Abstract
AIM: To investigate the effect of c9,t11-conjugated linoleic acid (c9,t11-CLA) on the invasion of human gastric carcinoma cell line and its possible mechanism of preventing metastasis.
METHODS: Using reconstituted basement membrane invasion, chemotaxis, adhesion, PAGE substrate zymography and RT-PCR assays, we analyzed the abilities of invasion, direct migration, adhesion of intracellular matrix, as well as the activity of type IV collagenase and expression of tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 mRNA in SGC-7901 cells which were treated with gradually increased concentrations (25, 50, 100 and 200 μmol/L) of c9,t11-CLA for 24 h.
RESULTS: At the concentrations of 200 μmol/L, 100 μmol/L and 50 μmol/L, c9,t11-CLA suppressed the invasion of SGC-7901 cells into the reconstituted basement membrane by 53.7%, 40.9% and 29.3%, respectively, in comparison with the negative control. Only in the 200 μmol/L c9,t11-CLA group, the chemotaxis of SGC-7901 cells was inhibited by 16.0% in comparision with the negative control. C9,t11-CLA also could inhibit the adhesion of SGC-7901 cells to laminin, fibronectin and Matrigel, increase the expression of TIMP-1 and TIMP-2 mRNA, and reduce type IV collagenase activities in the serum-free medium supernatant of SGC-7901 cells.
CONCLUSION: c9,t11-CLA can inhibit the invasion of SGC-7901 cells at multiple procedures in tumor metastasis cascade, which may be associated with the induction of TIMP-1 and TIMP-2 mRNA expression.
INTRODUCTION
Conjugated linoleic acid (CLA) refers to a class of positional and geometrical isomers of linoleic acid (18:2) with conjugated double bonds. The conjugated double bonds are mainly located at sites 9 and 11 or 10 and 12, and each double bond may be in the cis or trans configuration[1-5]. CLA exists in dairy products and meat of ruminants, the former is the principal source of CLA, of which c9,t11-CLA is the major isomer and represents 85%-90% of total CLA in bovine milk[1]. CLA is mainly produced from linoleic acid by rumen bacteria during biohydrogenation[6], although CLA can also be synthesized in non-ruminants by △9-desaturase from trans-1118:1, another intermediate in rumen biohydrogenation[7]. The level of CLA in human adipose tissue has been reported to be associated with the consumption of dairy fat[8]. Besides, CLA can be synthesized in the laboratory and commercially synthesized CLA is available as a dietary supplement and has been shown to be non-toxic[9].
CLA is a potent cancer preventive agent and has chemoprotective properties[10-20]. In animal models of chemical carcinogenesis, CLA has been shown to inhibit skin papillomas[10,11], forestomach neoplasia[12,13] and mammary tumors[14-20]. Several studies[21-28] suggest that CLA is cytostatic and cytotoxic to a variety of human cancer cells, including hepatoma, malignant melanoma, colorectal cancer, breast carcinomas, and gastric cancer cells. Moreover, CLA also plays a role in reducing the tumor size and inhibiting the metastasis of transplanted human breast cancer cells and prostate cancer cells in SCID mice[29,30].
Gastric cancer is one of the most common malignancies in China[31-34] and its metastasis is the major cause of death in cancer patients. Our previous studies have revealed that c9,t11-CLA is an effective agent to prevent gastric cancer[13,35,36], and could inhibit the invasion of mouse melanoma[37]. However, it is unclear whether c9,t11-CLA influences on the metastasis of gastric cancer. Thus, we investigated the effect of c9,t11-CLA on the metastasis of human gastric carcinoma cell line SGC-7901.
MATERIALS AND METHODS
Materials
c9,t11-CLA with 98% purity, was obtained from Dr. Rui-Hai Liu (Food Science and Toxicology, Department of Food Science, Cornell University, Ithaca, NY, USA). It was dissolved in ethanol and then diluted to the following concentrations: 25 μmol/L, 50 μmol/L, 100 μmol/L and 200 μmol/L, respectively.
Methods
Cell culture Human gastric adenocarcinoma cells (SGC-7901) purchased from Cancer Research Institute of Beijing (China) were cultured at 37 °C in PRMI 1640 (Gibco Co.) medium supplemented with fetal calf serum (100 mL/L), penicillin (100 × 103 U/L), streptomycin (100 mg/L) and L-glutamine (2 mmol/L). The pH was maintained at 7.2-7.4 by equilibration with 5% CO2. The SGC-7901 cells were sub-cultured with EDTA.
In vitro invasion assay Invasion assay assessing the ability of cells to invade a synthetic basement membrane was performed in transwell chambers (Costar Co. USA) with a polycarbonate filter of 8.0 μm pore size, separating the upper and lower chambers. The top surface of the polycarbonate filter was coated with Matrigel, the bottom with fibronectin. SGC-7901 cells (2 × 105) treated with different concentration of c9,t11-CLA (25, 50, 100, and 200 μmol/L) for 24 h were added to the upper transwell chamber in 100 μL of serum-free RPMI 1640 medium containing 0.1% bovine serum albumin (BSA, Gibco Co.) and 600 μL of serum-free BSA-RPMI 1640 medium was added to the lower chamber. After 4 h, the filters were fixed in methanol and stained with hematoxylin and eosin (HE). The noninvading cells on the top surface of the filter membrane were removed with a cotton swab. Cells on the bottom surface of the filter were counted and the cell means were obtained from five high-power fields under a light microscope. The inhibitory rate (IR) was calculated as follows:
IR(%) = (Number of invasive cells in negative control group - Number of invasive cells in test groups/Number of invasive cells in negative control group) × 100
Chemotaxied-motion assay The assay also was performed in transwell chambers (Costar Co. USA) with a polycarbonate filter of 8.0 μm pore size separating the upper and lower chambers. The bottom surface of filter was coated with fibronectin. The lower chambers were filled with 600 μL of serum-free BSA-RPMI 1640 medium. SGC-7901 cells (2 × 105) treated with different concentrations of c9,t11-CLA (25, 50, 100, and 200 μmol·L-1) for 24 h were added to the upper chambers, the chambers then were treated the same as invasion assay. SGC-7901 cells that migrated from the upper chamber to the bottom surface of the filter were counted and the means of cell numbers from five high-power fields under a light microscope were calculated. The inhibitory rate (IR) was calculated as follows:
IR(%) = (Number of cells in negative control group - Number of cells in test groups/Number of cells in negative control group) × 100
Cell adhesion assay 96-well plates (Nunc. Co.) were incubated at 37 °C with laminin or fibronectin or Matrigel for 1 h and then blocked with phosphate-buffered saline (PBS) containing 10 g/L BSA for another 1 h at the same temperature. The SGC-7901 cells exposed to different concentrations of c9,t11-CLA (25, 50, 100, and 200 μmol/L) for 24 h were suspended in serum-free medium at a density of 8 × 105 cells/mL. Then 0.1 mL of SGC-7901 cells suspension was added to each well, and incubated at 37 °C for 1 h. The plates were washed three times in PBS to remove the unattached cells, then the remaining SGC-7901 cells in 96-well plates were reacted with MTT for 4 h at 37 °C, then were solubilized with DMSO, and the absorbance of each well was measured at 570 nm with an ELX800 microplate reader (Bio-TEK Co.). Results were expressed as the percentage of total cells, assuming that the adhesion of cells in control represented 100%.
Zymography Zymography was used for the analysis of MMP activity secreted into the culture medium of cell lines as described[38]. SGC-7901 cells were seeded at a density of 8 × 104 cells/pore in 24 well plates (Nunc. Co.) and maintained in 400 μL of serum-free medium containing different concentrations of c9,t11-CLA (25, 50, 100, and 200 μmol/L) for 24 h. After centrifugation at 500 g for 10 min, the supernatant was collected and stored at -20 °C. SDS-PAGE was performed on gradient gels that contained 1.0 g/L gelatin (Amresco Co.) and run for 3-4 h under nondenaturing conditions. After electrophoresis, the gels were incubated in 2.5% Triton X-100 for 1 h and then incubated in substrate buffer [50 μmol/L Tris (pH7.5), 10 mmol/L CaCl2, 200 mmol/L NaCl and 1 μmol/L ZnCl2] for 12-16 h at 37 °C. After incubation, the gels were stained in a solution containing 1 g/L Coomassie blue R250 for 4 h, and destained with 45% methanol and 10% acetic acid untill clear bands were shown.
RT-PCR SGC-7901 cells were treated at different concentrations of c9,t11-CLA (25, 50, 100, and 200 μmol/L) for 24 h and collected by centrifugation. Total RNA was isolated using Trizol reagent according to the manufacturer’s instructions. The concentrations and purity of total RNA were determined by DUR 640 nucleic acid and protein analyzer (Beckman, USA). The first-strand cDNA was synthesized from 5 μg of total RNA using 50 pmol of oligo (dT) primers, 10 units of AMV reverse transcriptase XL) (TAKAKA Biotechnology, Dalian Co.), 20 units RNase Inhibitor, 5 × buffer and 10 mmol/L each dNTP in a total volume of 20 μL. PCRs were performed using respectively primers for TIMP-1, TIMP-2 and β-actin. The primer sequences are described in Table 1. PCR was carried out in 25 μL volume containing 4 μL of cDNA template, 10 × PCR buffer, 20 μmol/L each primer, 2.5 mmol/L dNTP mixture, 2.5 unit of Taq Polymerase. After denaturation at 94 °C for 5 min, the reaction mixtures were subjected to 35 cycles of PCR amplification in PCT-100TM programmable thermal controller (MJ Research Inc., USA). Each cycle consisted of 1 min of denaturation at 94 °C, a primer specific annealing temperature and period (at 58 °C for 45 s for TIMP-1, at 58 °C for 30 s for TIMP-2, at 55 °C for 30 s for β-actin) and extension at 72 °C (1.5 min for TIMP-1, 1 min for TIMP-2, 45 s for β-actin).
Table 1.
Primer sequence and size of expected PCR products
| Primer | Sequence | Lengh (bp) |
| β-actin sense | 5’-AAGGATTCCTATGTGGGC-3’ | 532 |
| antisense | 5’-CATCTCTTGCTCGAAGTC-3’ | |
| TIMP-1 sense | 5’-CTGTTGGCTGTGAGGAATGCACAG-3’ | 106 |
| antisense | 5’-TTCAGAGCCTTGGAGGAGCTGGTC-3’ | |
| TIMP-2 sense | 5’-AGACGTAGTGATCGGGCCA-3’ | 490 |
| antisense | 5’-GTACCACGCGCAAGAACCT-3’ |
The amplified products were separated in 20 g·L-1 agarose gel and stained with ethidium bromide. After electrophoresis, the gel was observed and photographed under ultraviolet reflector. The density and area of each band were analyzed using ChemiImagerTM 4000 digital system (Alpha Innotech Corporation, USA).
Statistical analysis
Analysis of data was performed using the Student’s t test. A value of P < 0.05 was considered as statistically significant.
RESULTS
Effect of c9,t11-CLA on invasion in SGC-7901 cells
As shown in Table 2, the invasive abilities of SGC-7901 cells treated with 50 μmol/L, 100 μmol/L, and 200 μmol/L of c9,t11-CLA were significantly lower than those of the negative control group (P < 0.01), and the inhibitory rates were 53.7%, 40.9% and 29.3%, respectively. At 25 μmol/L c9,t11-CLA, the invasive ability of SGC-7901 cells did not differ from that of the negative control group (P > 0.05). The effect of c9,t11-CLA on the invasive ability of SGC-7901 cells is shown in Figure 1.
Table 2.
Effect of c9,t11-CLA on invasive ability of SGC-7901 cells
| Groups | Invasive cell number (-x ± s) | Inhibitory frequency (%) |
| 200 μmol/L | 23.2 ± 2.9a | 53.7 |
| 100 μmol/L | 29.6 ± 3.3a | 40.9 |
| 50 μmol/L | 35.4 ± 2.8a | 29.3 |
| 25 μmol/L | 44.9 ± 3.1 | 10.4 |
| Negative control | 50.1 ± 4.6 | 0 |
P < 0.01, compared with the negative control group.
Figure 1.
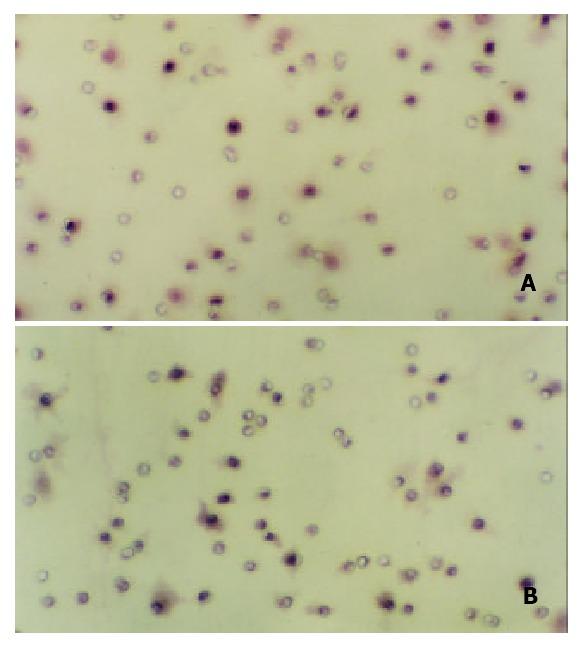
Effect of c9,t11-CLA on invasion of SGC-7901 cells detected by reconstituted basement membrane invasion assay (100 ×). A: There are more invading SGC-7901 cells in the nega-tive control group. B: There are less invading SGC-7901 cells in the 200 μmol/L c9,t11-CLA group.
Effect of c9,t11-CLA on Chemotaxic migration ability in SGC-7901 cells
The chemotaxic ability of SGC-7901 cells at 200 μmol/L c9,t11-CLA was lower than that of the negative control group (P < 0.05) and the inhibitory rate was 16.0%. At 100 μmol/L, 50 μmol/L and 25 μmol/L of c9,t11-CLA, the chemotaxic ability of SGC-7901 cells did not differ from that of the negative control group (P > 0.05). The effect of c9,t11-CLA on the chemotaxic migration ability of SGC-7901 cells is shown in Table 3 and Figure 2.
Table 3.
Effect of c9,t11-CLA on migration ability of SGC-7901 cells
| Groups | Cell number (-x ± s) | Inhibitory frequency (%) |
| 200 μmol/L | 50.8 ± 3.3a | 16.0 |
| 100 μmol/L | 52.5 ± 3.1 | 13.2 |
| 50 μmol/L | 54.4 ± 3.9 | 10.1 |
| 25 μmol/L | 55.0 ± 4.4 | 10.1 |
| Negative control | 60.5 ± 4.2 | 0 |
P < 0.05, compared with the negative control group.
Figure 2.
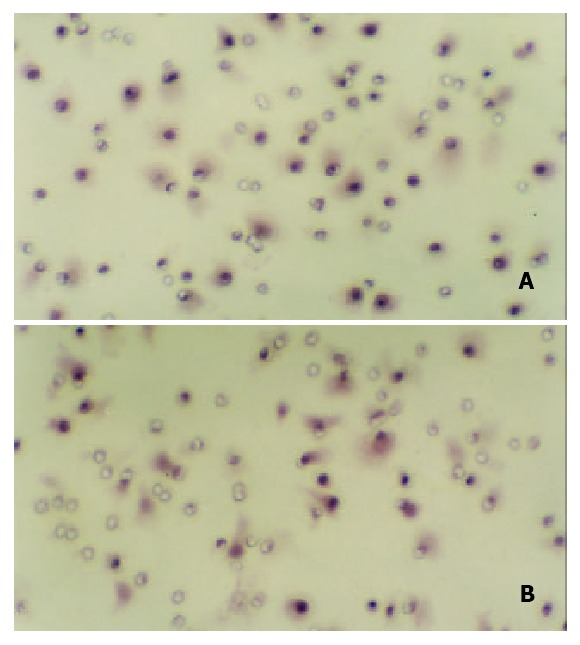
Effect of c9,t11-CLA on chemotaxic migration of SGC-7901 cells detected by chemotaxied-motion assay (100 ×). A: There are more invading SGC-7901 cells in the negative con-trol group. B: There are less invading SGC-7901 cells in the 200 μmol/L c9,t11-CLA group.
Effect of c9,t11-CLA on attachment ability in SGC-7901 cells
As shown in Figure 3, at levels of 25 μmol/L, 50 μmol/L, 100 μmol/L and 200 μmol/L c9,t11-CLA could decrease the attachment to FN, LN or Matrigel ability of SGC-7901 cells and the inhibitory effect was positively correlated with the concentration of c9,t11-CLA.
Figure 3.
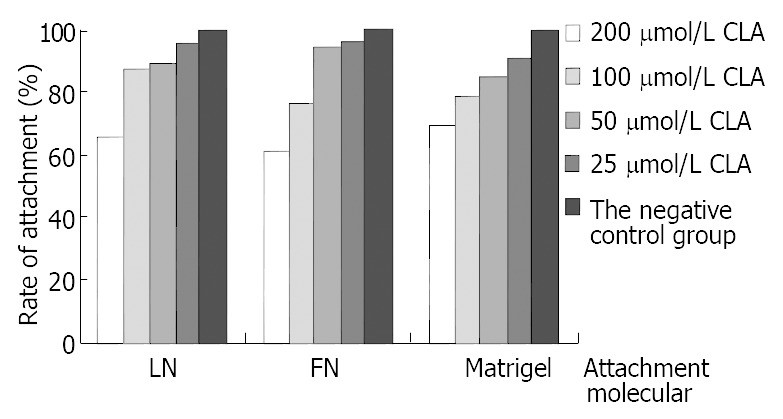
Effect of c9,t11-CLA on the attachment ability of SGC-7901 cells.
Effect of c9,t11-CLA on collagenase ability in SGC-7901 cells
As shown in Figure 4 and Figure 5, at levels of 200 μmol/L, 100 μmol/L and 50 μmol/L c9,t11-CLA significantly reduced 92 kDa type IV collagenase (MMP-9) activity in the serum-free medium supernatant of SGC-7901 cells, but at 25 μmol/L c9,t11-CLA, the collagenase ability did not differ from that in the negative control group. c9,t11-CLA did not influence on the 72 kDa collagenase (MMP2) activity.
Figure 4.
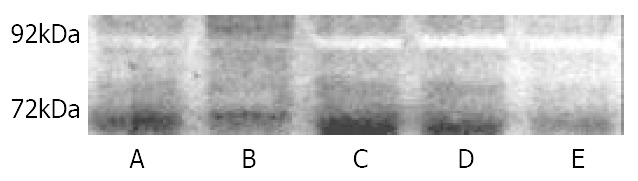
Effects of c9,t11-CLA on Gelatinase secretion in SGC-7901 cells detected by zymography. A, B, C, D are 200 μmol/L, 100 μmol/L, 50 μmol/L, 25 μmol/L c9,t11-CLA, respectively. E is the negative control group.
Figure 5.
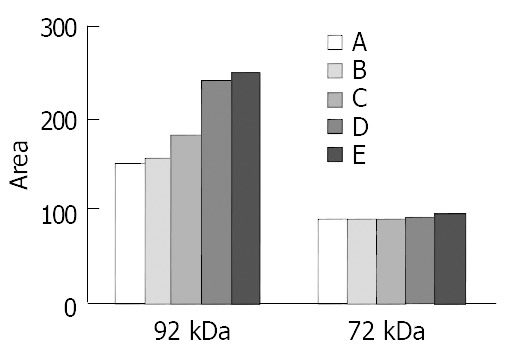
Quantitation of 92 and 72 kDa type IV Collagenase levels in SGC-7901 cells by ChemiImager 4000. A, B, C, D are 200 μmol/L, 100 μmol/L, 50 μmol/L, 25 μmol/L c9,t11-CLA, respectively. E is the negative control group.
Effect of c9,t11-CLA on expression of TIMP-1and TIMP-2 mRNA in SGC-7901 cells
The expression of TIMP-1 and TIMP-2 mRNA of SGC-7901 cells treated at different concentrations of c9,t11-CLA increased in comparison with that of the negative control group. As the concentrations of c9,t11-CLA increased, the expression of TIMP-1and TIMP-2 mRNA was upregulated. Moreover, the increase of TIMP-2 mRNA expression was more obvious (Figure 6, Figure 7).
Figure 6.
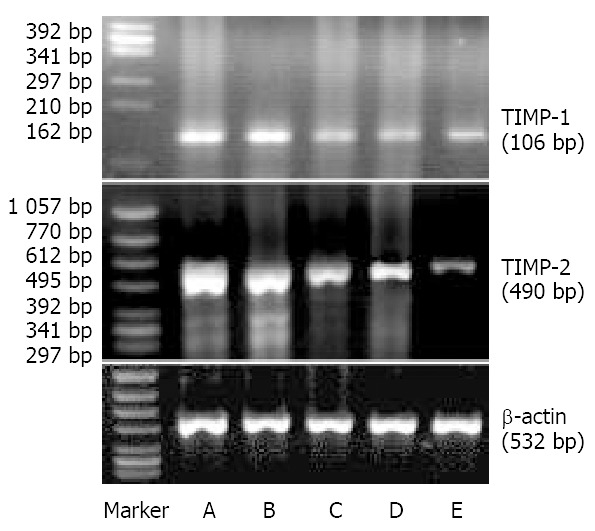
Effects of c9,t11-CLA on expression of TIMP-1 mRNA and TIMP-2 mRNA in SGC-7901 cells detected by RT-PCR. Top: Expression of TIMP-1 mRNA. Middle: Expression of TIMP-2 mRNA. A, B, C, D are 20 μmol/L, 100 μmol/L, 50 μmol/L, 25 μmol/L c9,t11-CLA, respectively. E is the negative control group.
Figure 7.
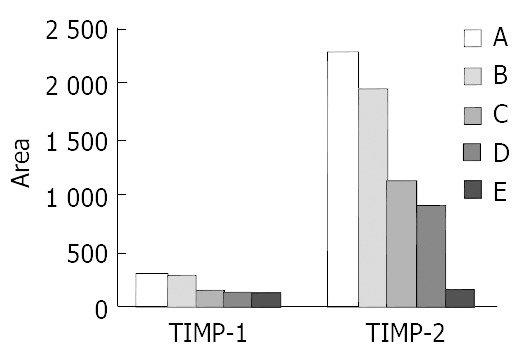
Quantitation of TIMP-1 and TIMP-2 mRNA levels in the SGC-7901 cells by ChemiImager 4000. A, B, C, D are 200 μmol/L, 100 μmol/L, 50 μmol/L, 25 μmol/L c9,t11-CLA, respectively. E is the negative control group.
DISCUSSION
Metastasis is a multistage process involving interactions between tumor cells and extracellular matrix (ECM). Metastasis of cancer cells required several sequent steps, including changes in cell-ECM interaction, disconnection of intercellular adhesions, separation of single cell from tumor tissue, degradation of ECM, migration of tumor cells into the ECM, invasion of lymph and blood vessels, immunologic escape in the circulatory system, adhesion to endothelial cells, extravasation from lymph and blood vessels, proliferation of cells and induction of angiogenesis[39]. The complex metastasis cascade could be described as cell attachment to the extracellular matrix, proteolytic dissolution of the matrix, and movement of cells through the digested barrier[40]. Therefore, tumor metastasis can be inhibited by blocking attachment, invasion and motility. In vitro invasion, attachment, proteolytic dissolution of the matrix and movement are required for tumor cell through Matrigel and polycarbonate, thus the reconstituted basement membrane invasion assay could better reflects the invasion ability of tumor. This study showed that the invasion ability of SGC-7901 cells treated with 200 μmol/L, 100 μmol/L and 50 μmol/L of c9,t11-CLA was significantly inhibited, which was consistent with the study result from mice melanoma[37]. We conclude that CLA can inhibit the invasion and metastasis of mice melanoma and human gastric adenocarcinoma.
Many studies indicated the importance of cancer cell-matrix interaction. Cell and matrix interactions promoted cell migration, proliferation, and ECM degradation[39-43]. Also, it has been shown that prevention of tumor cell adhesion and migration was related to the inhibition of tumor cell invasion into the basement membrane, and agents inhibiting cell attachment in vitro decreased the invasion and metastatic potential of tumor cells in vivo. Therefore, cellular interactions with ECM, which promote adhesion and migration, were thought to be required for tumor invasion, migration, and metastasis. We demonstrated that after incubated with 200 μmol/L, 100 μmol/L and 50 μmol/L of c9,t11-CLA for 1 h, the attachment to extracellular matrix component of SGC-7901 cells was significantly reduced, which was consistent with our previous finding[37], indicating CLA could inhibit the attachment to extracellular matrix component of tumor cells. However, in previous study we found that CLA could not affect the direct migration of B16-MB cells and in this study we observed that 200 μmol/L CLA could reduce the direct migration of SGC-7901 cells. Therefore, attachment of tumor cells to matrix inhibited by c9,t11-CLA may be a mechanism for the inhibition of invasion and needs further study.
Basement membrane is a barrier for tumor invasion and metastasis. Tumor cell invasion through the ECM was an essen tial p ro cess in can cer metastasis[42]. Matrix metalloproteinases (MMPs) were important enzymes for the proteolysis of extracellular matrix proteins such as collagen, laminin and fibronectin[44]. Most MMPs were synthesized and secreted from the cells as proenzymes[45]. Human MMP-2 (gelatinase A/72kD type IV collagenase) and MMP-9 (gelatinase B/92kD type IV collagenase) were thought to be the key enzymes for degrading IV collagen, which is a principal structural protein of the basement mmebrane[46]. Studies revealed that increased production of MMPs was correlated with the invasion, metastasis, and angiogenesis of tumors[47]. In this study, we found that c9,t11-CLA significantly reduced 92 kDa type (MMP9) activities in the serum-free medium supernatant of SGC-7901 cells, but had no effect on 72 kDa collagenase (MMP2), showing that CLA could inhibit the ability of tumor cells to degrade basement membrane via reduction of type IV collagenase activities. MMPs activity is regulated by tissue inhibitors of metalloproteinase (TIMPs). There have been four members of the TIMP family determined up to date, of which TIMP-1 and TIMP-2 were best characterized as inhibitors of all known MMPs[44]. We found that TIMP-1 and TIMP-2 mRNA expression increased in SGC-7901 cells treated with c9,t11-CLA, indicating that c9,t11-CLA may inhibit the MMPs activity via inducing the expression of TIMP-1 and TIMP-2 mRNA and then inhibit the metastasis of SGC-7901 cells.
In conclusion, c9,t11-CLA inhibits several essential steps of metastasis in SGC-7901 cells. It can inhibit cell-matrix component interaction, reduce the activity of MMPs and increase the expression of TIMP1 and TIMP2 mRNA. Its mechanism in SGC-7901 cells metastasis needs to be studied further.
ACKNOWLEDGEMENTS
We are grateful to Dr. Rui-Hai Liu from Cornell University for providing 98% purity of c9,t11-CLA.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30070658
Edited by Ren SY and Wang XL
References
- 1.Sébédio JL, Gnaedig S, Chardigny JM. Recent advances in conjugated linoleic acid research. Curr Opin Clin Nutr Metab Care. 1999;2:499–506. doi: 10.1097/00075197-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Pariza MW, Park Y, Cook ME. Mechanisms of action of conjugated linoleic acid: evidence and speculation. Proc Soc Exp Biol Med. 2000;223:8–13. doi: 10.1046/j.1525-1373.2000.22302.x. [DOI] [PubMed] [Google Scholar]
- 3.Pariza MW, Park Y, Cook ME. Conjugated linoleic acid and the control of cancer and obesity. Toxicol Sci. 1999;52:107–110. doi: 10.1093/toxsci/52.suppl_1.107. [DOI] [PubMed] [Google Scholar]
- 4.Whigham LD, Cook ME, Atkinson RL. Conjugated linoleic acid: implications for human health. Pharmacol Res. 2000;42:503–510. doi: 10.1006/phrs.2000.0735. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald HB. Conjugated linoleic acid and disease prevention: a review of current knowledge. J Am Coll Nutr. 2000;19:111S–118S. doi: 10.1080/07315724.2000.10718082. [DOI] [PubMed] [Google Scholar]
- 6.Kim YJ, Liu RH, Bond DR, Russell JB. Effect of linoleic acid concentration on conjugated linoleic acid production by Butyrivibrio fibrisolvens A38. Appl Environ Microbiol. 2000;66:5226–5230. doi: 10.1128/aem.66.12.5226-5230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santora JE, Palmquist DL, Roehrig KL. Trans-vaccenic acid is desaturated to conjugated linoleic acid in mice. J Nutr. 2000;130:208–215. doi: 10.1093/jn/130.2.208. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J, Wolk A, Vessby B. Relation between the intake of milk fat and the occurrence of conjugated linoleic acid in human adipose tissue. Am J Clin Nutr. 1999;70:21–27. doi: 10.1093/ajcn/70.1.21. [DOI] [PubMed] [Google Scholar]
- 9.Scimeca JA. Toxicological evaluation of dietary conjugated linoleic acid in male Fischer 344 rats. Food Chem Toxicol. 1998;36:391–395. doi: 10.1016/s0278-6915(97)00169-5. [DOI] [PubMed] [Google Scholar]
- 10.Belury MA, Nickel KP, Bird CE, Wu Y. Dietary conjugated linoleic acid modulation of phorbol ester skin tumor promotion. Nutr Cancer. 1996;26:149–157. doi: 10.1080/01635589609514471. [DOI] [PubMed] [Google Scholar]
- 11.Pariza MW, Hargraves WA. A beef-derived mutagenesis modulator inhibits initiation of mouse epidermal tumors by 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1985;6:591–593. doi: 10.1093/carcin/6.4.591. [DOI] [PubMed] [Google Scholar]
- 12.Ha YL, Storkson J, Pariza MW. Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990;50:1097–1101. [PubMed] [Google Scholar]
- 13.Zhu Y, Qiou J, Chen B. [The inhibitory effect of CLA on mice forestomach neoplasia induced by B(a)P] Zhonghua Yufang Yixue Zazhi. 2001;35:19–22. [PubMed] [Google Scholar]
- 14.Ip C, Jiang C, Thompson HJ, Scimeca JA. Retention of conjugated linoleic acid in the mammary gland is associated with tumor inhibition during the post-initiation phase of carcinogenesis. Carcinogenesis. 1997;18:755–759. doi: 10.1093/carcin/18.4.755. [DOI] [PubMed] [Google Scholar]
- 15.Ip C, Singh M, Thompson HJ, Scimeca JA. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212–1215. [PubMed] [Google Scholar]
- 16.Ip C, Banni S, Angioni E, Carta G, McGinley J, Thompson HJ, Barbano D, Bauman D. Conjugated linoleic acid-enriched butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J Nutr. 1999;129:2135–2142. doi: 10.1093/jn/129.12.2135. [DOI] [PubMed] [Google Scholar]
- 17.Thompson H, Zhu Z, Banni S, Darcy K, Loftus T, Ip C. Morphological and biochemical status of the mammary gland as influenced by conjugated linoleic acid: implication for a reduction in mammary cancer risk. Cancer Res. 1997;57:5067–5072. [PubMed] [Google Scholar]
- 18.Banni S, Angioni E, Casu V, Melis MP, Carta G, Corongiu FP, Thompson H, Ip C. Decrease in linoleic acid metabolites as a potential mechanism in cancer risk reduction by conjugated linoleic acid. Carcinogenesis. 1999;20:1019–1024. doi: 10.1093/carcin/20.6.1019. [DOI] [PubMed] [Google Scholar]
- 19.Kimoto N, Hirose M, Futakuchi M, Iwata T, Kasai M, Shirai T. Site-dependent modulating effects of conjugated fatty acids from safflower oil in a rat two-stage carcinogenesis model in female Sprague-Dawley rats. Cancer Lett. 2001;168:15–21. doi: 10.1016/s0304-3835(01)00459-1. [DOI] [PubMed] [Google Scholar]
- 20.Ip C, Ip MM, Loftus T, Shoemaker S, Shea-Eaton W. Induction of apoptosis by conjugated linoleic acid in cultured mammary tumor cells and premalignant lesions of the rat mammary gland. Cancer Epidemiol Biomarkers Prev. 2000;9:689–696. [PubMed] [Google Scholar]
- 21.Shultz TD, Chew BP, Seaman WR, Luedecke LO. Inhibitory effect of conjugated dienoic derivatives of linoleic acid and beta-carotene on the in vitro growth of human cancer cells. Cancer Lett. 1992;63:125–133. doi: 10.1016/0304-3835(92)90062-z. [DOI] [PubMed] [Google Scholar]
- 22.Igarashi M, Miyazawa T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett. 2000;148:173–179. doi: 10.1016/s0304-3835(99)00332-8. [DOI] [PubMed] [Google Scholar]
- 23.Igarashi M, Miyazawa T. The growth inhibitory effect of conjugated linoleic acid on a human hepatoma cell line, HepG2, is induced by a change in fatty acid metabolism, but not the facilitation of lipid peroxidation in the cells. Biochim Biophys Acta. 2001;1530:162–171. doi: 10.1016/s1388-1981(00)00180-3. [DOI] [PubMed] [Google Scholar]
- 24.Park Y, Allen KG, Shultz TD. Modulation of MCF-7 breast cancer cell signal transduction by linoleic acid and conjugated linoleic acid in culture. Anticancer Res. 2000;20:669–676. [PubMed] [Google Scholar]
- 25.O'Shea M, Devery R, Lawless F, Murphy J, Stanton C. Milk fat conjugated linoleic acid (CLA) inhibits growth of human mammary MCF-7 cancer cells. Anticancer Res. 2000;20:3591–3601. [PubMed] [Google Scholar]
- 26.O'Shea M, Stanton C, Devery R. Antioxidant enzyme defence responses of human MCF-7 and SW480 cancer cells to conjugated linoleic acid. Anticancer Res. 1999;19:1953–1959. [PubMed] [Google Scholar]
- 27.Cunningham DC, Harrison LY, Shultz TD. Proliferative responses of normal human mammary and MCF-7 breast cancer cells to linoleic acid, conjugated linoleic acid and eicosanoid synthesis inhibitors in culture. Anticancer Res. 1997;17:197–203. [PubMed] [Google Scholar]
- 28.Schønberg S, Krokan HE. The inhibitory effect of conjugated dienoic derivatives (CLA) of linoleic acid on the growth of human tumor cell lines is in part due to increased lipid peroxidation. Anticancer Res. 1995;15:1241–1246. [PubMed] [Google Scholar]
- 29.Visonneau S, Cesano A, Tepper SA, Scimeca JA, Santoli D, Kritchevsky D. Conjugated linoleic acid suppresses the growth of human breast adenocarcinoma cells in SCID mice. Anticancer Res. 1997;17:969–973. [PubMed] [Google Scholar]
- 30.Cesano A, Visonneau S, Scimeca JA, Kritchevsky D, Santoli D. Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res. 1998;18:1429–1434. [PubMed] [Google Scholar]
- 31.Liu LX, Liu ZH, Jiang HC, Qu X, Zhang WH, Wu LF, Zhu AL, Wang XQ, Wu M. Profiling of differentially expressed genes in human gastric carcinoma by cDNA expression array. World J Gastroenterol. 2002;8:580–585. doi: 10.3748/wjg.v8.i4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591–595. doi: 10.3748/wjg.v8.i4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi XY, Zhao FZ, Dai X, Ma LS, Dong XY, Fang J. Effect of jianpiyiwei capsule on gastric precancerous lesions in rats. World J Gastroenterol. 2002;8:608–612. doi: 10.3748/wjg.v8.i4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792–796. doi: 10.3748/wjg.v8.i5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Chen B, Liu R, Lu G. [Inhibitory effect of conjugated linoleic acid on human gastric carcinoma cell line] Weisheng Yanjiu. 1999;28:353–355. [PubMed] [Google Scholar]
- 36.Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901) World J Gastroenterol. 2002;8:224–229. doi: 10.3748/wjg.v8.i2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue Y, Chen B, Zheng Y, Yuan L. [Effects of conjugated linoleic acid on the metastasis of mouse melanoma B16-MB] Wei Sheng Yan Jiu. 2001;30:37–39. [PubMed] [Google Scholar]
- 38.Ries C, Loher F, Zang C, Ismair MG, Petrides PE. Matrix metalloproteinase production by bone marrow mononuclear cells from normal individuals and patients with acute and chronic myeloid leukemia or myelodysplastic syndromes. Clin Cancer Res. 1999;5:1115–1124. [PubMed] [Google Scholar]
- 39.Yoon SO, Kim MM, Chung AS. Inhibitory effect of selenite on invasion of HT1080 tumor cells. J Biol Chem. 2001;276:20085–20092. doi: 10.1074/jbc.M101143200. [DOI] [PubMed] [Google Scholar]
- 40.Ara T, Deyama Y, Yoshimura Y, Higashino F, Shindoh M, Matsumoto A, Fukuda H. Membrane type 1-matrix metalloproteinase expression is regulated by E-cadherin through the suppression of mitogen-activated protein kinase cascade. Cancer Lett. 2000;157:115–121. doi: 10.1016/s0304-3835(00)00494-8. [DOI] [PubMed] [Google Scholar]
- 41.Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJ. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA. 1992;89:1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakahara H, Nomizu M, Akiyama SK, Yamada Y, Yeh Y, Chen WT. A mechanism for regulation of melanoma invasion. Ligation of alpha6beta1 integrin by laminin G peptides. J Biol Chem. 1996;271:27221–27224. doi: 10.1074/jbc.271.44.27221. [DOI] [PubMed] [Google Scholar]
- 43.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 44.Kim MH, Kitson RP, Albertsson P, Nannmark U, Basse PH, Kuppen PJ, Hokland ME, Goldfarb RH. Secreted and membrane-associated matrix metalloproteinases of IL-2-activated NK cells and their inhibitors. J Immunol. 2000;164:5883–5889. doi: 10.4049/jimmunol.164.11.5883. [DOI] [PubMed] [Google Scholar]
- 45.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 46.Andela VB, Schwarz EM, Puzas JE, O'Keefe RJ, Rosier RN. Tumor metastasis and the reciprocal regulation of prometastatic and antimetastatic factors by nuclear factor kappaB. Cancer Res. 2000;60:6557–6562. [PubMed] [Google Scholar]
- 47.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]


