Abstract
AIM: To analyze the association of tobacco smoking, polymorphism of CYP1A1 (7th exon) and GSTM1 genotype and esophageal cancer(EC) in Xi’an.
METHODS: A hospital based case-control study, with molecular epidemiological method, was carried out. Polymorphism of CYP1A1 and GSTM1 of samples from 127 EC cases and 101 controls were detected by PCR method.
RESULTS: There were no significant difference of age and gender between cases and controls. Tobacco smoking was the main risk factor(OR = 1.97; 95%CI = 1.12-3.48) for EC in Xi’an. The proportions of CYP1A1 Ile/Ile, Ile/Val and Val/Val gene types in cases and controls was 19.7%, 45.7%, 34.6% and 30.7%, 47.5%, 21.8% respectively (P = 0.049).Individuals with CYP1A1 Val/Val genotype compared to those with CYP1A1 Ile/Ile genotype had higher risk for EC increased (OR = 2.48, 95%CI = 1.12-5.54). The proportions of GSTM1 deletion genotype in cases and controls were 58.3% and 43.6% (OR = 1.81, 95%CI = 1.03-3.18, P = 0.028). Analysis of gene-environment interaction showed that tobacco smoking and CYP1A1 Val/Val genotype; tobacco smoking and GSTM1 deletion genotype had synergism interaction respectively. Analysis of gene-gene interaction did not find synergistic interaction between these two genes. But in GSTM1 deletion group,there was significant difference of distribution of CYP1A1 genotype between cases and controls (P = 0.011).
CONCLUSION: CYP1A1 Val/Val and GSTM1 deletion genotypes are genetic susceptibility biomarkers for EC. The risk increases, when person with CYP1A1 Val/Val and/or GSTM1 deletion genotype. And these two-metabolic enzymes seem to have interactions with tobacco smoking, in which the mechanism still needs further study.
INTRODUCTION
Esophageal cancer (EC) is one of the most common malignant tumors of human being. The incidence of EC varies in different countries. China is the country with highest incidence and mortality rate of EC. Research showed that risks for EC in different countries or different places were different[1-6]. In western countries alcohol intake and tobacco smoking were studied deeply[7-12]. It was thought that besides tobacco smoking and alcohol drinking, nutrition factors, life style, viruses infection, heredity or exposure to nitrosamines, fungi or AFB1 maybe involved in the process of EC[1,3,13-19]. In China, researches showed risks for EC were different in areas with different incidence[1-5,16,18,20,21]. The mortality rate of EC of Xi’an city in Shaanxi province is about 24 per 100000, which ranks first in all cancer mortalities. Previous studies showed that both of tobacco smoking and family history of EC were main risk factors for EC in Xi’an city[2,22,23].
EC is a multi-etiology disease; environmental risks exposures and genetic susceptibility may take the role part[2,22-24]. Almost all of the environmental carcinogens (procarcinogens) are activated to be ultimate carcinogens before initiate the process of carcinogenesis. Some metabolic enzymes are closely related to the activation and detoxification of procarcinogens. Alterations of the key oncogene or tumor suppress gene can disturb the cycle of cell proliferation, which can also initiate the process of carcinogenesis[23,25,26]. Susceptibility of cancer is associated with the genetics polymorphism of related metabolic enzymes. Both certain susceptibility related biomarkers and certain environmental carcinogens perhaps are indispensable factors for EC[20,23,27,28]. To explore the bio-basis of genetic susceptibility of EC in Xi’an, we carried out a hospital based case- control study to analyze the associations of tobacco smoking, CYP1A1, GSTM1 gene polymorphism and EC.
MATERIALS AND METNODS
Seletion of patients and controls
All cases with esophageal cancer (confirmed by pathological diagnosis) came from inpatients of Tandu Hospital during half a year period (December, 1999 to April, 2000). All controls were stratified randomly selected from non-cancer inpatients from different department of the same hospital during the same period. Both cases and controls were confined to residents with long-term living in Xi’an (with similar proportion of gender and age).
Collected data
Trained interviewers using a structured questionnaire interviewed cases and controls in the hospital. The questionnaire obtained detailed information on residence, occupation, tobacco smoking habit and so on. Here tobacco smoking was defined as smoking at least one cigarette per day and persisting for more than one year. 127 cases (male 97, female 30) and 101 (male 78, female 23) controls were included. Blood samples were also collected for extraction of DNA genome. All blood samples had been stored at -70 °C before started DNA extraction.
PCR methods to detect polymorphism of CYP1A1 and GSTM1
Digested by Proteinase K, DNA genomes were extracted from blood clot of cases and controls with hydroxybenzene, chloroform method in a uninterrupted period. CYP1A1 and GSTM1 polymorphisms were identified by polymerase chain reaction (PCR) before which DNA samples were stored at 4 °C. Primers for GSTM1(P1: 5’-GTACCCTACTTGATTGATGGG-3’; P2: 5’-CTGGATTGTAGCAGATCATGC-3’) and for CYP1A1 (P3: 5’-CGGAAGTGTATCGGTGAGACCA-3’ P4: 5’-CGGAAGTGTATCGGTGAGACCG-3’; P5: 5’-GTAGACAGAGTCTAGGCCTCA-3’) were synthesized by Shenggong bio-technical company of Shanghai. PCR condition for GSTM1 as follows, 50 µL solution including 10 × buffer 5 µL, Mg2+ 2 µL, P1, P2 1 µL respectivly, template DNA 1.5 µL, dDNTPs 1 µL and Taq DNA polymerase 3U. After denaturation at 94 °C for 10 min, Taq DNA polymerase was added,followed by 30 cycles with 94 °C 1min, 60 °C 1 min, and 72 °C 1 min. 20 g·L-1 agar was used to electrophoresis PCR production, then observed under the violate light. GSTM1 exist genotype was characterized as had a 273 bp fragment; while GSTM1 deletion genotype had no fragment (Figure 1).
Figure 1.
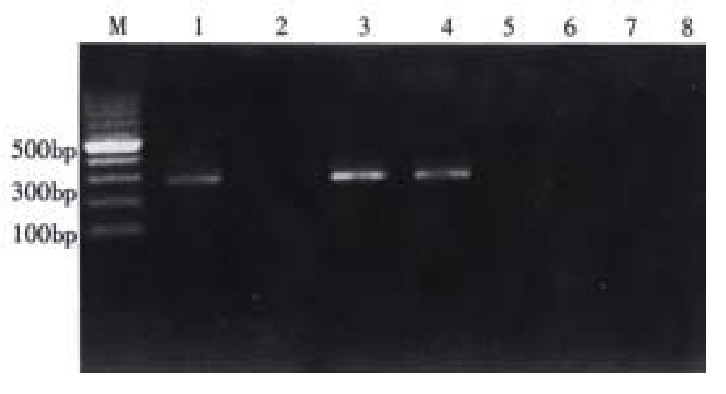
Identify the GSTM1 genotype. M:100 bp DNA ladder, 3,4 were GSTM1 exist 2,5 were GSTM1 deletion; 1 positive control, 6 negative control, 7 was blank control (without DNA template)
We used two pairs of primers to detect the polymorphism of CYP1A1 (7th exon). For each DNA sample two sets of PCR were carried out using P3, P5 (marked as tube A) and P4, P5 (marked as tube B) respectively. PCR conditions were the same:50 µL solution including 10 × buffer 5 µL, Mg2+ 2 µL, P3,P5 (or P4, P5) 1 µL, template DNA 1.5 µL, dDNTPs 1 µL and Taq DNA polymerase 3U, then 94 °C 10 min followed by 94 °C 1 min, 55 °C 1 min, 72 °C 1 min, 35 cycles, 72 °C extending 10 min. PCR products were observed. The PCR was conducted to detect the mutation of A-G in CYP1A1 7th exon, the mutation can leads to change of one amino acid (Ile to Val). If there was only tube A had the specifically fragment (200 bp), the DNA was regarded as CYP1A1 Ile/Ile genotype (pure wild genotype); if only tube B had the positive fragment, CYP1A1 Val/Val genotype (pure mutation) was considered; and CYP1A1 Ile/Val genotype was identified with both tube A and tube B had the fragment (Figure 2).
Figure 2.
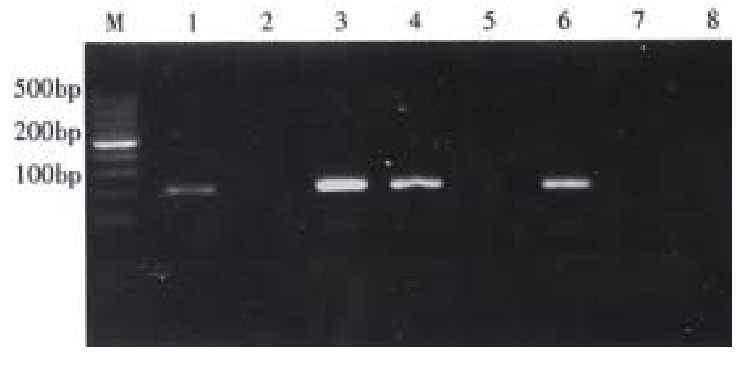
Identified the genotypes of CYP1A1. M: 100 bp DNA ladder; 1(A),2(B) represent Ile/Ile genotype; 3(A), 4(B) represent Ile/Val genotype; 5(A), 6(B) represent Val/Val genotype; 7(A),8(B) as blank control( without DNA template).
Quality control
DNA extraction and PCR were conducted in different period and places. The genotypes of DNA samples were identified blindly. Every PCR had were set controls as blanket control (without DNA template), positive control and negative control, and when any one of these controls was failure, PCR wasre-conducted.
Statistical analysis
Data were input into computer, then the values of χ2, odds ratio (OR) and 95%CI (confidence intermediate) were calculated. And ORs of gene-environment and gene-gene interaction were also estimated.
RESULTS
Comparability between cases and controls
The age and gender in cases and controls were comparable(Table 1).
Table 1.
Comparability of age and gender in cases and controls
| Factor | Case | Control | χ2 | P |
| Age(year) | ||||
| < 50 | 28 | 16 | ||
| 50- | 38 | 44 | ||
| ≥ 60 | 61 | 41 | 4.73 | 0.094 |
| Gender | ||||
| Male | 97 | 78 | ||
| Female | 30 | 23 | 0.02 | 0.80 |
Risk factors for EC
The proportions of tobacco smoking, GSTM1 deletion genotype and CYP1A1 genotype (Val/Val) in cases and controls were significantly different (P < 0.05) (Table 2).
Table 2.
Distributions of smoking, GSTM1 deletion and CYP1A1 genotypes
| Factors | Case | Control | OR | OR95%CI | χ2 | P |
| Smoking Yes | 69 | 38 | 1.97 | 1.12-3.48 | 6.28 | 0.012 |
| No | 58 | 63 | ||||
| GSTM1 | ||||||
| Deletion | 74 | 44 | 1.81 | 1.03-3.18 | 4.85 | 0.028 |
| Exist | 53 | 57 | ||||
| CYP1A1 | ||||||
| Ile/Ile | 25 | 31 | 1.00 | 1.00 | ||
| Ile/Val | 58 | 48 | 1.50 | 0.74-3.03 | 1.48 | 0.22 |
| Val/Val | 44 | 22 | 2.48 | 1.12-5.54 | 5.93 | 0.015 |
Interaction of tobacco smoking and GSTM1 deletion genotype or CYP1A1 Val/Val genotypes
Analysis showed that there was synergistic interaction between tobacco smoking and GSTM1 deletion genotype (Table 3). Tobacco smoking and CYP1A1 Val/Val genotype also appeared synergistic interaction(Table 4). But CYP1A1 mutation genotypes(Val/Val, Ile/Val) and GSTM1deletion genotype did not show significant interaction (Table 5). OR of individuals with CYP1A1 mutation genotype and GSTM1deletion genotype was greater than those with of any other forms of the two genotypes. But there did not show any synergistic interaction between CYP1A1 mutation genotypes and GSTM1deletion genotype.
Table 3.
Interaction of smoking and GSTM1 deletion genotype.
| Smoking | GSTM1 deletion | Case | Control | OR | OR95%CI | χ2 | P |
| No | No | 25 | 37 | 1.00 | |||
| Yes | No | 28 | 20 | 2.07 | 0.90-4.80 | 3.48 | 0.062 |
| No | Yes | 33 | 26 | 1.88 | 0.86-4.13 | 2.93 | 0.087 |
| Yes | Yes | 41 | 18 | 3.37 | 1.49-7.69 | 10.29 | 0.0013 |
SIA = 3.37/(1.88+2.07-1.00) = 1.14
Table 4.
Interaction of tobacco smoking and CYP1A1 Val/Val genotype
| Smoking | CYP1A1(Val/Val) | Case | Control | OR | OR95%CI | χ2 | P |
| No | No | 36 | 47 | 1.00 | |||
| Yes | No | 47 | 32 | 1.92 | 0.98-3.76 | 4.18 | 0.04 |
| No | Yes | 22 | 16 | 1.80 | 0.77-4.20 | 2.18 | 0.14 |
| Yes | Yes | 22 | 6 | 4.79 | 1.62-14.83 | 10.30 | 0.0013 |
SIM = 4.79 / (1.92 × 1.80) = 1.39
Table 5.
Interaction of CYP1A1 mutation genotypes(Val/Val, Ile/Val) and GSTM1 deletion genotype
| GSTM1 deletion | CYP1A1 mutation | Case | Control | OR | OR95%CI | χ2 | P |
| No | No | 10 | 20 | 1.00 | |||
| No | Yes | 43 | 37 | 2.32 | 0.89-6.14 | 3.61 | 0.057 |
| Yes | No | 15 | 11 | 2.73 | 0.81-9.42 | 3.28 | 0.070 |
| Yes | Yes | 59 | 33 | 3.58 | 1.39-9.38 | 8.66 | 0.0033 |
Stratified with GSTM1 deletion genotype to analyze the distributions of CYP1A1 genotypes in cases and controls. Results showed that there were significant different in cases and controls (P = 0.011) in GSTM1 exist genotype CYP1A1 genotypes, whereas there were no significant difference (P = 0.83) between cases and controls in GSTM1 deletion genotype (Table 6).
Table 6.
Analysis of CYP1A1 genotypes in cases and controls with Stratified GSTM1 deletion
| CYP1A1 genotype |
GSTM1 deletion |
existing GSTM1 |
||
| Case | Control | Case | Control | |
| Ile/Ile | 15 | 11 | 10 | 20 |
| Ile/Val | 35 | 19 | 23 | 29 |
| Val/Val | 24 | 14 | 20 | 8 |
| χ2 | 0.39 | 9.04 | ||
| P | 0.83 | 0.011 | ||
DISCUSSION
Under similar environmental carcinogens exposure only a few of individuals get neoplasm, for there were individual difference to environmental exposure. The different liability to cancer was called genetic susceptibility of cancer. Genetic susceptibility can affect on every step of carcinogenesis, including modify the effect of environmental carcinogens[24,29-35].Oncogenes and tumor suppressor genes can also affect individual’s susceptibility to cancer.Cancer susceptibility genes includes typeI, typeII metabolism enzyme gene,DNA repair gene and those affect cell proliferation rate gene. In recent years evidence has accumulated to support the hypothesis that cancer susceptibility gene may be of importance in determining individual susceptibility to cancer[34,36-46].
EC is a multi-factor determined disease; including environmental risk factors and genetic factors. In recent years, more and more researches considered environmental and genetic susceptibility factors and their interactions in evaluating the risks of cancer[2,17,43,47-50]. Investigations showed the mortality rate of EC in Shaanxi province did not decreased during the late 20 years, and risks factors for EC in Xi’an city were discussed in several researches[2,22,23]. In this hospital based case- control study, the results showed that tobacco smoking was a risk factor; and tobacco smoking had interactions with GSTM1 deletion genotype and CYP1A1 Val/ Val genotype.
Most chemical carcinogens in environment are pro-carcinogens. And aromatic hydrocarbons (AHs) in tobacco smoking are pro-carcinogens, they need to be activated to reactive electrophilic forms by type ¢ñ metabolic enzymes (CYP450s), then initiate the carcinogenesis. On the other hand the reactive electrophilic forms of carcinogen can be detoxified and excreted by type I metabolic enzymes such as GSTM1. Although theoretically the increase of activity of type II metabolic enzymes and/or decrease of activity of type I metabolic enzymes can increase the risk for cancer, there were different results in different researches, some supported this hypothesis and others did not[27,34,35,37,40-42,51-56]. Our results showed that individuals with the GSTM1 deletion genotype or/and CYP1A1 Val/Val genotype had increased risks for EC.
P450 CYP1A1 gene located in chromosome 15q22 mainly metabolizes pro-carcinogens. There are three kinds of polymorphism of CYP1A1: MspI site, 7th exon (Ile-Val) and AA polymorphism . MspI polymorphism include three genotypes: without MspI enzyme cleavage site allele gene m1 (m1/m1) as A genotype;having MspI cleavage site allele (m2/m2) as C genotype and m1/m2 as B genotype. In different populations the distribution of these three genotypes were different. CYP1A1 Ile-Val polymorphism caused by 7th exon 4889th base difference (A or G), transition of A to G results in 462th amino turned from isoleucine to valine[13],then form three kinds of genotypes: homozygote wild genotype (Ile/Ile), mutation genotype(Val/Val) and heterozygote Ile/Val genotype. Polymorphism of 7th exon correlated with polymorphism of MspI in Asia and Caucasian populations, and in Americans from Africa these two kind of CYP1A1 polymorphism were independent, CYP1A1 7th exon polymorphism and Msp I site were incomplete linkage. Research showed CYP1A1 Val/Val genotype have higher ability to activate pro-carcinogen than CYP1A1 Ile/Ile genotype. PAH-DNA adducts in leukocyte were higher in heavy smoking population with CYP1A1 Val/Val genotype than those with CYP1A1 Ilel/Val or Ile/Ile genotype. AA polymorphism was new special MspI polymorphism, which still under discussion.
Although evidence showed that CYP1A1 mutation genotype (Val/Val) had the strongest ability to activate pro-carcinogens, the associations between CYP1A1 genotype and susceptibility to cancers were varied[30-33,37,57,58]. Data from Guandong province in China showed that MspI C correlated with no-smoking population’s lung cancer susceptibility[52]. Study in Shanghai and Haerbin no significant relation was discovered between CYP1A1 (Ile-Val) polymorphism and lung cancer susceptibility in non-smoking female patients[51].CYP1A1 Val/Val genotype only appear about 3.2%-5% in white population,while in Japanese it was about 19.8%, in Chinese it was 22.3%. Our study showed that distributions of CYP1A1 genotypes in cases and controls were different (P = 0.049), CYP1A1 Val/Val genotype was associated with EC (OR = 2.48, 95%CI = 1.12-5.54) and there was interaction of tobacco smoking and CYP1A1 Val/Val genotype.
GSTM1 can detoxify a number of reactive electrophilic compound substances, including the carcinogens PAHs. If individuals with GSTM1 deletion genotype, the ability of detoxify the carcinogens decreased. Individuals with GSTM1 deletion can have the increased risk of cancers[24,43,46]. In China there were similar research on GSTM1 deletion genotype and the risks of lung cancer(OR = 2.56)[53],and stomach cancer(OR = 1.90, 95%CI = 1.01-3.56)[54].Researches showed that in Henan province, high incidence of EC in China, GSTM1 deletion gene polymorphisms had not significant relation with EC susceptibility[20]. Results of our study indicated GSTM1 deletion genotype was significant different in cases and controls (P = 0.028) and the OR was 1.81(95%CI = 1.03-3.18). GSTM1 deletion genotype had synergistic interaction with tobacco smoking.
In summary, we found tobacco smoking, CYP1A1 Val/Val genotype; GSTM1 deletion genotype had associations with EC in Xi’an area. Gene-environment interaction analysis showed that tobacco smoking had synergistic interactions with CYP1A1 Val/Val genotype, and with GSTM1 deletion genotype. Gene-gene interaction analysis did not find synergistic interaction between CYP1A1 mutation genotypes and GSTM1 deletion genotype, though individuals with these two genotypes had increased risk for EC. The synergistic interactions and their mechanisms of tobacco smoking with these two metabolic enzymes gene polymorphisms still need further study with large (population-based) samples and modified designs.
ACKNOLEDGMENTS
We would like to thank Bing-Quan Gu for their help in collecting blood sample.
Footnotes
Supported by National Natural Science Foundation of China, No.39670651
Edited by Wang JH and Xu XQ
References
- 1.Zhang W, Bailey-Wilson JE, Li W, Wang X, Zhang C, Mao X, Liu Z, Zhou C, Wu M. Segregation analysis of esophageal cancer in a moderately high-incidence area of northern China. Am J Hum Genet. 2000;67:110–119. doi: 10.1086/302970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li LS, Sun CS, Zhang XL, Qiao GB, Xu DZ, Han CL, Yang WX, Chang GS, Yan MX, Wang Y, et al. A comparative molecular epidemiological study on esophageal cancer between Xi'an and Lizhou. Jiefangjun Yufangyixue Zazhi. 1999;17:255–259. [Google Scholar]
- 3.Zhuo XG, Watanabe S. Factor analysis of digestive cancer mortality and food consumption in 65 Chinese counties. J Epidemiol. 1999;9:275–284. doi: 10.2188/jea.9.275. [DOI] [PubMed] [Google Scholar]
- 4.Wang LD, Zou JX, Hong JY, Zhou Q, Deng CJ, Xie DW, Holly C. Identification of a novel genetic polymorphism of human O-6-alkyguanine -DNA alkyltransferase in patients with esophagus cancer. Huren Xiaohua Zazhi. 1998;6:560–463. [Google Scholar]
- 5.Lu J, Lian S, Sun X, Zhang Z, Dai D, Li B, Cheng L, Wei J, Duan W. [A case-control study on the risk factors of esophageal cancer in Linzhou] Zhonghua Liuxingbingxue Zazhi. 2000;21:434–436. [PubMed] [Google Scholar]
- 6.Li W, Wang X, Zhang C. [Esophageal carcinoma in part of population of Yangquan City] Zhonghua Yixue Zazhi. 1998;78:203–206. [PubMed] [Google Scholar]
- 7.Castellsagué X, Muñoz N, De Stefani E, Victora CG, Quintana MJ, Castelletto R, Rolón PA. Smoking and drinking cessation and risk of esophageal cancer (Spain) Cancer Causes Control. 2000;11:813–818. doi: 10.1023/a:1008984922453. [DOI] [PubMed] [Google Scholar]
- 8.Lagergren J, Bergström R, Lindgren A, Nyrén O. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer. 2000;85:340–346. [PubMed] [Google Scholar]
- 9.Launoy G, Milan C, Faivre J, Pienkowski P, Gignoux M. Tobacco type and risk of squamous cell cancer of the oesophagus in males: a French multicentre case-control study. Int J Epidemiol. 2000;29:36–42. doi: 10.1093/ije/29.1.36. [DOI] [PubMed] [Google Scholar]
- 10.Talamini G, Capelli P, Zamboni G, Mastromauro M, Pasetto M, Castagnini A, Angelini G, Bassi C, Scarpa A. Alcohol, smoking and papillomavirus infection as risk factors for esophageal squamous-cell papilloma and esophageal squamous-cell carcinoma in Italy. Int J Cancer. 2000;86:874–878. doi: 10.1002/(sici)1097-0215(20000615)86:6<874::aid-ijc18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Castellsagué X, Muñoz N, De Stefani E, Victora CG, Castelletto R, Rolón PA, Quintana MJ. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999;82:657–664. doi: 10.1002/(sici)1097-0215(19990827)82:5<657::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Castellsagué X, Muñoz N, De Stefani E, Victora CG, Castelletto R, Rolón PA. Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer. 2000;88:658–664. doi: 10.1002/1097-0215(20001115)88:4<658::aid-ijc22>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon PK, Farrow DC, Vaughan TL, Chow WH, Risch HA, Gammon MD, Mayne ST, Stanford JL, Schoenberg JB, Ahsan H, et al. Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer. 2001;93:148–152. doi: 10.1002/ijc.1294. [DOI] [PubMed] [Google Scholar]
- 14.Nayar D, Kapil U, Joshi YK, Sundaram KR, Srivastava SP, Shukla NK, Tandon RK. Nutritional risk factors in esophageal cancer. J Assoc Physicians India. 2000;48:781–787. [PubMed] [Google Scholar]
- 15.Chang F, Syrjänen S, Shen Q, Cintorino M, Santopietro R, Tosi P, Syrjänen K. Evaluation of HPV, CMV, HSV and EBV in esophageal squamous cell carcinomas from a high-incidence area of China. Anticancer Res. 2000;20:3935–3940. [PubMed] [Google Scholar]
- 16.Li T, Lu ZM, Chen KN, Guo M, Xing HP, Mei Q, Yang HH, Lechner JF, Ke Y. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis. 2001;22:929–934. doi: 10.1093/carcin/22.6.929. [DOI] [PubMed] [Google Scholar]
- 17.Shi Q, Xu D, Sun C. [Study on family aggregation of esophageal cancer in Linzhou city] Zhonghua Yufang Yixue Zazhi. 2000;34:269–270. [PubMed] [Google Scholar]
- 18.Shen YP, Gao YT, Dai Q, Hu X, Xu TL, Xiang YB, Tang ZL, Li WL. A case-control study on esophageal cancer in Huaian city, Jiansu province(I): role of the cigarette smoking and alcohol drinking. Zhongliu. 1999;19:363–367. [Google Scholar]
- 19.Lagergren J, Ye W, Lindgren A, Nyrén O. Heredity and risk of cancer of the esophagus and gastric cardia. Cancer Epidemiol Biomarkers Prev. 2000;9:757–760. [PubMed] [Google Scholar]
- 20.Lin DX, Tang YM, Lu SX, Kadlubar FF. Glutathione S-trans-ferase M1, T1genetypes and risks of esophageal cancer: a case-control study. Zhonghua Liuxingbing Zazhi. 1998;19:195–199. [PubMed] [Google Scholar]
- 21.Gao Y, Den J, Xiang Y, Ruan Z, Wang Z, Hu B, Guo M, Teng W, Han J, Zhang Y. [Smoking, related cancers, and other diseases in shanghai: a 10-year prospective study] Zhonghua Yufang Yixue Zazhi. 1999;33:5–8. [PubMed] [Google Scholar]
- 22.Zhang H, Sun C, Li L, Yan M. [Cytochrome P450IA1 and the genetic susceptibility to esophageal carcinoma] Zhonghua Yufang Yixue Zazhi. 2000;34:69–71. [PubMed] [Google Scholar]
- 23.Wang AH, Zhang HY, Wang Y, Yan MX, Sun CS, Li LS, Huang JY, Cheng QS, Zhu YF. Molecular epidemiological study on esophageal cancer in Xi'an. Disi Junyi Daxue Xuebao. 2001;22:61–63. [Google Scholar]
- 24.Tan W, Song N, Wang GQ, Liu Q, Tang HJ, Kadlubar FF, Lin DX. Impact of genetic polymorphisms in cytochrome P450 2E1 and glutathione S-transferases M1, T1, and P1 on susceptibility to esophageal cancer among high-risk individuals in China. Cancer Epidemiol Biomarkers Prev. 2000;9:551–556. [PubMed] [Google Scholar]
- 25.Hu N, Huang J, Emmert-Buck MR, Tang ZZ, Roth MJ, Wang C, Dawsey SM, Li G, Li WJ, Wang QH, et al. Frequent inactivation of the TP53 gene in esophageal squamous cell carcinoma from a high-risk population in China. Clin Cancer Res. 2001;7:883–891. [PubMed] [Google Scholar]
- 26.Tanière P, Martel-Planche G, Puttawibul P, Casson A, Montesano R, Chanvitan A, Hainaut P. TP53 mutations and MDM2 gene amplification in squamous-cell carcinomas of the esophagus in south Thailand. Int J Cancer. 2000;88:223–227. doi: 10.1002/1097-0215(20001015)88:2<223::aid-ijc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 27.Shao GZ, Hu Z, Li EM, Li J, Wen BG. Relatyionship between the GSTM1 genetic polymorphisim and susceptibility to squamous cell carcinoma of esophagus. Shantou DaxueYixueyuan Xuebao. 1999;12:1–3. [Google Scholar]
- 28.Mizobuchi S, Furihata M, Sonobe H, Ohtsuki Y, Ishikawa T, Murakami H, Kurabayashi A, Ogoshi S, Sasaguri S. Association between p53 immunostaining and cigarette smoking in squamous cell carcinoma of the esophagus. Jpn J Clin Oncol. 2000;30:423–428. doi: 10.1093/jjco/hyd114. [DOI] [PubMed] [Google Scholar]
- 29.van Lieshout EM, Roelofs HM, Dekker S, Mulder CJ, Wobbes T, Jansen JB, Peters WH. Polymorphic expression of the glutathione S-transferase P1 gene and its susceptibility to Barrett's esophagus and esophageal carcinoma. Cancer Res. 1999;59:586–589. [PubMed] [Google Scholar]
- 30.Roth MJ, Dawsey SM, Wang G, Tangrea JA, Zhou B, Ratnasinghe D, Woodson KG, Olivero OA, Poirier MC, Frye BL, et al. Association between GSTM1*0 and squamous dysplasia of the esophagus in the high risk region of Linxian, China. Cancer Lett. 2000;156:73–81. doi: 10.1016/s0304-3835(00)00442-0. [DOI] [PubMed] [Google Scholar]
- 31.Morita S, Yano M, Tsujinaka T, Akiyama Y, Taniguchi M, Kaneko K, Miki H, Fujii T, Yoshino K, Kusuoka H, et al. Genetic polymorphisms of drug-metabolizing enzymes and susceptibility to head-and-neck squamous-cell carcinoma. Int J Cancer. 1999;80:685–688. doi: 10.1002/(sici)1097-0215(19990301)80:5<685::aid-ijc9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 32.Butler WJ, Ryan P, Roberts-Thomson IC. Metabolic genotypes and risk for colorectal cancer. J Gastroenterol Hepatol. 2001;16:631–635. doi: 10.1046/j.1440-1746.2001.02501.x. [DOI] [PubMed] [Google Scholar]
- 33.Rojas M, Cascorbi I, Alexandrov K, Kriek E, Auburtin G, Mayer L, Kopp-Schneider A, Roots I, Bartsch H. Modulation of benzo[a]pyrene diolepoxide-DNA adduct levels in human white blood cells by CYP1A1, GSTM1 and GSTT1 polymorphism. Carcinogenesis. 2000;21:35–41. doi: 10.1093/carcin/21.1.35. [DOI] [PubMed] [Google Scholar]
- 34.Tanimoto K, Hayashi S, Yoshiga K, Ichikawa T. Polymorphisms of the CYP1A1 and GSTM1 gene involved in oral squamous cell carcinoma in association with a cigarette dose. Oral Oncol. 1999;35:191–196. doi: 10.1016/s1368-8375(98)00094-3. [DOI] [PubMed] [Google Scholar]
- 35.Sato M, Sato T, Izumo T, Amagasa T. Genetic polymorphism of drug-metabolizing enzymes and susceptibility to oral cancer. Carcinogenesis. 1999;20:1927–1931. doi: 10.1093/carcin/20.10.1927. [DOI] [PubMed] [Google Scholar]
- 36.Xing DY, Tan W, Song N, Lin DX. Genetic polymorphism in hOGG1 and susceptibility to esophageal cancer in Chinese. ZhonghuaYiue Yichuanxue Zazhi. 2000;17:377–380. [PubMed] [Google Scholar]
- 37.Chen S, Xue K, Xu L, Ma G, Wu J. Polymorphisms of the CYP1A1 and GSTM1 genes in relation to individual susceptibility to lung carcinoma in Chinese population. Mutat Res. 2001;458:41–47. doi: 10.1016/s1383-5726(01)00011-5. [DOI] [PubMed] [Google Scholar]
- 38.Song C, Xing D, Tan W, Wei Q, Lin D. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res. 2001;61:3272–3275. [PubMed] [Google Scholar]
- 39.Lee JM, Lee YC, Yang SY, Yang PW, Luh SP, Lee CJ, Chen CJ, Wu MT. Genetic polymorphisms of XRCC1 and risk of the esophageal cancer. Int J Cancer. 2001;95:240–246. doi: 10.1002/1097-0215(20010720)95:4<240::aid-ijc1041>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Chao YC, Wang LS, Hsieh TY, Chu CW, Chang FY, Chu HC. Chinese alcoholic patients with esophageal cancer are genetically different from alcoholics with acute pancreatitis and liver cirrhosis. Am J Gastroenterol. 2000;95:2958–2964. doi: 10.1111/j.1572-0241.2000.02328.x. [DOI] [PubMed] [Google Scholar]
- 41.Sato M, Sato T, Izumo T, Amagasa T. Genetically high susceptibility to oral squamous cell carcinoma in terms of combined genotyping of CYP1A1 and GSTM1 genes. Oral Oncol. 2000;36:267–271. doi: 10.1016/s1368-8375(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 42.Gsur A, Haidinger G, Hollaus P, Herbacek I, Madersbacher S, Trieb K, Pridun N, Mohn-Staudner A, Vetter N, Vutuc C, et al. Genetic polymorphisms of CYP1A1 and GSTM1 and lung cancer risk. Anticancer Res. 2001;21:2237–2242. [PubMed] [Google Scholar]
- 43.Dong CH, Yu SZ, Chen GC, Zhao DM, Hu Y. Association of poly-morphisms of glutathione S transferase M1 and T1 genotypes with elevated aflatoxin and increased risk of primary liver cancer. Huaren Xiaohua Zazhi. 1998;6:463–466. [Google Scholar]
- 44.Bian JC, Shen FM, Shen L, Wang TR, Wang XH, Chen GC, Wang JB. Susceptibility to hepatocellular carcinoma associated with null genotypes of GSTM1 and GSTT1. World J Gastroenterol. 2000;6:228–230. doi: 10.3748/wjg.v6.i2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai L, Yu SZ, Zhang ZF. Glutathione S-transferases M1, T1 genotypes and the risk of gastric cancer: a case-control study. World J Gastroenterol. 2001;7:506–509. doi: 10.3748/wjg.v7.i4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai L, Yu SZ. A molecular epidemiologic study on gastric cancer in Changle,Fujian province. Shijie Huaren Xiaohua Zazhi. 1999;7:652–655. [Google Scholar]
- 47.Lee JM, Lee YC, Yang SY, Shi WL, Lee CJ, Luh SP, Chen CJ, Hsieh CY, Wu MT. Genetic polymorphisms of p53 and GSTP1,but not NAT2,are associated with susceptibility to squamous-cell carcinoma of the esophagus. Int J Cancer. 2000;89:458–464. doi: 10.1002/1097-0215(20000920)89:5<458::aid-ijc10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 48.Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- 49.Butkiewicz D, Cole KJ, Phillips DH, Harris CC, Chorazy M. GSTM1, GSTP1, CYP1A1 and CYP2D6 polymorphisms in lung cancer patients from an environmentally polluted region of Poland: correlation with lung DNA adduct levels. Eur J Cancer Prev. 1999;8:315–323. doi: 10.1097/00008469-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Liu G, Zhou Q, Wang LD, Hong JY, Deng CJ, Wang YY, Zou JX. Blood clot as a DNA source for studying genetic polymorphism of human carcinogen-metabolizing enzymes. World J Gastroenterol. 1998;4(Suppl 2):108–109. [Google Scholar]
- 51.Qu YH, Shi YB, Peter S, Zhong LJ, Sun L, Sun XW, Cheng JR, Lin YJ, Xian YB, Dai XD, et al. The genotpyes of cytochrome P4501A1 and GST M1 in non-smoking female lung cancer. Zhongliu. 1998;18:80–82. [Google Scholar]
- 52.Hu YL, Zhang Q. Genetic Polymorphisms of CYP1A1and susceptibil-ity of lung cancer. Zhonghua YixueYichuangxue Zzhi. 1999;16:26–28. [PubMed] [Google Scholar]
- 53.Gao J, Ren C, Zhang Q. [CYP2D6 and GSTM1 genetic polymorphism and lung cancer susceptibility] Zhonghua Zhongliu Zazhi. 1998;20:185–186. [PubMed] [Google Scholar]
- 54.Cai L, Yu SZ. Preliminary studies on cytochrome P4502E1 and glu-tathione stransferase M1 polymorphisms and susceptibility to gastric cancer. Zhongguo Gonggong Weisheng. 1999;15:895–897. [Google Scholar]
- 55.Olshan AF, Weissler MC, Watson MA, Bell DA. GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:185–191. [PubMed] [Google Scholar]
- 56.London SJ, Yuan JM, Coetzee GA, Gao YT, Ross RK, Yu MC. CYP1A1 I462V genetic polymorphism and lung cancer risk in a cohort of men in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2000;9:987–991. [PubMed] [Google Scholar]
- 57.Murata M, Watanabe M, Yamanaka M, Kubota Y, Ito H, Nagao M, Katoh T, Kamataki T, Kawamura J, Yatani R, et al. Genetic polymorphisms in cytochrome P450 (CYP) 1A1, CYP1A2, CYP2E1, glutathione S-transferase (GST) M1 and GSTT1 and susceptibility to prostate cancer in the Japanese population. Cancer Lett. 2001;165:171–177. doi: 10.1016/s0304-3835(01)00398-6. [DOI] [PubMed] [Google Scholar]
- 58.Shen J, Wang RT, Xing HX, Wang LW, Wang ZX, Wang BY, Li MS, Wang JM, Hua ZL, Guo CH, et al. Research on the interaction models of cytochrome P450 1A1 polymorphism(s) in the agents of stomach cancer. Zhonghua Yufang Yixue Zazhi. 2001;35:167–170. [Google Scholar]


