Abstract
AIM: To conduct a randomized trial to evaluate the role of using high-dose iodized oil transcatheter arterial chemoembolization(TACE) in the treatment of large hepatocellular carcinoma (HCC).
METHODS: From January 1993 to June 1998, 473 patients with unresectable hepatocellular carcinoma were divided into two groups: 216 patients in group A received more than 20mL iodized oil during the first TACE treatment; 257 patients in group B received 5-15 mL iodized oil in the same way. The Child’s classification and ICG-R15 for evaluating the liver function of the patients were done before the treatment. During the TACE procedure the catheters was inserted into the target artery selectively and the tumor vessels were demonstrated with contrast medium in the hepatic angiography. The anticancer drug mixed with iodized oil (Lipiodol) were Epirubicin and Mitomycin. In group A, 112 cases received 20-29 mL Lipiodol in the first procedure, 85 cases 30-39 mL, 19 cases more than 40 mL. The largest dose was 53 mL and the average dose was 28.3 mL. In group B, 119 cases received 5-10 mL Lipiodol, 138 cases received 11-15 mL, and the average dose was 11.8 mL.
RESULTS: High-dose Lipiodol chemoembolization caused tolerable side effects and a little hurt to the liver function in the patients with Child grade A or ICG-R15 < 20. But the patients with child grade B or ICG-R15 > 20 had higher risk of liver failure after high-dose TACE. More type I and type II lipiodol accumulations in CT scan after 4 weeks of TACE were seen in the group A patients than those in the group B patients (P < 0.01). The resection rate and complete tumor necrosis rate in group A were higher than those of group B (P < 0.05). The 1-,2-,3-year survival rates of group A patients with Child grade A were 79.2%, 51.8% and 34.9%, respectively, better than those of group B (P < 0.001).
CONCLUSION: High-dose Lipiodol can result in more complete tumor necrosis by blocking both arteries and small portal vein of the tumor. High-dose TACE for treatment of large and hypervascular hepatocellular carcinoma is practically acceptable with the better effect than the routine dose. For the patients with large and hypervascular tumor of Child grade A liver function or ICG-R15 less than 20%, oily chemoembolization with 20-40 mL Lipiodol is recommended.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in human beings. It was estimated that in 1985, about 315000 new cases of primary liver cancer occurred worldwide, accounting for 4.1% of all human cancer cases[1]. In the same year, 312000 patients died as a consequence of the disease. In China, HCC is responsible for 130000 deaths every year and is the second cause of the cancer deaths[2]. The crude mortality of HCC was 20.4 per 100000 population, accouning for 18.8% of the total cancer deaths in 1990-1992[3].
About 80% of HCC are associated with cirrhosis that makes treatment more difficult[4]. Surgical resection is the best options for the treatment of HCC and the 5-year survival rate after hepatectomy was about 20%-40%[5]. But in only 20% of HCC patients the surgical resection is feasible. About 60%-70% HCC were in late stages at the time of diagnosis and had lost the chance of operation[6-8]. Transcatheter arterial chemoembolization (TACE) is one of the most common method for the treatment of the unresectable HCC[9,10]. The results achieved by using TACE were much better than those of systemic or regional chemotherapy[11,12]. TACE with anticancer agent suspended in an oily substance has become one of the standard forms of treatment for advanced HCC. In the treatment, iodized oil is used as an embolic agent and carrier of anticancer drugs[13,14]. After entering into the small arteries and peritumoral sinusoid of HCC through a catheter, the iodized oil can be retain there to block the terminal blood flow. The more iodized oil entering into the small arteries and peritumoral sinusoid of HCC, the more complete blocking of the terminal blood supply to the cancer will occur[15,16]. But in such ease more iodized oil may flow into the portal vein causing infarction or necrosis in the noncancerous hepatic tissue, and thus lead to the more liver dysfunction. Generally the amount of iodized oil recommended is about 5-15 mL at each procedure for fear that more iodized oil could lead to liver dysfunction[17,18]. In clinical practice, most patients who received TACE were those with large HCC. For the large and hypervascular liver tumor, using 5-15 mL iodized oil is not enough to attain complete filling of tumor vessel bed. To get a full blocking of the tumor vessels, a larger volume of iodized oil is necessary. Basing on the experience of our clinical practice and the good evaluation of liver function, we conducted a high-dose (more than 20 mL) iodized oil TACE for the large HCC.
MATERIALS AND METHODS
Eligibility of patients
From January 1993 to June 1998, 473 patients with large HCC who were eligible for this study were treated at the Tumor Hospital of Sun Yat-sen University of Medical Sciences. The eligibility criteria for entering this study were as follows: ① a diagnosis of HCC was established on the basis of the patient’s high serum alpha-fetoprotein (AFP) value, findings obtained at computed tomography(CT) scan and clinical manifestations; ② the largest diameter of the tumor exceeded 5 cm; ③ there was no evidence of portal trunk occlusion by thrombosis, extrahepatic metastasis, jaundice or ascites; ④ during the TACE procedure the catheter was successfully inserted into the target artery selectively as proved by angiography. We excluded patients who had Child’s C grade liver function or ICG-R15 (indocyanine green retention rate in 15 min) > 30%. All patients were randomly divided into two treatment groups. In Group A, 216 patients received more than 20 mL of iodized oil in the first TACE, and in Group B, 257 patients received 5-15 mL.
Clinical Characteristics of the patients
The clinical characteristics of the patients including age, sex, size of tumor, thrombosis in portal branch, AFP, serum alanine aminotransferase(ALT), Child’s grading, ICG-R15 were collected before the treatment. The patients in the two groups did not differ significantly with respect to these clinical characteristics (Table 1).
Table 1.
Pretreatment clinical characteristics of 473 patients
| Characteristics | Group A (N = 216) | Group B (N = 257) | Probability value |
| Age | |||
| Mean | 44.6 | 45.1 | 0.348 |
| Range | 22-67 | 24-71 | |
| Sex | |||
| Mate | 213 | 246 | 0.065 |
| Female | 3 | 11 | |
| Diameter of tumor | |||
| 6-9.9 cm | 32 | 9 | 0.133 |
| 10-14.9 cm | 105 | 135 | |
| ≥ 15 cm | 79 | 73 | |
| Thrombosis | |||
| Yes | 54 | 81 | 0.118 |
| No | 162 | 176 | |
| AFP (μg/L) | |||
| ≥ 400 | 91 | 114 | 0.626 |
| < 400 | 125 | 143 | |
| ALT | |||
| < 40 | 117 | 109 | 0.023 |
| 40-79 | 78 | 103 | |
| 80-119 | 17 | 39 | |
| ≥ 120 | 4 | 7 | |
| Child’s class | |||
| A | 203 | 238 | 0.553 |
| B | 13 | 19 | |
| ICG-R15* | |||
| < 10 | 71 | 89 | 0.314 |
| 10-19 | 127 | 137 | |
| 20-29 | 18 | 31 |
*ICG-R15 refers to the indocyanine green retention rate at 15 minutes
Methods of chemoembolization
During the hepatic angiography that demonstrated the tumor vessels and tumor contour, the catheter was inserted selectively into the targetartery. The catheter tip was placed as close as possible to the tumor for attaining the high-selective embolization[19]. The first 5-10 mL iodized oil (Lipiodol, Laboratorie Guerbet, Aulnay-sous-Bois, France) were mixed with anticancer drugs, Epirubicin(50 mg·m-2) and Mitomycin(8 mg·m-2), to form an emulsion. The emulsion was slowly injected under fluoroscopic control. The remainder Lipiodol was added according to the state of accumulation of the emulsion within the tumor at the first injection, speed of blood flowing to the tumor, and appearance of portal vein around the tumor (Figure 1). Gelatin sponge impregnated with contrast medium was injected into the tumor, if the blood flow to the tumor wasn’t slowed down enough after the Lipiodol injection[20]. Any embolization was terminated when the blood flow to the tumor had slowed down.
Figure 1.
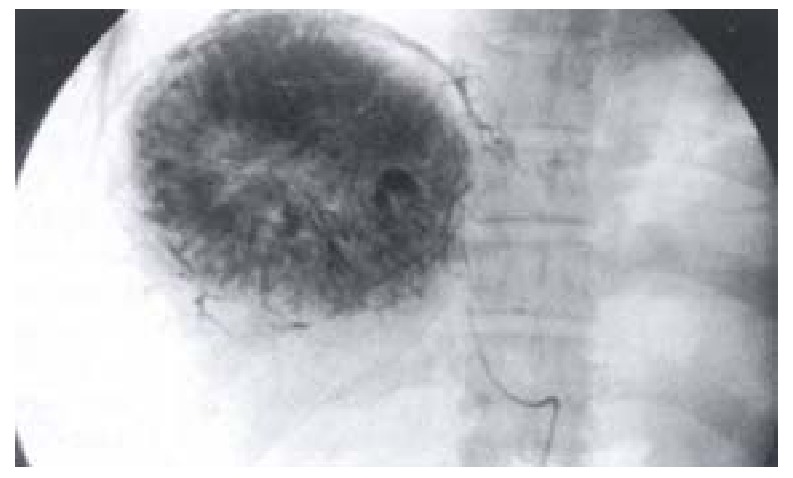
Portal vein branches around the tumor as visualized by high-dose iodized oil
In group A, 112 cases received 20-29 mL Lipiodol in the first procedure, 85 cases 30-39 mL, 19 cases more than 40 mL; the largest dose was 53 mL and the average dose was 28.3 mL. In group B, 119 cases received 5-10 mL Lipiodol and 138 cases received 11-15 mL; the average dose was 11.8 mL.
Follow-up
Four weeks after TACE, the therapeutic effect was assessed by double phase CT scan and AFP test. The CT was performed after a bolus administration of 50-100 mL of contrast material[21]. The pattern of Lipiodol accumulation on CT were classified into following four kinds according to Nishimine[22]: type I, homogeneous; type II, defective; type III, inhomogeneous; and type IV, only slight accumulation, if any (Table 2). Type I was further subgrouped into type Ia (with accumulation around the tumor) and type Ib (without accumulation around the tumor). AFP was tested at 4 wk intervals after first TACE.
Table 2.
The comparison of liver dysfunction** in two group
| Group |
Child-Pugh Classification |
ICG-R15 |
|||
| A | B | < 10% | 10%-19% | ≥ 20% | |
| Group A | 5.9%(12/203) | 92.3%(12/13) | 1.4%(1/71) | 7.9%(10/127) | 72.2%(13/18) |
| Group B | 5.5%(13/238) | 84.2%(15/19) | 2.2%(2/89) | 5.8%(8/137) | 58.1%(18/31) |
**Liver dysfunction is refereed to ascites, jaundice(Tbil > 34.2 μmol/L), or hepatic encephalopaphy
Math 1
Math 1.
Math(A1).
A repeat TACE and other therapies were performed depending on the conditions of the patient after the first TACE. 11 patients underwent totally 5 times TACE; 32 underwent 4; 139 underwent 3; 207 underwent 2; 84 underwent 1; 59 patients underwent percutaneous ethonal injection(PEI) after TACE and 46 patients received surgical resection. Survival was measured from date of the first treatment until death. The causes of death were determined for 347 patients during the follow-up period. When a patient died from advanced carcinoma, hepatic failure or a deterioration of his or her general condition shortly after the procedure, the patients death was considered to be due to treatment failure.
Statistical analysis
The changes of liver dysfunction and CT type was analyzed by chi-square test. Cumulative survival rates were estimated by the Kaplan-Meier method. The relationship between each of the variable and survival was assessed by log rank test. “Significant” indicated a calculated two-tailed value of < 0.05.
RESULTS
Side Effects
The most frequent side effects of TACE were fever and abdominal pain. In Group A abdominal pain occurred in 53.7% of the patients, fever in 71.3%, vomiting in 20.8%, appetite loss in 41.7% and upper digestive tract breeding in 0.93%. In group B, abdominal pain occurred in 49.8% patients, fever in 47.5%, vomiting in 14.8%, appidit loss in 42.8% and upper digestive breeding in 1.17%. The toxic effect of the drugs on the hemopoietic system reflected in slight decreases in the white blood cell counts, platelet counts, and hemoglobin levels of peripheral blood in both groups. Elevations in the serum alanine aminotransferase, alkaline phosphatase, or total bilirubin levels were seen in the patients of both groups, but the changes were not severe and no significant differences were found between the two groups. Liver dysfunction occurred in 24 patients of group A and in 28 patients of group B. The patients with child’s grade B or ICG-R15 > 20 had higher risk of liver failure after high-dose TACE (Table 2).
CT and AFP
CT scan was done four weeks after TACE: In group A there were 77 cases of type I, 130 cases of type II, 9 cases of type III; whereas in group B there were 27 cases of type I,108 cases of type II,90 cases of type II and 32 cases of typeIV (group A vs group B P < 0.001, Figure 2). In group A, the AFP level in 78 of 91 AFP-positive patients ( ≥ 400 μg·L-2) had decreased and in 12 of them down to normal; in group B, 81 of 114 AFP-positive patients had their AFP decrease and in 9 down to normal (P < 0.01).
Figure 2.
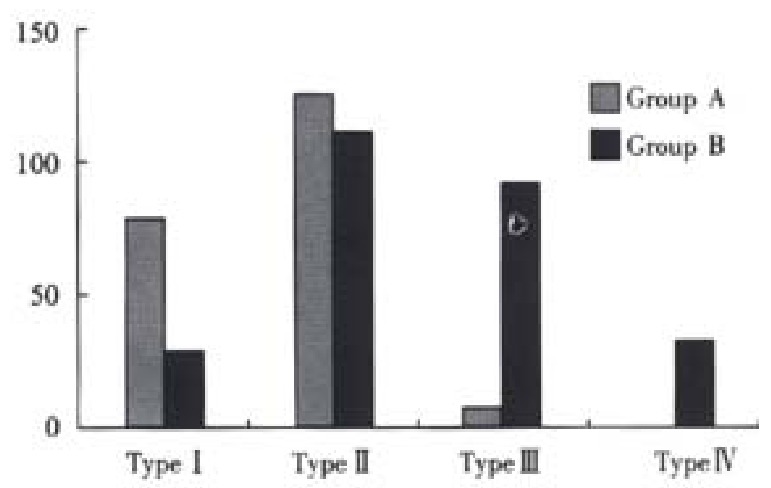
Comparison of CT type between the two groups.
Resection
After TACE, 47 cases achieved surgical resection in group A, and 19 of them were found to have complete tumor necrosis pathologically. In group B, 25 patients received surgical resection and only 4 cases were found to have complete tumor necrosis. The resection rate and complete tumor necrosis rate of group A were higher than those of group B (P < 0.05).
Survival Rates
The 1-,2-,3-year cumulative survival rates of the patients with Child’s grade A in group A were 79.2%, 51.8%, 34.9% and in group B was 59.1%, 26.7%, 14.9%, respectively (Figure 3). The cumulative survival rates were significantly better in group A than in groupB(P < 0.001). The 1-,2-,3-year cumulative survival rates of the patients with Child’s grade B in group A were 42.1%, 21.1%, 7.7% and in group B was 46.8%, 23.7%, 0%, respectively (no significantly difference in between the two groups).
Figure 3.
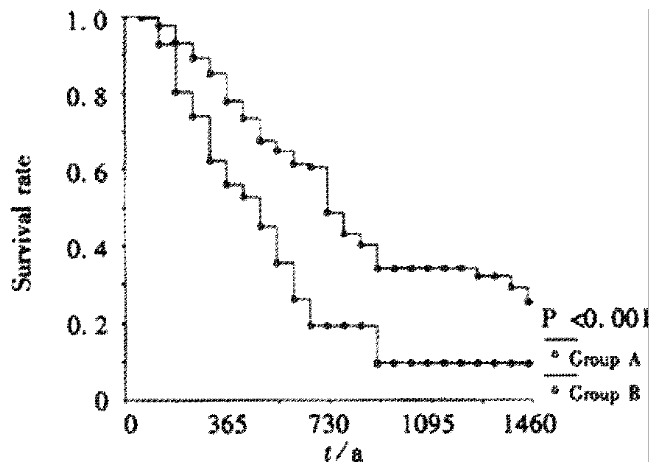
Cumulative survival rates of patients with Child’s A in two groups.
DISCUSSION
For use in TACE, Iodized oil (mostly lipiodol) mixed with anticancer drugs has been reported to be one of the most effective agents[23,24]. Iodized oil plays an important role as an embolic agent. It not only occludes the small arteries supplying the tumor but also acts as the carrier bringing the drug to the tumor. The lipiodol can enter into the microcirculation of the tumor and stay there to stop the blood flow[25]. The experimental investigations in animal and human resected specimens revealed that the lipiodol could stay in the small artery, sinusoid, and small portal vein within the tumor. In general, the recommended amount of lipiodol is 5-15 mL. The use of Iarge volume of iodized oil is more prone to invade the normal liver parenchyma, causing more live injury[26]. Matsuo[27] reported the relationship between the lipiodol dose and the tumor necrosis rates in 198 HCC cases. He found that the prognosis was better in the group of patients with bigtumors in which the doses of lipiodol were correspondingly larger tumor diameter, but the total dose must not be more than 10mL in small HCC. Other authors[28,29] comfirmed that the dose of iodized oil played an important role in TACE. A sufficiently high dose of Lp-TACE would be a factor of good tumor response, if the tumor was large. Therefore the volume of lipiodol is an important a factor influencing the antitumor effect of TACE. The large and hypervascular liver tumors have vast vessel bed. So it is necessary to inject high-dose lipiodol to attain complete filling of tumor vessel bed. While the tumor 5 cm in diameter needs 5 mL lipiodol to fill the vessel bed, the 10 cm tumor would need at least 8 times of lipiodol to do the same work. In this situation a high-dose of iodized oil must be used for complete filling of the large tumor[30,31]. Some portal branches around the tumors are displayed after the high- dose lipiodol pooled into the tumor; this is considered to be the sign of complete arterial block. After a certain amount of lipiodol is pooled into the hepatic microcirculation and sinusoid, any additional volume of lipiodol enter into the branches of portal vein via the sinus between hepatic artery and portal vein. And then some lipiodol is seen in the small branches of portal vein around the tumor. As the amount of lipiodol delivered into artery increase, the portal vein branches become more prominent[32]. Prominent portal vein appearances were seen in 29% patients given 10 mL or less of lipiodol, in 67% with 10-20 mL,and in 86% with more than 20 mL. It was known that infiltrating portion and nonencapsulated daughter nodules of the tumor are nourished by both the portal vein and the hepatic artery. So even if the tumor arteries are successfully embolized, some tumor cells can survive because the portal vein blood supply still exists. This may be the reason for incomplete tumor necrosis with subsequent tumor recurrence[28,15]. High-dose lipiodol fully fills the sinus and stops the portal vein supply to tumor cells after the arteries are embolized. Embolization of both hepatic artery and small portal veins may cause complete necrosis of the tumor infiltrating portion and nonencapsulated daughter nodules[13,14]. The visualization of some portal vein tributaries around the tumor may be a sign of complete embolization.
After Lp-TACE it is important to estimate the amount and state of iodized oil accumulating within the tumor, because this has much bearingon the prognosis of the patients[33]. Matsuo[27] found that the volume of lipiodol infusion correlated with the CT type. If the lipiodol volume is greater than the tumor mass in the small HCC, more type I lipiodol accumulation will be seen in the CT scan after Lp-TACE. He suggested that the lipiodol volume should be greater than the tumor mass, but the maximum dose should be less than 10 mL for fear of liver dysfunction even in the large HCC. Nishimine[22] reported that tumors of CT type I have the highest cumulative survival rates and those of the typeI have higher cumulative survival rates than type III and type IV. The iodized oil retention was again evaluated by using CT scan one month after TACE. It was found that the patients with iodized oil retention in the tumor greater than 50 per cent of tumor size survived longer than patients with retention of less than 50 per cent[34-36] . Our study showed that high-dose lipiodol could bring about more type I and type II lipiodol accumulation than a routine dose. Pathological sgudy of resected specimen after TACE showed that the area of lipiodol retention in CT was the necrotic area of the tumor. The high-dose lipiodol would lead to more lipiodol retention in the necrotic tumor[37,38].
For preventing severe side effects, attention must be paid. The liver reserve function must be evaluated by Child’s grading and ICG-R15[39,40,41,42]. Our data showed that the liver dysfunction most frequently occurred in the patients with child’s B or ICG-R15 > 20. The patients with Child’s A or ICG-R15 < 20 were usually tolerant of 20-40 mL lipiodol. Nonetheless, a high-dose lipiodol is contraindicated for the patients with child’s B or ICG-R15 > 20. Second, to avoid normal liver parenchyma damage a high-selective placement of the catheter is crucial for injecting high-dose lipiodol. We mainly used the 5F Yasilo catheter or 5F RH catheter to perform the high selective catheterization. Subsegemental or segmental embolization is necessary for the high-dose lipiodol infusion[43,44]. If the tip of catheter can’t pass over all the arteries that flow to normal tissue and organ, high-dose lipiodol injection should be done with caution. Finally, slow injection under fluorescopic guidance is also important to let all the lipiodol flow clearly into the tumor vessels. Whenever the lipiodol flows to the outside of tumor, injection should be stopped immedialely. In the condition that all the lipiodol selectively enters into the tumor and doesn’t flow to the normal liver tissue, the hepatic function will not be deteriorated[45,46]. We safely injected 53 mL into a large HCC with a diameter > 20 cm. If the blood flow is still fast toward the tumor after lipiodol infusion, Gelatin sponge should be prescribed[47]. High-dose lipiodol TACE can bring about more tumor necrosis. So high-dose lipiodol TACE results in higher survival rate.
It is concluded that high-dose lipiodol can bring about more completely tumor necrosis by blocking both the arteries and small portal veins of the tumor. High-dose TACE for treatment of large and hypervascular hepatocellular carcinoma is practically acceptable and gives a better effect than those using a routine dose. For the patients with large and hypervascular tumors and with Child A or ICG-R15 less than 20%, chemoembolization with 20-40 mL lipiodol is recommended.
Footnotes
Supported by the “9·5” National Major Project of National Committee of Sciences and Technology, No.96-907-03-02
Edited by Lu HM
References
- 1.Wu MC, Shen F. Progress in research of liver surgery in China. World J Gastroenterol. 2000;6:773–776. doi: 10.3748/wjg.v6.i6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang ZY, Yu YQ, Zhou XD, Ma ZC, Wu ZQ. Progress and prospects in hepatocellular carcinoma surgery. Ann Chir. 1998;52:558–563. [PubMed] [Google Scholar]
- 3.Zhang S, Li L, Lu F. [Mortality of primary liver cancer in China from 1990 through 1992] Zhonghua Zhongliu Zazhi. 1999;21:245–249. [PubMed] [Google Scholar]
- 4.Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, Caselmann WH. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:208–215. doi: 10.3748/wjg.v7.i2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu MC. Progresses in surgical treatment of primary hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:921–923. [Google Scholar]
- 6.Sithinamsuwan P, Piratvisuth T, Tanomkiat W, Apakupakul N, Tongyoo S. Review of 336 patients with hepatocellular carcinoma at Songklanagarind Hospital. World J Gastroenterol. 2000;6:339–343. doi: 10.3748/wjg.v6.i3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yip D, Findlay M, Boyer M, Tattersall MH. Hepatocellular carcinoma in central Sydney: a 10-year review of patients seen in a medical oncology department. World J Gastroenterol. 1999;5:483–487. doi: 10.3748/wjg.v5.i6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu SP, Wu DM, Yuan YZ, Wu YL, Jiang SH, Wu YX. Treatment of hepatocellular carcinoma by transcanther arterial chemoembolization with hydroxycamptothecin. Shijie Huaren Xiaohua Zazhi. 1999;7:158–160. [Google Scholar]
- 10.Zhang XQ, Yang XM, Sun HL, Li ZR. Effects of hepatic artery chemoembolization on hepatic carcinoma. Xin Xiaohuabingxue Zazhi. 1997;5:112–113. [Google Scholar]
- 11.Fan J, Ten GJ, He SC, Guo JH, Yang DP, Wang GY. Arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 1998;4:33–37. doi: 10.3748/wjg.v4.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okusaka T, Okada S, Ueno H, Ikeda M, Yoshimori M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Iwata R, et al. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. On-cology. 2000;58:2093–2099. doi: 10.1159/000012115. [DOI] [PubMed] [Google Scholar]
- 13.Ono Y, Yoshimasu T, Ashikaga R, Inoue M, Shindou H, Fuji K, Araki Y, Nishimura Y. Long-term results of lipiodol-transcatheter arterial embolization with cisplatin or doxorubicin for unresectable hepatocellular carcinoma. Am J Clin Oncol. 2000;23:564–568. doi: 10.1097/00000421-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Ueno K, Miyazono N, Inoue H, Nishida H, Kanetsuki I, Nakajo M. Transcatheter arterial chemoembolization therapy using iodized oil for patients with unresectable hepatocellular carcinoma: evaluation of three kinds of regimens and analysis of prognostic factors. Cancer. 2000;88:1574–1581. [PubMed] [Google Scholar]
- 15.Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Muramatsu Y, Moriyama N, Okusaka T, Okada S, Ueno H. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol. 2001;176:681–688. doi: 10.2214/ajr.176.3.1760681. [DOI] [PubMed] [Google Scholar]
- 16.Mizoe A, Yamaguchi J, Azuma T, Fujioka H, Furui J, Kanematsu T. Transcatheter arterial embolization for advanced hepatocellular carcinoma resulting in a curative resection: report of two cases. Hepatogastroenterology. 2000;47:1706–1710. [PubMed] [Google Scholar]
- 17.Vogl TJ, Trapp M, Schroeder H, Mack M, Schuster A, Schmitt J, Neuhaus P, Felix R. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214:349–357. doi: 10.1148/radiology.214.2.r00fe06349. [DOI] [PubMed] [Google Scholar]
- 18.Ernst O, Sergent G, Mizrahi D, Delemazure O, Paris JC, L'Herminé C. Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization: comparison of planned periodic chemoembolization and chemoembolization based on tumor response. AJR Am J Roentgenol. 1999;172:59–64. doi: 10.2214/ajr.172.1.9888740. [DOI] [PubMed] [Google Scholar]
- 19.Inoue H, Ito T, Siraki K, Sugimoto K, Sakai T, Oomori S, Takase K, Nakano T. Effect of segmental transcatheter arterial chemoembolization on branched chain amino acids and tyrosine ratio in patients with hepatocellular carcinoma. Int J Oncol. 2000;17:977–980. doi: 10.3892/ijo.17.5.977. [DOI] [PubMed] [Google Scholar]
- 20.Kwok PC, Lam TW, Chan SC, Chung CP, Wong WK, Chan MK, Lo HY, Lam WM. A randomized clinical trial comparing autologous blood clot and gelfoam in transarterial chemoembolization for inoperable hepatocellular carcinoma. J Hepatol. 2000;32:955–964. doi: 10.1016/s0168-8278(00)80100-2. [DOI] [PubMed] [Google Scholar]
- 21.Katyal S, Oliver JH, Peterson MS, Chang PJ, Baron RL, Carr BI. Prognostic significance of arterial phase CT for prediction of response to transcatheter arterial chemoembolization in unresectable hepatocellular carcinoma: a retrospective analysis. AJR Am J Roentgenol. 2000;175:1665–1672. doi: 10.2214/ajr.175.6.1751665. [DOI] [PubMed] [Google Scholar]
- 22.Nishimine K, Uchida H, Matsuo N, Sakaguchi H, Hirohashi S, Nishimura Y, Guo Q, Ohishi H, Nagano N, Yoshioka T. Segmental transarterial chemoembolization with Lipiodol mixed with anticancer drugs for nonresectable hepatocellular carcinoma: follow-up CT and therapeutic results. Cancer Chemother Pharmacol. 1994;33 Suppl:S60–S68. doi: 10.1007/BF00686670. [DOI] [PubMed] [Google Scholar]
- 23.Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Dao T, Van Steenbergen W, Buffet C, Rougier P, Adler M, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129–134. doi: 10.1016/s0168-8278(98)80187-6. [DOI] [PubMed] [Google Scholar]
- 25.Savastano S, Miotto D, Casarrubea G, Teso S, Chiesura-Corona M, Feltrin GP. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with Child's grade A or B cirrhosis: a multivariate analysis of prognostic factors. J Clin Gastroenterol. 1999;28:334–340. doi: 10.1097/00004836-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Kamada K, Nakanishi T, Kitamoto M, Aikata H, Kawakami Y, Ito K, Asahara T, Kajiyama G. Long-term prognosis of patients undergoing transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: comparison of cisplatin lipiodol suspension and doxorubicin hydrochloride emulsion. J Vasc Interv Radiol. 2001;12:847–854. doi: 10.1016/s1051-0443(07)61510-3. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo N, Uchida H, Sakaguchi H, Nishimine K, Nishimura Y, Hirohashi S, Ohishi H. Optimal lipiodol volume in transcatheter arterial chemoembolotherapy for hepatocellular carcinoma: study based on lipiodol accumulation patterns and histopathologic findings. Semin Oncol. 1997;24:S6–61-S6-S6-61-70. [PubMed] [Google Scholar]
- 28.Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, Sato M, Yamanaka N, Shimamura Y, Ohto M. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699–704. doi: 10.2214/ajr.175.3.1750699. [DOI] [PubMed] [Google Scholar]
- 29.Bizollon T, Rode A, Bancel B, Gueripel V, Ducerf C, Baulieux J, Trepo C. Diagnostic value and tolerance of Lipiodol-computed tomography for the detection of small hepatocellular carcinoma: correlation with pathologic examination of explanted livers. J Hepatol. 1998;28:491–496. doi: 10.1016/s0168-8278(98)80324-3. [DOI] [PubMed] [Google Scholar]
- 30.Oi H, Kim T, Kishimoto H, Matsushita M, Tateishi H, Okamura J. Effective cases of transcatheter arterioportal chemoembolization with high-dose iodized oil for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33 Suppl:S69–S73. doi: 10.1007/BF00686671. [DOI] [PubMed] [Google Scholar]
- 31.Oi H, Kishimoto H, Matsushita M, Katsushima S, Tateishi H, Okamura J. Antitumor effect of transcatheter oily chemoembolization for hepatocellular carcinoma assessed by computed tomography: role of iodized oil. Semin Oncol. 1997;24:S6–56-S6-S6-56-60. [PubMed] [Google Scholar]
- 32.Nakayama A, Imamura H, Matsuyama Y, Kitamura H, Miwa S, Kobayashi A, Miyagawa S, Kawasaki S. Value of lipiodol computed tomography and digital subtraction angiography in the era of helical biphasic computed tomography as preoperative assessment of hepatocellular carcinoma. Ann Surg. 2001;234:56–62. doi: 10.1097/00000658-200107000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caturelli E, Siena DA, Fusilli S, Villani MR, Schiavone G, Nardella M, Balzano S, Florio F. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue-long-term prospective study. Radiology. 2000;215:123–128. doi: 10.1148/radiology.215.1.r00ap21123. [DOI] [PubMed] [Google Scholar]
- 34.Colagrande S, Fargnoli R, Dal Pozzo F, Bindi A, Rega L, Villari N. Value of hepatic arterial phase CT versus lipiodol ultrafluid CT in the detection of hepatocellular carcinoma. J Comput Assist Tomogr. 2000;24:878–883. doi: 10.1097/00004728-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Higashi S, Tabata N, Kondo KH, Maeda Y, Shimizu M, Nakashima T, Setoguchi T. Size of lipid microdroplets effects results of hepatic arterial chemotherapy with an anticancer agent in water-in-oil-in-water emulsion to hepatocellular carcinoma. J Pharmacol Exp Ther. 1999;289:816–819. [PubMed] [Google Scholar]
- 36.Vogl TJ, Schroeder H, Trapp M, Straub R, Schuster A, Schuster M, Mack M, Souchon F, Neuhaus P. [Multi-sequential arterial chemoembolization of advanced hepatocellular carcinomas: computerized tomography follow-up parameters for evaluating effectiveness of therapy] Rofo. 2000;172:43–50. doi: 10.1055/s-2000-279. [DOI] [PubMed] [Google Scholar]
- 37.Kamada K, Nakanishi T, Kitamoto M, Aikata H, Kawakami Y, Ito K, Asahara T, Kajiyama G. Long-term prognosis of patients undergoing transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: comparison of cisplatin lipiodol suspension and doxorubicin hydrochloride emulsion. J Vasc Interv Radiol. 2001;12:847–854. doi: 10.1016/s1051-0443(07)61510-3. [DOI] [PubMed] [Google Scholar]
- 38.Okusaka T, Okada S, Ueno H, Ikeda M, Yoshimori M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Iwata R, et al. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. Oncology. 2000;58:293–299. doi: 10.1159/000012115. [DOI] [PubMed] [Google Scholar]
- 39.Jiao LR, El-Desoky AA, Seifalian AM, Habib N, Davidson BR. Effect of liver blood flow and function on hepatic indocyanine green clearance measured directly in a cirrhotic animal model. Br J Surg. 2000;87:568–574. doi: 10.1046/j.1365-2168.2000.01399.x. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto M, Watanabe G. Hepatic parenchymal cell volume and the indocyanine green tolerance test. J Surg Res. 2000;92:222–227. doi: 10.1006/jsre.2000.5893. [DOI] [PubMed] [Google Scholar]
- 41.Tang ZY. Advances in clinical research of hepatocellular carcinoma in China. Huaren Xiaohua Zazhi. 1998;6:1013–1016. [Google Scholar]
- 42.Zhou HG, Gu GW. New trend in clinicopathology of primary liver cancer. Huaren Xiaohua Zazhi. 1998;6:714–715. [Google Scholar]
- 43.Wu ZQ, Fan J, Qiu SJ, Zhou J, Tang ZY. The value of postoperative hepatic regional chemotherapy in prevention of recurrence after radical resection of primary liver cancer. World J Gastroenterol. 2000;6:131–133. doi: 10.3748/wjg.v6.i1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Wu PH, Li JQ, Zhang WZ, Lin HG, Zhang YQ. Segmental transcatheter arterial embolization for primary hepatocellular carcinoma. World J Gastroenterol. 1998;4:511–512. doi: 10.3748/wjg.v4.i6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarazov PG, Polysalov VN, Prozorovskij KV, Grishchenkova IV, Rozengauz EV. Ischemic complications of transcatheter arterial chemoembolization in liver malignancies. Acta Radiol. 2000;41:156–160. doi: 10.1080/028418500127344966. [DOI] [PubMed] [Google Scholar]
- 46.Caturelli E, Siena DA, Fusilli S, Villani MR, Schiavone G, Nardella M, Balzano S, Florio F. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue-long-term prospective study. Radiology. 2000;215:123–128. doi: 10.1148/radiology.215.1.r00ap21123. [DOI] [PubMed] [Google Scholar]
- 47.Ono Y, Yoshimasu T, Ashikaga R, Inoue M, Shindou H, Fuji K, Araki Y, Nishimura Y. Long-term results of lipiodol-transcatheter arterial embolization with cisplatin or doxorubicin for unresectable hepatocellular carcinoma. Am J Clin Oncol. 2000;23:564–568. doi: 10.1097/00000421-200012000-00006. [DOI] [PubMed] [Google Scholar]


