human monoblastic leukemia cells in vitro[5], murine B-16 melanoma cells in vitro[6], hamster buccal pouch tumor cells in vivo[7], avian lymphoid cells in vitro[8,9], murine EL4 T lymphoma cells in vitro[4,10], human gastric cancer cells in vitro and in vivo[11-13], and human breast cancer cells in vitro and in vivo[14,15].
The exact mechanisms are not clearly known, but the inhibitory effect of VES on the proliferation of rapidly dividing cells can be attributed to the induction of cell cycle blockage[16], increased secretion and activation of transforming growth factor-βs (TGF-βs) and TGF-β receptor II[9,14,17], and the induction of apoptosis[18-20]. In many instances, growth inhibition following terminal differentiation[21-23]or anticancer drug treatment[24-26] results in apoptosis. Apoptosis, namely, programmed cell death, is an active and physiological process characterized by a series of morphological and biological alterations including condensation of cytoplasm, loss of plasma membrane microvilli, segmentation of nucleus and extensive degradation of chromosomal DNA into oligomers of 180bp[27-30]. The exact mechanisms of apoptosis are still unclear, but our earlier studies indicated that VES can secrete and activate biologically active TGF-β and then TGF-β increases the kinase activity of c-Jun N-terminal kinase(JNK) followed by phosphorylation of c-Jun, and finally activated c-Jun triggers apoptosis in human gastric cancer SGC-7901 cells[31].
Gastric cancer is common in China[32-41]. Since VES is a potential tumor chemopreventive and chemotherapeutic agent, it is worthwhile to investigate the manner in which VES inhibits the cell growth and thereafter gain a better understanding of the molecular events involved. In this study, we demonstrate the ability of VES to inhibit cell proliferation, arrest DNA synthesis and induce human gastric cancer SGC-7901 cells to undergo apoptosis, address the involvement of certain apoptosis-related events in this process and prove that VES-triggered apoptosis is different from VES-induced DNA synthesis arrest.
MATERIALS AND METHODS
Materials
VES, DAPI (4’,6-diamidine-2’-phenylindole dihydrochloride, succinic acid, MTT [3-(4,5-dimethylthiazole-2-yl)-2,5-dipenyltetrazolium bromide] and propidium iodide were purchased from Sigma Co. Ltd. RPMI 1640 media was obtained from Gibco, BRL. In situ cell death detection kit was purchased from Boehringer Mannheim, Indianapolis, Inc. 3H-TdR was supplied by Chinese Academy of Sciences.
Methods
Cell culture Human gastric cancer cell lines SGC-7901 were maintained in RPMI 1640 medium supplemented with 100 mL·L-1 fetal calf serum (FCS), 100 kU·L-1 penicillin, 100 mg·L-1 streptomycin and 2 mmol·L-1 L-glutamine under 50 mL·L-1 CO2 in a humidified incubator at 37 °C. SGC-7901 cells were incubated for different time periods in the presence of VES at 5, 10 and 20 mg·L-1 (VES was dissolved in absolute ethanol and diluted in RPMI 1640 complete condition media correspondingly to a final concentration of VES and 1 mL·L-1 ethanol), succinic acid and ethanol equivalents as vehicle (VEH) control and condition media only as untreated (UT) control.
Growth curve Exponentially growing SGC-7901 cells were trypsinized and aliquoted into 24-well flat-bottomed tissue culture plates at 5 × 10 4·well-1. The cells were allowed to attach overnight and then incubated for seven days in the presence or absence of VES. Cell number and viability were determined by trypan blue dye exclusion analysis.
MTT assay The cells were inoculated in a 96-well plate (5 × 103·well-1) as described previously[42]. In brief, cells after 24 h of incubation were treated with VES and controls and then cultured for six days. The cells in eight wells at every dose were supplemented with 0.2 mL of 5 g·L-1 MTT every day. After 4 h, culture media were discarded followed by addition of 0.2 mL of DMSO and vibration for 10 min. The absorbance (A) was measured at 570 nm using a microplate reader. The percentage of viable cells was calculated as follow:(A of experimental group /A of control group) × 100%. [3H] Thymidine incorporation. The cells were treated with VES for 24 or 48 h as described previously[43]. 37 kBq of tritiated thymidine were added to cells during the last 6 h of culture and harvested onto glass fiber filters. [3H] TdR uptake was measured in a Beckman LS5000 TD liquid scintillation counter.
Electron microscopy The cells treated with VES or VEH were trypsinized and harvested after 24 h and 48 h , respectively. Subsequently the cells were immersed with Epon 821, imbedded in capsules and converged for 72 h at 60 °C, the cells were prepared into ultrathin section (60 nm) and stained with uranyl acetate and lead citrate . Cell morphology was examined by transmission electron microscopy.
DAPI staining Treated cells were pelleted and washed three times with distilled water, and then stained with 2 mg·L-1 DAPI in 100% methanol for 15min at 37 °C. Cells were viewed using a fluorescence microscope with ultraviolet (UV) excitation at 300-500 nm. Cells with nuclei that contained clear condensed chromatin or cells with fragmented nuclei were scored as apoptosis.
TUNEL assay Apoptosis of SGC-7901 cells was analyzed by using in situ cell death detection kit. The method is essentially based on the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) technique, and it can detect apoptosis at very early stages. In brief, cells were treated in the presence or absence of VES and fixed overnight in 100 g·L-1 formaldehyde, treated with proteinase K and then H2O2, labeled with fluorescein dUTP in a humid box for 1h at 37 °C. The cells were then combined with POD-Horseradish peroxidase, colorized with DAB (3,3-diaminobenzidine) and counterstained with methyl green. Cells were visualized with light microscope.
Flow cytometry Cells were incubated with various doses of VES, harvested, washed with phosphate-buffered saline (PBS) twice and fixed with 700 mL·L- 1 ethanol at 4 °C overnight. Fixed cells were washed twice with PBS and stained with 800 μL propidium iodide and 200 μL deoxyribonulcease-free ribonuclease A in PBS. The fluorescence indensity of propidium iodide-stained nuclei was determined by a FACscan.
Statistical analysis Student’s t test was used to assess statistical significance of differences. If P < 0.05, the difference was considered significant.
RESULTS
Inhibition of Human Gastric Cancer SGC-7901 Cell Proliferation by VES
Growth curve VES has been previously shown to function as a growth inhibitory agent. In this study, VES inhibited SGC-7901 cell growth in a dose-dependent manner. The growth curve showed that cell growth was suppressed by 24.7%, 49.2% and 68.7% following 24 h of VES treatment at 5, 10 and 20 mg·L-1, respectively. The percentage of inhibition was 100% on day 4 with the treatment of VES at 20 mg·L-1 (Figure 1).
Figure 1.
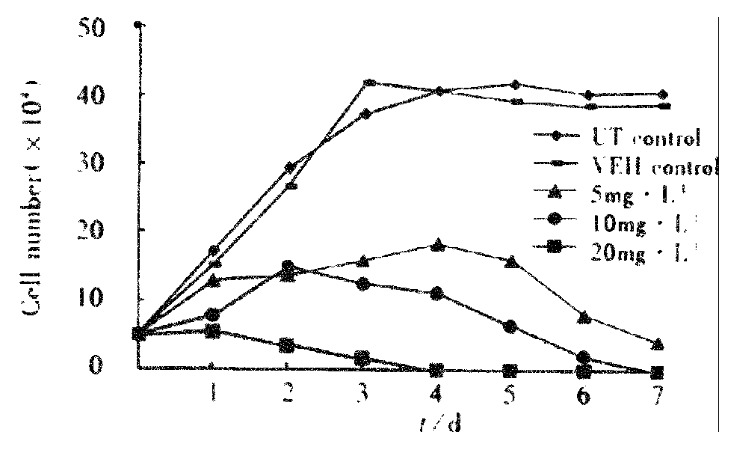
Growth curve of SGC-7901 cell treated with VES.
MTT assay SGC-7901 cells were treated in the presence or absence of VES for six days and cell viability was daily measured by MTT proliferation analysis. VES significantly inhibited cell growth and VES at 10 and 20 mg·L-1 had an obviously inhibitory effect by 100% on the 5th and 4th days, respectively (Table 1), similar to the findings from growth curve.
Table 1.
Effect of VES on the viability of SGC-7901 cells
| Group |
Rate of viable cells/% |
|||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| UT control | 100 | 100 | 100 | 100 | 100 | 100 |
| VEH control | 105.5 | 102.4 | 92.1 | 95.3 | 105.7 | 97.5 |
| 5 mg·L-1 VES | 94.8 | 101.2 | 91.4 | 144.3 | 91.4 | 74.6 |
| 10 mg·L-1 VES | 91.4 | 64.6 | 20.8 | 4.2 | 0 | 0 |
| 20 mg·L-1 VES | 53.4 | 32.9 | 4.3 | 0 | 0 | 0 |
DNA synthesis arrest by VES
VES treatment of SGC-7901 cells inhibited [3H] thymidine uptake with a dose-response relationship. DNA synthesis was evidently reduced by 35%, 45% and 98% after 24 h VES treatment at 20 mg·L-1 and 48 h at 10 and 20 mg·L-1 , respectively, compared with UT control (P < 0.01). On the other hand, VEH control did not affect SGC-7901 cell proliferation (Table 2).
Table 2.
Inhibitory effect of VES on DNA synthesis incorporated with 3H-TdR (-x ± s, n = 6)
| Group |
Radioactive intensity/Bq·104cell-1) |
|
| 24 h(% of inhibition ratio) | 48 h(% of inhibition ratio) | |
| UT control | 64.1 ± 10.2(0) | 143.2 ± 13.7(0) |
| VEH control | 63.8 ± 10.3(1) | 141.9 ± 14.7(0) |
| 5 mg·L-1 VES | 61.8 ± 10.4(4) | 137.1 ± 16.3(4) |
| 10 mg·L-1 VES | 57.2 ± 10.7(11) | 79.3 ± 10.0(45)b |
| 20 mg·L-1 VES | 41.4 ± 8.0(35)b | 2.2 ± 0.3(98)b |
P < 0.01, vs UT control.
VES induction of apoptosis
Morphological changes Apoptotic characteristics including chromatin condensation, chromatin crescent formation/ margination, DNA fragmentation and apoptotic body formation were seen by electron microscopy (Figure 2) and by fluorescence microscopy of DAPI-labelled cells (Figure 3). The cells undergoing apoptosis evidently increased when the concentration of VES was elevated and reaction time was prolonged.
Figure 2.
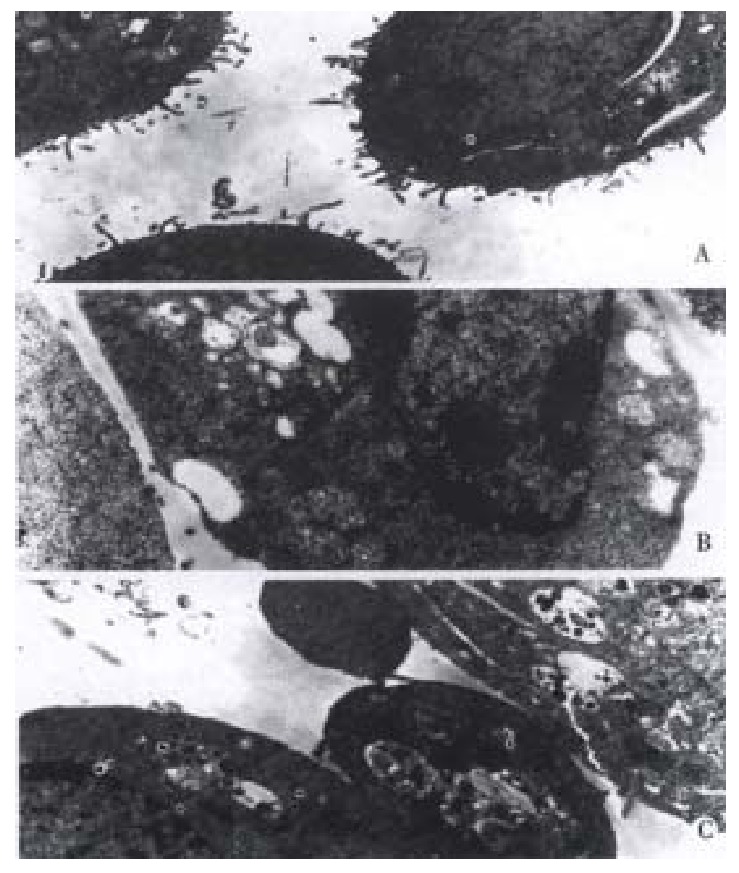
VES-induced apoptosis in SGC-7901 cells with transmission elec-tron microscope. A: Normal cell; B: Apoptotic cell with chromatin condensation, chromatin crescent formation/margination; C: Cell with apoptotic body.
Figure 3.
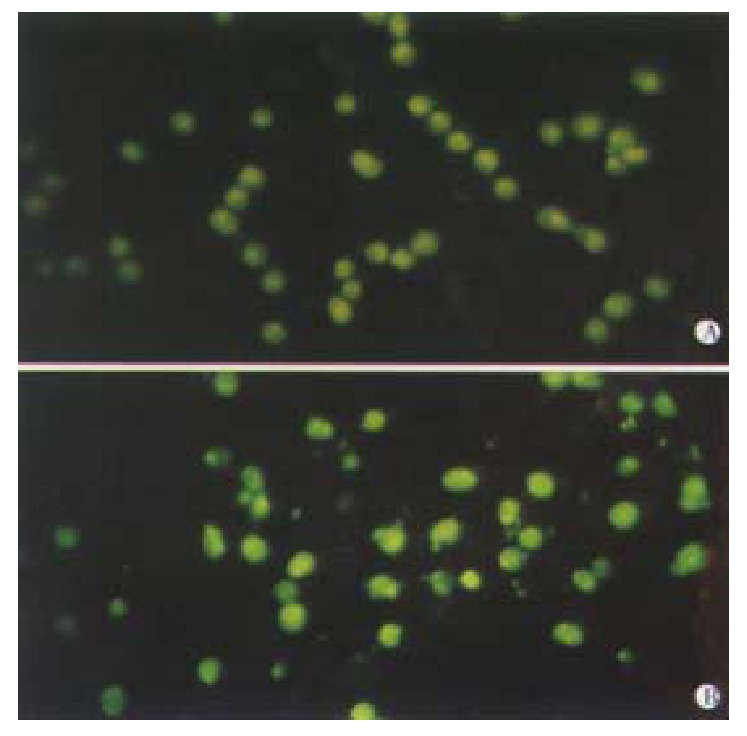
VES-induced apoptosis in SGC-7901 cells with DAPI staining UT control; VES at 20 mg·L-1.
Flow cytometry VES-induced apoptosis was studied further in SGC-7901 cells using flow ctyometry. Cell cycle analysis after VES treatment revealed the presence of a sub-G1 apoptotic peak (Figure 4).
Figure 4.
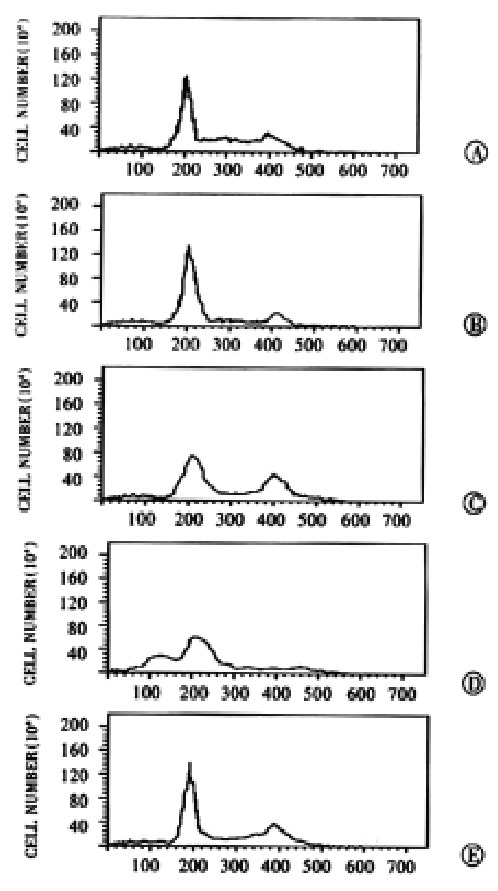
VES-induced apoptosis in SGC-7901 cells with flow cytometry. A: UT control; B: VEH control; C: VES at 5 mg·L-1; D: VES at 10 mg·L-1; E: VES at 20 mg·L-1.
TUNEL assay Apoptotic cell death was determined by TUNEL assay according to the manufacturer’s instructions. The results showed that apoptotic cells increased with the increase of the concentration of VES in an obviously dose-dependent manner. The percentage of apoptosis was 90% after VES treatment at 20 mg·L-1 (Figure 5 and Table 3).
Figure 5.
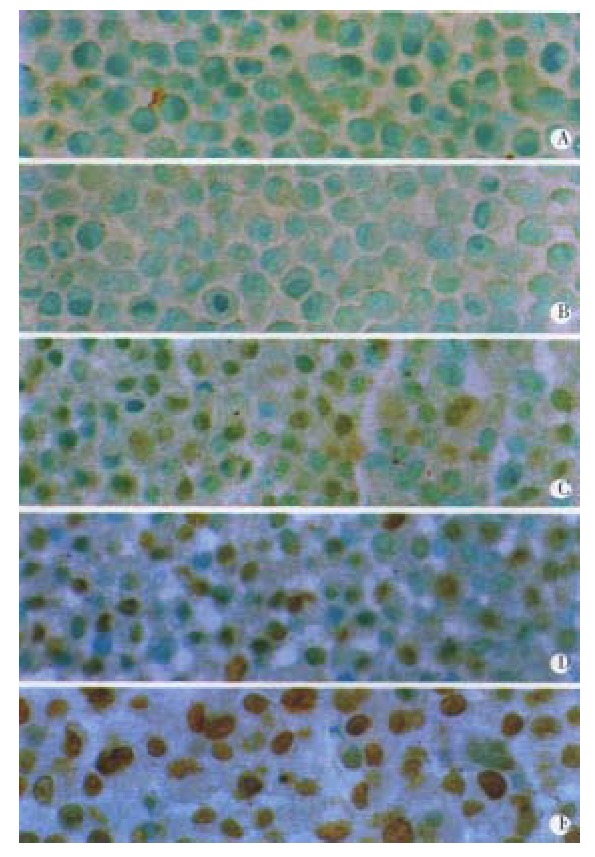
VES-induced apoptosis by TUNEL assay. A: UT control; B: VEH control; C: VES at 5 mg·L-1; D: VES at 10 mg·L-1; E: VES at 20 mg·L-1.
Table 3.
Induction of apoptosis after 48 h of VES treatment in SGC-7901 cells
| Group | Apoptotic cells/104 cells | Rate of apoptosis/% |
| UT control | 6 | 0.06 |
| VEH control | 28 | 0.28 |
| 5 mg·L-1 VES | 1248b | 12.48 |
| 10 mg·L-1 VES | 5736b | 57.63 |
| 20 mg·L-1 VES | 8996b | 89.96 |
P < 0.01, vs UT control.
DISCUSSION
Cell growth is regulated by the interaction of cell proliferation and cell death. When cell growth is inhibited, the decrease of cell proliferation and increase of cell death will occur. Cell proliferation is performed by continuously proceeding into cell cycle and will be suppressed if cell cycle is disturbed[44-46]. The disorder of DNA synthesis, however, is a common reason for the disturbance of cell cycles[47,48]. Cell death is another principal reason for cell growth inhibition and is relevant to cell proliferation. Apoptotic cell death is a naturally occurring process of cell suicide which plays a crucial role in the development and homeostasis of metazons by eliminating superfluous or unwanted cells (reviewed by[49-53]).
Previous studies showed that VES can block DNA synthesis in tumor cells[43,54]. The percent of DNA synthesis arrest is more than 90% when human breast cancer cells are treated with VES at 10 mg·L-1, similar to the rate of breast cancer cell growth inhibition which exceeds 80%[15]. A recent study shows that VES can function as an apoptosis inducer in tumor cells. In this study, VES was found to inhibit cell proliferation in human gastric cancer SGC-7901 cells by trypan blue exclusion analysis and MTT assay. Cell growth was inhibited by 100% with treatment of VES at 10 mg·L-1 on day 7 and at 20 mg·L-1 on day 4 in the former experiment, while on the 6th and 4th days in the latter one. Meanwhile, VES significantly suppressed cell growth by DNA synthesis arrest via preventing SGC-7901 cells from progressinginto S-phase, ultimately blocking the cell proliferation. In order to further elucidate the mechanisms of inhibitory effects of VES on cell growth, we determined the incidence of VES-mediated apoptosis in SGC-7901 cells by electron microscopy, DAPI staining, flow cytometry and TUNEL assay on the basis of morphological and molecular level. The results demonstrated that VES-treated SGC-7901 cells were characteristic by typical apoptotic alterations, including morphological changes by electron microscopy and DAPI staining, typical apoptotic sub-G1 peak observed by flow cytometry; increase of apoptotic cells with the elevation of the concentration of VES in a clearly dose-dependent manner by TUNEL assay.The data above implicated that VES inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest.
Apoptosis is a complex and programmed process which is regulated by a variety of factors[55-60]. The antiproliferative actions of VES may be due, in part, to the ability of VES to induce autocrine acting biologically active TGF-βs[9,14,17]. In addition, VES-mediated apoptosis is inhibited when c-Jun mutant is transfected into breast cancer cells[61]. JNK, mitogen-activated protein kinase (MAPK) family member, plays an important role in the course of VES-triggered apoptosis[31,61]. Although the exact mechanisms involved in VES-induced apoptosis have not been well known up to now, the ability of a compound to induce 90% apoptosis in a tumor cell population within 48 h is noticeable. It is also noteworthy that VES can selectively inhibit the growth of tumor cells but not normal cells[8,62-64]. The research in our laboratory demonstrated that VES inhibited benzo (a)pyrene (B(a)P)-induced forestomach carcinogenesis in female mice[11], while the inhibition and inhibitory mechanisms of VES in vivo are worth further investigating.
Footnotes
Supported by National Natural Science Foundation of China, No.39870662
Edited by Wang JH and Xu XQ
References
- 1.Wang YF, Li QF, Wang H, Mao Q, Wu CQ. Effects of vitamin E on experimental hepatic fibrosis in rats. Huaren Xiaohua Zazhi. 1998;6:207–209. [Google Scholar]
- 2.Jiang ZS, Gao Y. Biological feature of matrix metalloproteinase and its action in metastasis of liver cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:1403–1404. [Google Scholar]
- 3.Kline K, Yu W, Zhao B, Israel K, Charpentier A, Simmons-Menchaca M, Sanders BG. Vitamin E Succinate: Mechanisms of action as tumor cell growth inhibitor. In: Prasad KN. Santamaria L, Williams RM, eds. Nutrients in Cancer Prevention and Treatment. Totowa, NY: Humana. 1995:39–56. [Google Scholar]
- 4.Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits EL4 thymic lymphoma cell growth by inducing apoptosis and DNA synthesis arrest. Nutr Cancer. 1997;27:92–101. doi: 10.1080/01635589709514508. [DOI] [PubMed] [Google Scholar]
- 5.Fariss MW, Fortuna MB, Everett CK, Smith JD, Trent DF, Djuric Z. The selective antiproliferative effects of alpha-tocopheryl hemisuccinate and cholesteryl hemisuccinate on murine leukemia cells result from the action of the intact compounds. Cancer Res. 1994;54:3346–3351. [PubMed] [Google Scholar]
- 6.Ottino P, Duncan JR. Effect of alpha-tocopherol succinate on free radical and lipid peroxidation levels in BL6 melanoma cells. Free Radic Biol Med. 1997;22:1145–1151. doi: 10.1016/s0891-5849(96)00529-1. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz J, Shklar G. The selective cytotoxic effect of carotenoids and alpha-tocopherol on human cancer cell lines in vitro. J Oral Maxillofac Surg. 1992;50:367–373; discussion 373-374. doi: 10.1016/0278-2391(92)90400-t. [DOI] [PubMed] [Google Scholar]
- 8.Kline K, Yu W, Sanders BG, Vitamin E. Mechanisms of action as tumor cell growth inhibitors. In: Prasad KN, Cole WC, eds. Cancer and Nutrition. Amsterdam: IOS. 1998:37–53. doi: 10.1093/jn/131.1.161S. [DOI] [PubMed] [Google Scholar]
- 9.Simmons-Menchaca M, Qian M, Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits DNA synthesis and enhances the production and secretion of biologically active transforming growth factor-beta by avian retrovirus-transformed lymphoid cells. Nutr Cancer. 1995;24:171–185. doi: 10.1080/01635589509514405. [DOI] [PubMed] [Google Scholar]
- 10.Yu W, Sanders BG, Kline K. Modulation of murine EL-4 thymic lymphoma cell proliferation and cytokine production by vitamin E succinate. Nutr Cancer. 1996;25:137–149. doi: 10.1080/01635589609514436. [DOI] [PubMed] [Google Scholar]
- 11.Wu K, Shan YJ, Zhao Y, Yu JW, Liu BH. Inhibitory effects of RRR-alpha-tocopheryl succinate on benzo(a)pyrene (B(a)P)-induced forestomach carcinogenesis in female mice. World J Gastroenterol. 2001;7:60–65. doi: 10.3748/wjg.v7.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu K, Guo J, Shan YJ, Liu BH. The effects of vitamin E succinate on apoptosis in human gastric cancer. Weisheng Dulixue Zazhi. 1999;13:84–90. [Google Scholar]
- 13.Wu K, Ren Y, Guo J. The effects of vitamin E succinate on the cyclic regulation protein of human gastric cancer cells. Weisheng Dulixue Zazhi. 1998;12:203–207. [Google Scholar]
- 14.Turley JM, Ruscetti FW, Kim SJ, Fu T, Gou FV, Birchenall-Roberts MC. Vitamin E succinate inhibits proliferation of BT-20 human breast cancer cells: increased binding of cyclin A negatively regulates E2F transactivation activity. Cancer Res. 1997;57:2668–2675. [PubMed] [Google Scholar]
- 15.Malafa MP, Neitzel LT. Vitamin E succinate promotes breast cancer tumor dormancy. J Surg Res. 2000;93:163–170. doi: 10.1006/jsre.2000.5948. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Bang OS, Lee YS, Kang SS. Production of inducible nitric oxide is required for monocytic differentiation of U937 cells induced by vitamin E-succinate. J Cell Sci. 1998;111(Pt 4):435–441. doi: 10.1242/jcs.111.4.435. [DOI] [PubMed] [Google Scholar]
- 17.Ariazi EA, Satomi Y, Ellis MJ, Haag JD, Shi W, Sattler CA, Gould MN. Activation of the transforming growth factor beta signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999;59:1917–1928. [PubMed] [Google Scholar]
- 18.Neuzil J, Weber T, Schröder A, Lu M, Ostermann G, Gellert N, Mayne GC, Olejnicka B, Nègre-Salvayre A, Stícha M, et al. Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. FASEB J. 2001;15:403–415. doi: 10.1096/fj.00-0251com. [DOI] [PubMed] [Google Scholar]
- 19.Turley JM, Fu T, Ruscetti FW, Mikovits JA, Bertolette DC, Birchenall-Roberts MC. Vitamin E succinate induces Fas-mediated apoptosis in estrogen receptor-negative human breast cancer cells. Cancer Res. 1997;57:881–890. [PubMed] [Google Scholar]
- 20.Yu W, Israel K, Liao QY, Aldaz CM, Sanders BG, Kline K. Vitamin E succinate (VES) induces Fas sensitivity in human breast cancer cells: role for Mr 43,000 Fas in VES-triggered apoptosis. Cancer Res. 1999;59:953–961. [PubMed] [Google Scholar]
- 21.Foehr ED, Bohuslav J, Chen LF, DeNoronha C, Geleziunas R, Lin X, O'Mahony A, Greene WC. The NF-kappa B-inducing kinase induces PC12 cell differentiation and prevents apoptosis. J Biol Chem. 2000;275:34021–34024. doi: 10.1074/jbc.C000507200. [DOI] [PubMed] [Google Scholar]
- 22.Sementchenko VI, Watson DK. Ets target genes: past, present and future. Oncogene. 2000;19:6533–6548. doi: 10.1038/sj.onc.1204034. [DOI] [PubMed] [Google Scholar]
- 23.Steff AM, Trop S, Maira M, Drouin J, Hugo P. Opposite ability of pre-TCR and alpha beta TCR to induce apoptosis. J Immunol. 2001;166:5044–5050. doi: 10.4049/jimmunol.166.8.5044. [DOI] [PubMed] [Google Scholar]
- 24.Liu HF, Liu WW, Fang DC, Men RP. Relationship between Fas antigen expression and apoptosis in human gastric carcinoma and adjacent noncancerous tissues. Huaren Xiaohua Zazhi. 1998;6:321–322. [Google Scholar]
- 25.Xu HY, Yang YL, Guan XL, Song G, Jiang AM, Shi LJ. Expression of regulating apoptosis gene and apoptosis index in primary liver cancer. World J Gastroenterol. 2000;6:721–724. doi: 10.3748/wjg.v6.i5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacour S, Hammann A, Wotawa A, Corcos L, Solary E, Dimanche-Boitrel MT. Anticancer agents sensitize tumor cells to tumor necrosis factor-related apoptosis-inducing ligand-mediated caspase-8 activation and apoptosis. Cancer Res. 2001;61:1645–1651. [PubMed] [Google Scholar]
- 27.Liang WJ, Huang ZY, Ding YQ. Time effect of TNFá on apoptosis of Lovo cells of human colorectal carcinoma. Huaren Xiaohua Zazhi. 1998;6:27–29. [Google Scholar]
- 28.Nagata S. Fas-induced apoptosis. Intern Med. 1998;37:179–181. doi: 10.2169/internalmedicine.37.179. [DOI] [PubMed] [Google Scholar]
- 29.Leblanc V, Delumeau I, Tocqué B. Ras-GTPase activating protein inhibition specifically induces apoptosis of tumour cells. Oncogene. 1999;18:4884–4889. doi: 10.1038/sj.onc.1202855. [DOI] [PubMed] [Google Scholar]
- 30.Lu SY, Pan XZ, Peng XW, Shi ZL, Lin L, Chen MH. Effect of Hp infec-tion on gastric epithelial cell kinetics in stomach diseases. Shijie Huaren Xiaohua Zazhi. 2000;8:386–388. [Google Scholar]
- 31.Wu K, Liu BH, Zhao DY, Zhao Y. Effect of vitamin E succinate on expression of TGF-beta1, c-Jun and JNK1 in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2001;7:83–87. doi: 10.3748/wjg.v7.i1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao ZB, Meng XY, Ge ZJ, Li RZ, Shi GS. Expression of arylamidase isoenzyme in gastric carcinoma and precancerous lesions. Huaren Xiaohua Zazhi. 1998;6:323–325. [Google Scholar]
- 33.Wang LP, Yu JY, Deng YJ, Tian YW, Wu X, Liu G, Ding HY. Relation-ship between the expression of somatostatin and epidermal growth factor receptor in gastric carcinoma. Huaren Xiaohua Zazhi. 1998;6:606–609. [Google Scholar]
- 34.Zhang QX, Dou YL, Shi XY, Ding Y. Expression of somatostatin mRNA in various differentiated types of gastric carcinoma. World J Gastroenterol. 1998;4:48–51. doi: 10.3748/wjg.v4.i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu ZM, Shou NH. Expression significance of mdr1 gene in gastric carcinoma tissue. Shijie Huaren Xiaohua Zazhi. 1999;7:145–146. [Google Scholar]
- 36.Yu WL, Huang ZH. Progress in studies on gene therapy for gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:887–889. [Google Scholar]
- 37.Chen GY, Wang DR. The expression and clinical significance of CD44v in human gastric cancers. World J Gastroenterol. 2000;6:125–127. doi: 10.3748/wjg.v6.i1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang RQ, Fang DC, Liu WW. MUC2 gene expression in gastric cancer and preneoplastic lesion tissues. Shijie Huaren Xiaohua Zazhi. 2000;8:285–288. [Google Scholar]
- 39.Guo YQ, Zhu ZH, Li JF. Flow cytometric analysis of apoptosis and proliferation in gas tric cancer and precancerous lesion. Shijie Huaren Xiaohua Zazhi. 2000;8:983–987. [Google Scholar]
- 40.Chen SY, Wang JY, Ji Y, Zhang XD, Zhu CW. Effects of Helicobacter pylori and protein kinase C on gene mutation in gastric cancer and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2001;9:302–307. [Google Scholar]
- 41.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B, Wu K, Zhao D. [Inhibition of human gastric carcinoma cell growth by vitamin E succinate] Weisheng Yanjiu. 2000;29:172–174. [PubMed] [Google Scholar]
- 43.Liu BH, Wu K. Study on the growth inhibition of Vitamin E Succinate in human gastric cancer cell. Aibian Jibian Tubian. 2000;12:79–81. [Google Scholar]
- 44.Sturla LM, Westwood G, Selby PJ, Lewis IJ, Burchill SA. Induction of cell death by basic fibroblast growth factor in Ewing's sarcoma. Cancer Res. 2000;60:6160–6170. [PubMed] [Google Scholar]
- 45.Wang H, Chow DA. Natural antibody-induced intracellular signalling and growth control in C3H 10T1/2 fibroblast variants. Immunology. 2000;101:458–466. doi: 10.1046/j.1365-2567.2000.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen MR, Droogmans G, Eggermont J, Voets T, Ellory JC, Nilius B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J Physiol. 2000;529 Pt 2:385–394. doi: 10.1111/j.1469-7793.2000.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller C, Krude T. Requirement of Cyclin/Cdk2 and protein phosphatase 1 activity for chromatin assembly factor 1-dependent chromatin assembly during DNA synthesis. J Biol Chem. 2000;275:35512–35521. doi: 10.1074/jbc.M003073200. [DOI] [PubMed] [Google Scholar]
- 48.Khan SH, Moritsugu J, Wahl GM. Differential requirement for p19ARF in the p53-dependent arrest induced by DNA damage, microtubule disruption, and ribonucleotide depletion. Proc Natl Acad Sci USA. 2000;97:3266–3271. doi: 10.1073/pnas.050560997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 50.Xue XC, Fang GE, Hua JD. Gastric cancer and apoptosis. Shijie Huaren Xiaohua Zazhi. 1999;7:359–361. [Google Scholar]
- 51.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 52.Wu K, Zhao Y, Yu W. Study on apoptosis. Guowai Yixue Yichuanxue Fence. 2001;24:134–138. [Google Scholar]
- 53.Li Y, Wu K. Effectors of apoptosis. Guowai Yixue Weishengxue Fence. 2001;28:50–54. [Google Scholar]
- 54.Wu K, Guo J, Shan YJ. Inhibitory effects of VES on the growth of human squamous gastric carcinoma cells. In: Johnson IT and Fenwick GR, eds. Dietary anticarcinogens and antimutagens-Chemical and biological aspects. RS•C,UK: Athenaeum Press. 2000:304–307. [Google Scholar]
- 55.Yang WC, Wang LD. The mechanisms of cell proliferation control by tumor inhibitory genes and oncogenes. Xin Xiaohuabingxue Zazhi. 1997;5:262–263. [Google Scholar]
- 56.Aravind L, Dixit VM, Koonin EV. The domains of death: evolution of the apoptosis machinery. Trends Biochem Sci. 1999;24:47–53. doi: 10.1016/s0968-0004(98)01341-3. [DOI] [PubMed] [Google Scholar]
- 57.Gu XH, Li QF, Wang YM. Expression of hepatocyte apoptosis and Fas/FasL in liver tissues of patients with hepatitis D. Shijie Huaren Xiaohua Zazhi. 2000;8:35–38. [Google Scholar]
- 58.Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223–227. doi: 10.3748/wjg.v6.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y, Wu K. Cell death molecule Fas/CD95 and apoptosis. Aibian Jibian Tubian. 2001;13:55–58. [Google Scholar]
- 60.Yang JQ, Yang LY, Zhu HC. Mitomycin C induced apoptosis of human hepatoma cell. Shijie Huaren Xiaohua Zazhi. 2001;9:268–272. [Google Scholar]
- 61.Yu W, Simmons-Menchaca M, You H, Brown P, Birrer MJ, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate induction of prolonged activation of c-jun amino-terminal kinase and c-jun during induction of apoptosis in human MDA-MB-435 breast cancer cells. Mol Carcinog. 1998;22:247–257. [PubMed] [Google Scholar]
- 62.Simmons-Menchaca M, Qian M, Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits DNA synthesis and enhances the production and secretion of biologically active transforming growth factor-beta by avian retrovirus-transformed lymphoid cells. Nutr Cancer. 1995;24:171–185. doi: 10.1080/01635589509514405. [DOI] [PubMed] [Google Scholar]
- 63.Kline K, Sanders BG. RRR-alpha-tocopheryl succinate inhibition of lectin-induced T cell proliferation. Nutr Cancer. 1993;19:241–252. doi: 10.1080/01635589309514255. [DOI] [PubMed] [Google Scholar]
- 64.Neuzil J, Weber T, Gellert N, Weber C. Selective cancer cell killing by alpha-tocopheryl succinate. Br J Cancer. 2001;84:87–89. doi: 10.1054/bjoc.2000.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]


