Abstract
AIM: To investigate the apoptosis-inducing effect of Caspases-3 expressed by constructed eukaryotic vector on gastric cancer cell line SGC7901.
METHODS: PCR was employed to amplify the sequences of both small and large subunits of Caspases-3. Its products were separately cloned into the Sma I site of pBluescript KS+ to generate both plasmids pBS/SS and pBS/LS. The small subunit fragment was excised from plasmid pBS/SS with BamH I and then inserted into the BamH I site of plasmid pBS/LS preceding that of the large subunit to yield plasmid pBS/Rev-Caspase-3. Rev-Caspase-3 cDNA was excised with Kpn I + Xba I and then subcloned into plasmid pcDNA3.1 (+) to construct Rev-Caspase-3 eukaryotic expression vector pcDNA/Rev-Caspase-3, which was used to transiently transfect SGC7901 cell line. Cell count, MTT assay and electron microscopy were used to confirm the antiproliferation and apoptosis-inducing effect of Rev-Caspase-3 expression on gastric cancer cells.
RESULTS: Plasmid pBS/Rev-Caspase-3 and eukaryotic expression vector pcDNA/Rev-Caspase-3 were successfully constructed. SGC7901 cells were transiently transfected by either pcDNA/Rev-Caspase-3 or pcDNA3.1 (+) for 24, 48, 72, and 96 h respectively. Cell growth was measured by cell count and MTT assay. In cell count assay, the cell numbers were 1.8 × 106, 1.55 × 106, 2.0 × 106, and 3.1 × 106 in the experimental group and 2.5 × 106, 3.1 × 106, 4.0 × 106, and 5.7 × 106 in the control group at 24, 48, 72 and 96 h respectively. The growth of SGC7901 cells was suppressed by Rev-Caspase-3 in a time-dependent manner (P < 0.05). The results of MTT assay were similar to that of cell count (P < 0.05). The characteristics of apoptosis such as chromatin condensation, crescent formation and margination were seen and more obvious with time in the given-experimental period in the experimental group, but not easily observed in the control group.
CONCLUSION: The expression of Rev-Caspase-3 by the constructed eukaryotic vector can significantly induce apoptosis of gastric cancer cell line SGC7901, which may exhibit a potential way in gastric cancer gene therapy.
INTRODUCTION
Apoptosis is closely related to tumor[1-10]. Although there are many factors involved in apoptotic program[11-14], Caspases are shown to play a major role in the transduction of the apoptotic signal and the execution of apoptosis in mammalian[15-19]. Caspases belong to cysteine proteases family[20] and share several common features such as all homologous to interleukin-1-β-converting enzyme (ICE)[21] containing conserved QACR/QGC sequence[22] existing as inactive zymogens activated by cleavages specific internal ASP residues in interdomain linkers being able to cleave their substrates ASP residues, and consisting of prodomain, small subunit and large subunit.
So far 15 members of Caspases have been reported in the literature. According to their structure and function, Caspases are divided into two classes, the initiator and executor Caspases. The initiator Caspases carry long prodomain, and can process and activate their own and other inactive Caspase zymogens when triggered by a death signal[23,24]. The executor Caspases such as Caspases-3, however, lack the long prodomain and remain to be dormant until the initiator Caspases activate them by direct proteolysis[25]. Once activated, they dismantle cell regulatory components rapidly, leading to the typical changes observed in cell apoptosis[26-28].
Interestingly, by making small subunit fragment preceding that of the large subunit, Srinivasula et al[29] constructed a recombinant Caspases-3, which could simulate the three-dimensional structure of activated Caspases-3 and was capable of being catalyzed and inducing MCF-7 cell apoptosis without initiator Caspases’ proteolysis. In this study, the eukaryotic expression vector of recombinant Caspases-3 was constructed and the apoptosis-inducing effect of its expression on SGC7901 cell line was observed.
MATERIALS AND METHODS
Subclone of both small and large subunits
Plasmid pcDNA/Caspases-3 contains all the cDNA sequences of Caspases-3 gene. It was used as template to amplify the sequences of small and large subunits of Caspases-3 by PCR. The sequences of four pairs of primers were as follows: LS-forward (P1), 5’-ATGGAGAACACTGAAAACTCAG-3’;LS-reverse(P2), 5’-GTCATCATCAACACCTCAGTCT-3’; SS-forward (P3), 5’-GGATCCATGATTGAGACAGACAGTGG-3’; SS-reverse (P4), 5’-ATCAACTTCATCGTGATAAAAATAGAGTTC-3’.
PCR was performed in 50 μL reactive volume containing 2 μL cDNA, 5 μL PCR buffer, 2 μL dNTP, 1 μL primer, and 1 μL Taq DNA polymerase. The samples were subjected to 30 thermal cycles for 5 min at 95 °C for pre-denaturation, for 30 s at 94 °C for denaturing, for 30 s at 56 °C for annealing, for 50 s at 72 °C for extension, and for 7 min at 72 °C for final extension after the last cycle. The PCR products were separately cloned into the Sma I site of pBluescript KS+ to construct pBS/SS and pBS/LS, which were confirmed by Sma I digestion and DNA sequencing.
Construction of recombinant Caspases-3 (Rev-Caspaes-3)
The small subunit fragment was excised from plasmid pBS/SS with BamH I and then inserted into the BamH I site of plasmid pBS/LS to yield plasmid pBS/Rev-Caspases-3 with the small subunit fragment preceding that of the large subunit, which was verified with BamH I + Kpn I digestion and PCR method. The primers were SS-forward and LS-reverse.
Construction of Rev-Caspases-3 eukaryotic expression vector pcDNA/Rev-Caspases-3
Eukaryotic expression vector pcDNA3.1 (+) was cleaved and linearized with Kpn I + Nhe I, meanwhile, Rev-Caspases-3 cDNA was excised from plasmid pBS/Rev-Caspases-3 with Kpn I + Xba I. Both fragments were ligated each other with T4 DNA ligase to produce plasmid pcDNA/Rev-Caspases-3, which was proved with Stu I + Kpn I.
Cell line and cell curltrue
Cell line SGC7901 was derived from a moderately-differentiated gastric adenocarcinoma and characterized extensively. It was maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 200 IU·mL-1 penicillin and 50 μg·mL-1 streptomycin at 37 °C in a humidified atmosphere of 5% CO2.
Cell transfection, cell count and electron microscopy
Cells were seeded in a 6-well plate with 2 × 105 cells per well 24 h prior to transfection when they were cultured to a confluency of about 90%. Cell transfection was performed according to the manufacturers’ instructions. Briefly, a transfection mixture was prepared by adding 6 μg of plasmid DNA and 20 μL lipofectame (GIBCO BRL) to 500 μL serum-free RPMI1640. After incubated at room temperature for 20 min, the transfection mixture was added to the cells to be cultivated for 4 h at 37 °C when the media containing the transfection mixture was exchanged for growth medium. Cells were harvested and counted at 24, 48, 72, and 96 h after transfection. Some cells were fixed in 40 g·L-1 glutaraldehyde, then post-fixed in osmium tetroxide and embedded in Epon. Ultrathin sections were prepared, stained with wranyl acetate and lead citrate, examined under a transmission electron microscope to identify the morphological changes of apoptosis.
MTT assay
Antiproliferation effects of recombinant Caspases-3 were measured by MTT assay. SGC7901 cells were seeded in 96-well microtitre plates with 1 × 104 cells per well and incubated overnight in 100 μL of culture media. Then the cells were transiently transfected with 0.3 μg DNA of plasmid pcDNA/Rev-Caspases-3 for 24, 48, 72, and 96 h respectively. The cells were added with 100 μL MTT (1g·L-1) and further incubated for 4 h. After supernatant was removed, the cells were added with 100 μL DMSO per well and cultivated for 30 min. The absorbance at 490 nm was measured by a micro-ELISA reader. At the same time, the SGC7901 cells transfected with plasmid pcDNA3.1 (+) were served as control. Each assay was repeated three times.
Statistics
Comparison of the data among the groups was carried out using the Bonferroni-Dun multiple comparisons. In each case, P values less than 0.05 were considered statistically significant.
RESULTS
Construction of Rev-Caspaes-3 and pcDNA/Rev-Caspases-3
The construction strategies of recombinant Caspases-3 and eukaryotic expression plasmid pcDNA/Rev-Caspases-3 are shown in Figure 1.
Figure 1.
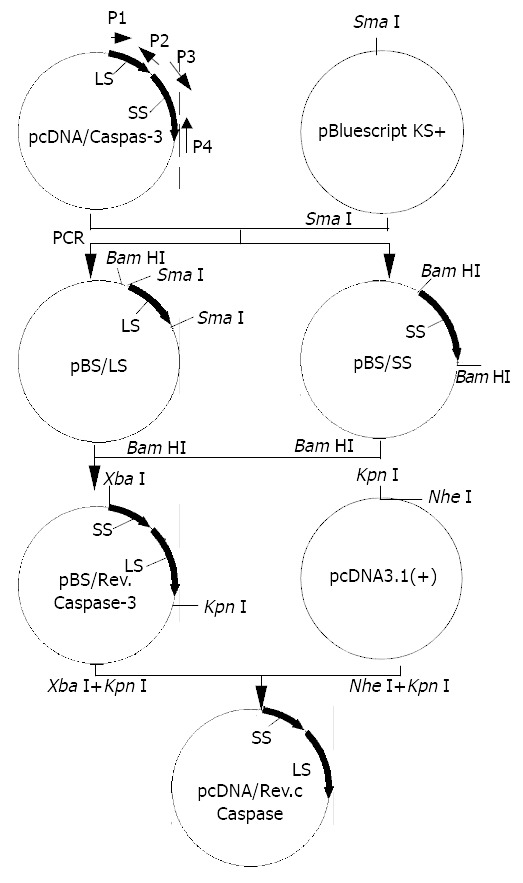
Schematic map of constructions of recombinant Caspases-3 and eukaryotic expression plasmid pcDNA/Rev-Caspases-3.
Both small and large subunit sequences of Caspases-3 were successfully amplified by PCR and subsequently cloned into Sma I site of pBluescript KS+ to yield pBS/SS and pBS/LS respectively, which were confirmed by Sma I digestion and DNA sequencing. The small subunit fragment was excised from plasmid pBS/SS with BamH I and cloned into BamH I site of plasmid pBS/LS to form recombinant plasmid pBS/Rev-Caspases-3 with the small subunit fragment located in-frame 5’ to that of the large subunit, which was identified by PCR using SS-forward and LS-reverse as primers. The length of PCR product was 882 bp (Figure 2). Rev-Caspases-3 cDNA was excised from plasmid pBS/Rev-Caspases-3 with Kpn I + Xba I and orientatively cloned into Kpn I and Nhe I site of eukaryotic expression vector pcDNA3.1 (+) to construct plasmid pcDNA/Rev-Caspases-3, which was proved by the digestion of Stu I + Kpn I with a 550 bp fragment release (Figure 3).
Figure 2.
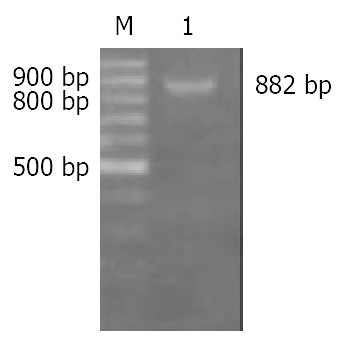
Identification of plasmid pBS/Rev-Caspase-3 by PCR with SS-forward and LS-reverse as primers. M: 100 bp ladder DNA marker, 1: PCR product, 882 bp.
Figure 3.
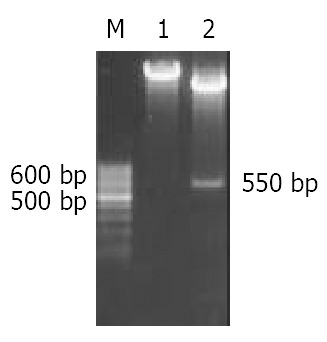
Enzyme digestion analysis of plasmid pcDNA/Rev-Caspase-3. M: 100 bp ladder DNA marker, 1: pcDNA/Rev-Caspase-3 cut with Stu I, 2: pcDNA/Rev-Caspase-3 cut with
Effect of Rev-Caspases-3 on SGC7901 cell growth
SGC7901 cells were transiently transfected by either pcDNA/Rev-Caspases-3 (the experimental group) or pcDNA3.1 (+) (the control group) for 24, 48, 72, and 96 h respectively. Cell growth was measured by cell count and MTT assay. In cell count assay, the cell numbers were 1.8 × 106, 1.55 × 106, 2.0 × 106, and 3.1 × 106 in the experimental group and 2.5 × 106, 3.1 × 106, 4.0 × 106, and 5.7 × 106 in the control group at 24, 48, 72, and 96 h respectively. The growth of SGC7901 cells was suppressed by Rev-Caspases-3 in a time-dependent manner (Figure 4, P < 0.05). The results of MTT assay were similar to that of cell count (Figure 5, P < 0.05).
Figure 4.
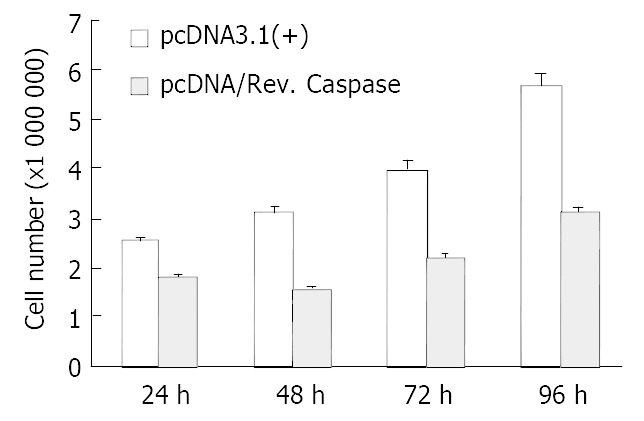
Comparison of cell count between SGC7901 cells trans-fected with pcDNA3.1 (+) and pcDNA/Rev. Caspase.
Figure 5.
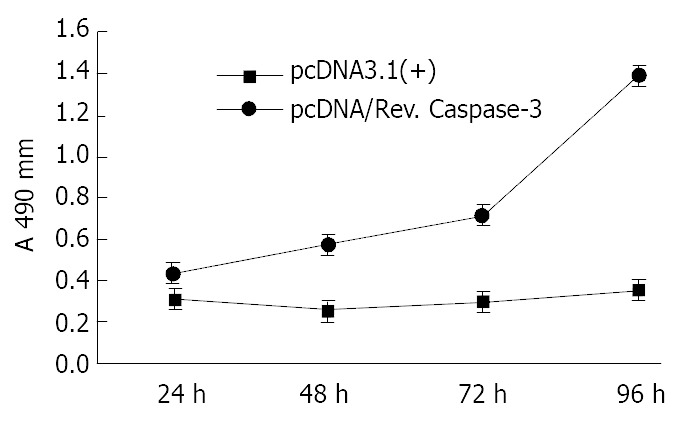
Comparison of MTT assays between SGC7901 cells transfected with pcDNA3.1 (+) and pcDNA/Rev.Caspase-3.
Induction of apoptosis in SGC7901 by Rev-Caspases-3
The ultrastructural characteristics of apoptosis such as chromatin condensation, crescent formation, and margination were seen by electron microscopy in the experimental group, which were more obvious with time in the given-experimental period and not easily observed in the control group (Figure 6).
Figure 6.
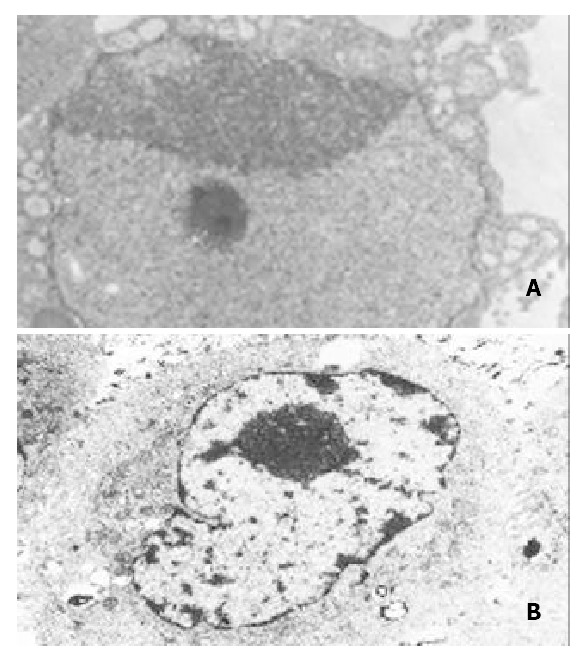
Electron microscopy of SGC7901 cells transfected with pcDNA3.1 (+) or pcDNA/Rev.Caspases-3. A: SGC7901 trans-fected with pcDNA/Rev. Caspases-3. Notice the chromatin condensation, crescent formation, original margination (× 5000); B: SGC7901 transfected with pcDNA3.1 (+), Notice no characteristics of apoptosis (× 5000).
DISCUSSION
Caspases belong to cysteine protease family. So far, more than 15 members of Caspases have been reported in the literature, and most of them play a major role in apoptosis, participating in the initiation and execution of programmed cell death[30-33]. Based on their structure and function, Caspases are classified into two groups, initiator (such as Caspases-2,-8 and 10) and executor Caspases (such as Caspases-3, -6 and 7)[33-35]. Structurally, Caspase is made up of prodomain, large and small subunits, of which prodomain is located in N-terminus, small subunit in C-terminus and large subunit between. Initiator Caspases bear a long prodomain, whereas the executor Caspases do not[36-38].
Caspases are synthesized as harmless inactive zymogens and generally activated by cleavages specific internal ASP residues present in interdomain linkers, which manifests as a hierarchical process. When triggered by a death signal, the initiator Caspases are recruited through their long prodomain by specialized adaptor molecules to form the death-inducing signaling complex (DISC) or Apaf. Because of the trimeric nature of the DISC, three Caspase molecules are brought close, which will be useful to activate initiator Caspases[39-40]. When activated, the initiator Caspase can activate itself and other inactive caspase zymogens including executor Caspases that are activated by direct proteolysis. The activated executors then rapidly dismantle important cellular components, leading to cell apoptosis.
The three-dimensional structure of activated Caspases-3 has been shown that the C-terminus of large subunit and the N-terminus of small subunit are separated far from each other, whereas the N-terminus of large subunit and C-terminus of small subunit are closed to each other[41,42]. Based on these observations, Srinivasula et al[29] successfully engineered recombinant Caspases-3 and -6 precursors by switching their subunits order at a gene level, in which the small subunit fragment was fused in frame N terminal to that of the large subunit. Thus the N terminus of small subunit and the C terminus of large subunit are free, while the C terminus of the small subunit and the N terminus of the large subunit are connected each other through a linker. These recombinant molecules could be activated spontaneously to induce apoptosis in MCF-7 cells independent of initiator Caspases. Jia et al[43] have also confirmed the apoptosis-inducing effect of recombinant Caspases-3 on HeLa cells.
Gastric carcinoma is one of the most common causes of malignancy-related death in China[44-48]. In the present study, to test the effect of recombinant Caspases-3 on gastric cancer cell, we constructed a eukaryotic expression vector of constitutively active recombinant Caspases-3 and used it to transiently transfect gastric cancer cell SGC7901. The results showed that the expressed recombinant Caspases-3 could inhibit the growth of SGC7901 in a time dependent manner. In addition, the apoptosis-inducing effect of recombinant Caspases-3 on SGC7901 was also evident. This study demonstrates the possible use of recombinant Caspases-3 in gastric cancer gene therapy. But the effects of growth inhibition and apoptosis induction conducted by recombinant Caspases-3 on other cell lines of gastric cancer or on gastric cancer cell in vivo need to be further investigated.
Footnotes
Edited by Zhu L, Zhang JZ and Wang XL
References
- 1.Sauer G, Deissler H, Kurzeder C, Kreienberg R. New molecular targets of breast cancer therapy. Strahlenther Onkol. 2002;178:123–133. doi: 10.1007/s00066-002-0957-0. [DOI] [PubMed] [Google Scholar]
- 2.Castiglioni P, Martin-Fontecha A, Milan G, Tomajer V, Magni F, Michaelsson J, Rugarli C, Rosato A, Bellone M. Apoptosis-dependent subversion of the T-lymphocyte epitope hierarchy in lymphoma cells. Cancer Res. 2002;62:1116–1122. [PubMed] [Google Scholar]
- 3.Paczesny S, Beranger S, Salzmann JL, Klatzmann D, Colombo BM. Protection of mice against leukemia after vaccination with bone marrow-derived dendritic cells loaded with apoptotic leukemia cells. Cancer Res. 2001;61:2386–2389. [PubMed] [Google Scholar]
- 4.Amin S, Robins RA, Maxwell-Armstrong CA, Scholefield JH, Durrant LG. Vaccine-induced apoptosis: a novel clinical trial end point. Cancer Res. 2000;60:3132–3136. [PubMed] [Google Scholar]
- 5.Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-kB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431–435. doi: 10.3748/wjg.v8.i3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng RH, Zhu ZG, Li JF, Liu BY, Yan M, Yin HR, Lin YZ. Inhibition of human telomerase in MKN-45 cell line by antisense hTR expression vector induces cell apoptosis and growth arrest. World J Gastroenterol. 2002;8:436–440. doi: 10.3748/wjg.v8.i3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao HQ, Zou SC. Effect of preoperative regional artery chemotherapy on proliferation and apoptosis of gastric carcinoma cells. World J Gastroenterol. 2002;8:451–454. doi: 10.3748/wjg.v8.i3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu K, Zhao Y, Liu BH, Li Y, Liu F, Guo J, Yu WP. RRR-alpha-tocopheryl succinate inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2002;8:26–30. doi: 10.3748/wjg.v8.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779–782. doi: 10.3748/wjg.v7.i6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry F, Bretaudeau L, Hequet A, Barbieux I, Lieubeau B, Meflah K, Grégoire M. Role of antigen-presenting cells in long-term antitumor response based on tumor-derived apoptotic body vaccination. Pathobiology. 1999;67:306–310. doi: 10.1159/000028086. [DOI] [PubMed] [Google Scholar]
- 11.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 12.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 13.Wei XC, Wang XJ, Chen K, Zhang L, Liang Y, Lin XL. Killing effect of TNF-related apoptosis inducing ligand regulated by tetracycline on gastric cancer cell line NCI-N87. World J Gastroenterol. 2001;7:559–562. doi: 10.3748/wjg.v7.i4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golstein P. Cell death: TRAIL and its receptors. Curr Biol. 1997;7:R750–R753. doi: 10.1016/s0960-9822(06)90000-1. [DOI] [PubMed] [Google Scholar]
- 15.Choi YH, Kim MJ, Lee SY, Lee YN, Chi GY, Eom HS, Kim ND, Choi BT. Phosphorylation of p53, induction of Bax and activation of caspases during beta-lapachone-mediated apoptosis in human prostate epithelial cells. Int J Oncol. 2002;21:1293–1299. [PubMed] [Google Scholar]
- 16.Grabarek J, Du L, Johnson GL, Lee BW, Phelps DJ, Darzynkiewicz Z. Sequential activation of caspases and serine proteases (serpases) during apoptosis. Cell Cycle. 2002;1:124–131. [PubMed] [Google Scholar]
- 17.Knoblach SM, Nikolaeva M, Huang X, Fan L, Krajewski S, Reed JC, Faden AI. Multiple caspases are activated after traumatic brain injury: evidence for involvement in functional outcome. J Neurotrauma. 2002;19:1155–1170. doi: 10.1089/08977150260337967. [DOI] [PubMed] [Google Scholar]
- 18.Shiraga S, Adamus G. Mechanism of CAR syndrome: anti-recoverin antibodies are the inducers of retinal cell apoptotic death via the caspase 9- and caspase 3-dependent pathway. J Neuroimmunol. 2002;132:72–82. doi: 10.1016/s0165-5728(02)00314-4. [DOI] [PubMed] [Google Scholar]
- 19.Hetz CA, Hunn M, Rojas P, Torres V, Leyton L, Quest AF. Caspase-dependent initiation of apoptosis and necrosis by the Fas receptor in lymphoid cells: onset of necrosis is associated with delayed ceramide increase. J Cell Sci. 2002;115:4671–4683. doi: 10.1242/jcs.00153. [DOI] [PubMed] [Google Scholar]
- 20.Harvey NL, Kumar S. The role of caspases in apoptosis. Adv Biochem Eng Biotechnol. 1998;62:107–128. doi: 10.1007/BFb0102307. [DOI] [PubMed] [Google Scholar]
- 21.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 22.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes-Alnemri T, Armstrong RC, Krebs J, Srinivasula SM, Wang L, Bullrich F, Fritz LC, Trapani JA, Tomaselli KJ, Litwack G, et al. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 27.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 28.Meller R, Skradski SL, Simon RP, Henshall DC. Expression, proteolysis and activation of caspases 6 and 7 during rat C6 glioma cell apoptosis. Neurosci Lett. 2002;324:33–36. doi: 10.1016/s0304-3940(02)00166-0. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasula SM, Ahmad M, MacFarlane M, Luo Z, Huang Z, Fernandes-Alnemri T, Alnemri ES. Generation of constitutively active recombinant caspases-3 and -6 by rearrangement of their subunits. J Biol Chem. 1998;273:10107–10111. doi: 10.1074/jbc.273.17.10107. [DOI] [PubMed] [Google Scholar]
- 30.Huo J, Luo RH, Metz SA, Li G. Activation of caspase-2 mediates the apoptosis induced by GTP-depletion in insulin-secreting (HIT-T15) cells. Endocrinology. 2002;143:1695–1704. doi: 10.1210/endo.143.5.8810. [DOI] [PubMed] [Google Scholar]
- 31.Distelhorst CW. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ. 2002;9:6–19. doi: 10.1038/sj.cdd.4400969. [DOI] [PubMed] [Google Scholar]
- 32.Heinke MY, Yao M, Chang D, Einstein R, dos Remedios CG. Apoptosis of ventricular and atrial myocytes from pacing-induced canine heart failure. Cardiovasc Res. 2001;49:127–134. doi: 10.1016/s0008-6363(00)00242-x. [DOI] [PubMed] [Google Scholar]
- 33.Riedl SJ, Fuentes-Prior P, Renatus M, Kairies N, Krapp S, Huber R, Salvesen GS, Bode W. Structural basis for the activation of human procaspase-7. Proc Natl Acad Sci USA. 2001;98:14790–14795. doi: 10.1073/pnas.221580098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 35.Cuvillier O, Edsall L, Spiegel S. Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J Biol Chem. 2000;275:15691–15700. doi: 10.1074/jbc.M000280200. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Shin NH, Sun Y, Wang KK. Molecular cloning and characterization of a novel caspase-3 variant that attenuates apoptosis induced by proteasome inhibition. Biochem Biophys Res Commun. 2001;283:762–769. doi: 10.1006/bbrc.2001.4871. [DOI] [PubMed] [Google Scholar]
- 37.Seol DW, Billiar TR. A caspase-9 variant missing the catalytic site is an endogenous inhibitor of apoptosis. J Biol Chem. 1999;274:2072–2076. doi: 10.1074/jbc.274.4.2072. [DOI] [PubMed] [Google Scholar]
- 38.Meergans T, Hildebrandt AK, Horak D, Haenisch C, Wendel A. The short prodomain influences caspase-3 activation in HeLa cells. Biochem J. 2000;349:135–140. doi: 10.1042/0264-6021:3490135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Chang HY, Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell. 1998;1:319–325. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 40.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 41.Mittl PR, Di Marco S, Krebs JF, Bai X, Karanewsky DS, Priestle JP, Tomaselli KJ, Grütter MG. Structure of recombinant human CPP32 in complex with the tetrapeptide acetyl-Asp-Val-Ala-Asp fluoromethyl ketone. J Biol Chem. 1997;272:6539–6547. doi: 10.1074/jbc.272.10.6539. [DOI] [PubMed] [Google Scholar]
- 42.Rotonda J, Nicholson DW, Fazil KM, Gallant M, Gareau Y, Labelle M, Peterson EP, Rasper DM, Ruel R, Vaillancourt JP, et al. The three-dimensional structure of apopain/CPP32, a key mediator of apoptosis. Nat Struct Biol. 1996;3:619–625. doi: 10.1038/nsb0796-619. [DOI] [PubMed] [Google Scholar]
- 43.Jia LT, Yu CJ, Xu YM, Zhu F, Peng WD, Su CZ, Wang CJ, Yang AG. Construction and effect of Caspases-3 fusion proteins and their apoptosis induction in HeLa cells. Disi Junyi Daxue Xuebao. 2001;12:1057–1060. [Google Scholar]
- 44.Wang CD, Chen YL, Wu T, Liu YR. Association between lowe expression of somatostatin receptor II gene and lymphoid me-tastasis in patients with gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:864–866. [Google Scholar]
- 45.Gu HP, Ni CR, Zhan RZ. Relationship between CD15 mRNA and its protein expression and gastric carcinoma invasion. Shijie Huaren Xiaohua Zazhi. 2000;8:851–854. [Google Scholar]
- 46.Wang DX, Fang DC, Liu WW. Study on alteration of multiple genes in intestinal metaplasia, atypical hyperplasia and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:855–859. [Google Scholar]
- 47.Guo SY, Gu QL, Liu BY, Zhu ZG, Yin HR, Lin YZ. Experimental study on the treatment of gastric cancer by TK gene combined with mIL-2 gene. Shijie Huaren Xiaohua Zazhi. 2000;8:974–978. [Google Scholar]
- 48.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]


