Abstract
AIM: To study the effect of hydroxyapatite (HAP) nanoparticles on human hepatoma cell line BEL-7402 in vitro.
METHODS: The human hepatoma cell line BEL-7402 was cultured and treated with HAP nanoparticles at various concentrations. Growth suppression was detected with MTT colorimetric assay, cell apoptotic alterations were evaluated by cytochemical staining (Hoechst 33258), transmission electron microscopy (TEM), and flow cytometry (FCM).
RESULTS: HAP nanoparticles inhibited the growth of hepatoma cells in a dose-dependent manner, with IC50 values of 29.30 mg/L. Treated with 50-200 mg/L HAP nanoparticles for 48 h, BEL-7402 cells apoptosis with nuclear chromatin condensation and fragmentation as well as cell shrinkage and the formation of apoptotic bodies were observed under cytochemical staining and transmission electron microscopy. FCM analysis showed hypodiploid peaks on histogram, the apoptotic rates at the concentrations of 50, 75, 100, 150 and 200 mg/L of HAP nanoparticles were 20.35 ± 2.23%, 25.35 ± 1.92%, 29.34 ± 4.61%, 44.92 ± 3.78% and 53.64 ± 3.49%, respectively, which were all significantly higher than that of control group 2.23 ± 0.14%. There was a significant correlation between HAP nanoparticle concentration and apoptotic rate (r = 0.994, P < 0.01).
CONCLUSION: HAP nanoparticles not only inhibit proliferation but also induce apoptosis of human hepatoma cell line BEL-7402 in vitro.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver. Although progress in diagnosis and treatment of hepatic carcinoma has been made in recent years, its prognosis is still poor. Exploring new materials for treatment and investigating the mechanism are important. With the development of nanometer technology, hydroxyapatite nanoparticle, a novel inorganic material, was found to be able to inhibit tumor cell proliferation. However, there was yet no report about the effects of hydroxyapatite nanoparticles on the growth and apoptosis of human hepatoma cell line BEL-7402. Therefore, the present study was designed to study the inhibitory effect of hydroxyapatite nanoparticles on human hepatoma cells and its mechanism to provide a theoretical basis for its clinical use.
MATERIALS AND METHODS
Hydroxyapatite nanoparticles were obtained from Institute of Biomaterials, East China University of Science and Technology. Stock solution was made at the concentration of 400 mg/L with RPMI 1640 and diluted to working concentration before use. RPMI 1640, fetal calf serum (FCS) and MTT were purchased from GIBCO. RNase, proteinase K, propidium iodide (PI) and Hoechst 33258 were products of Sigma Chemical Co.
Cell line and culture
Human hepatoma BEL-7402 cells were provided by China Center for Type Culture Collection of Wuhan University (CCTCC). The cells were grown as monolayers in RPMI1640 medium supplemented with 10% heat-inactived fetal calf serum and incubated in humidified incubator with 5% CO2 in air at 37 °C.
Assay of cell proliferation
MTT[3-(4,5-dimethythiazoyl-2-yl)2,5-diphenylte-trozoliumbromide] colorimetric analysis was used to measure the cytocide rate of HAP nanoparticles. Well-growing BEL-7402 cells were collected and seeded in 96-well plates at 1 × 105/mL density 100 μl cell suspension per well. When the cells anchored to the plates, the culture medium was replaced with fresh medium containing various concentrations of HAP nanoparticles (0, 12.5, 25, 50, 75, 100, 150 and 200 mg/L). Eight duplicate wells were set up in each sample. After incubated at 37 °C, 5% CO2 for 48 h, 5 g/L MTT 20 μl was added to each well and cultured for another 4 h. The MTT medium was discarded and warm dimethylsulfoxide (DMSO) 200 μl was added. Absorbance was measured at 490 nm. Cellular proliferation inhibition rate (CPIR) was calculated using the following formula: CPIR = (1 - average A value of experimental group/average A value of control group) × 100%.
Cytochemical staining of apoptotic cells
Cells treated with 50, 75, 100, 150 and 200 mg/L HAP nanoparticles for 48 h were harvested and fixed with 1:3 glacial acetic acid/methanol twice, first for 5 min and then for 10 min and washed with phosphate buffer solution (PBS). Cells were resuspended in Hoechst 33258 solution (Hoechst 33258 dissolved in PBS, 5 mg/L) and incubated at room temperature for 45 min in the dark. Aliquots of 100 μL/mL were placed on a glass slide and observed under an Olympus BH-2 fluorescence microscope after dried thoroughly.
Transmission electron microscopy assay
The BEL-7402 cells were seeded in culture flasks. Three culture bottles were divided into treatment group and control group. When the cells anchored to the plates, various concentrations (0, 50 and 100 mg/L) of HAP nanoparticles were added and the cells were incubated at 37 °C, 5% CO2 for 48 h. Hepatoma cells were then digested by 0.25% trypsinase and collected. After rinsed with PBS, the cells were prefixed with 3% glutaraldehyde for 30 min, post-fixed with 1% osmic acid, dehydrated in graded ethanol, embedded in Epon 812 mixture, and cut into sections on an ultramicrotome. The cells were observed under Hitachi H-600 electron microscopy.
Flow cytometric analysis
The suspended single cell solutions subjected to treatment with HAP nanoparticles at different concentrations (0, 50, 75, 100, 150 and 200 mg/L) for 48 h were harvested. Each group had three culture bottles. Cells were washed with PBS, fixed with 70% ethanol at -20 °C for 30 min and stored at 4 °C overnight, then washed with PBS again, treated with 100 mg/L RNase 100 μL at 37 °C for 30 min and stained with 50 mg/L PI 100 μL at 4 °C for 30 min in darkness. Apoptotic cells were assayed using FACSort Becton Dickinson Flow Cytometer at 488nm and data were analyzed with CELLQuest Software. For each sample, 6000 cells were measured.
Statistical analysis
All data were expressed as mean ± standard deviation. Statistical analysis was performed by t test using software SPSS11.0 for Windows. P < 0.05 was considered significant.
RESULTS
Effect of HAP nanoparticles at various concentrations on the growth of BEL-7402 cells
After 48 h treatment, HAP nanoparticles produced a dose-dependent inhibition of cell growth (Table 1). Correlation analysis displayed a significantly positive correlation between the concentration of HAP and inhibition rate (IR) (r = 0.931, P < 0.01). Regression equation was as follows: yconcentration = 966.09 x3IR-292.20xIR+54.63, the half effective inhibitory concentration (IC50) = 29.30 mg/L.
Table 1.
Inhibitory effect of HAP nanoparticles on hepatoma cell line BEL-7402
| Concentrations (mg/L) | A490nm (-x ± s) | Inhibition rate (%) | t vs control |
| 0 (control) | 0.9325 ± 0.0337 | ||
| 12.5 | 0.5275 ± 0.0440bd | 43.29 ± 6.11 | 16.68 |
| 25 | 0.4025 ± 0.0440b | 56.90 ± 3.71 | 51.20 |
| 50 | 0.3950 ± 0.0593b | 57.74 ± 5.37 | 37.43 |
| 75 | 0.4038 ± 0.0245b | 56.61 ± 3.61 | 29.10 |
| 100 | 0.3913 ± 0.0323b | 58.05 ± 3.05 | 46.20 |
| 150 | 0.3188 ± 0.0236bd | 65.78 ± 2.83 | 42.30 |
| 200 | 0.2588 ± 0.0270bd | 72.25 ± 7.75 | 53.79 |
aP < 0.05,
P < 0.01 vs 0 mg/L group; cP < 0.05,
P < 0.01 vs 25 mg/L group.
Fluorescence microscopic observation
Hoechst 33258 is a specific DNA-binding fluorochrome and under the fluorescence microscope it exhibits a green fluorescence. As shown in Figure 1, in the control group, the nucleus was big and round, with a smooth nuclear membrane, free of condensation and fragmentation. However, smaller nuclei, increased density of nuclear chromatin, fragmentation of nucleus and apoptotic body formation were easily identified in HAP nanoparticles treated groups. The higher the concentration of HAP nanoparticles, the more apoptotic cells there were. Apoptotic rate was counted under fluorescence microscopy, there were 18.68%, 22.27%, 33.49%, 49.03%, 57.16% at 50, 75, 100, 150 and 200 mg/L concentration, respectively.
Figure 1.
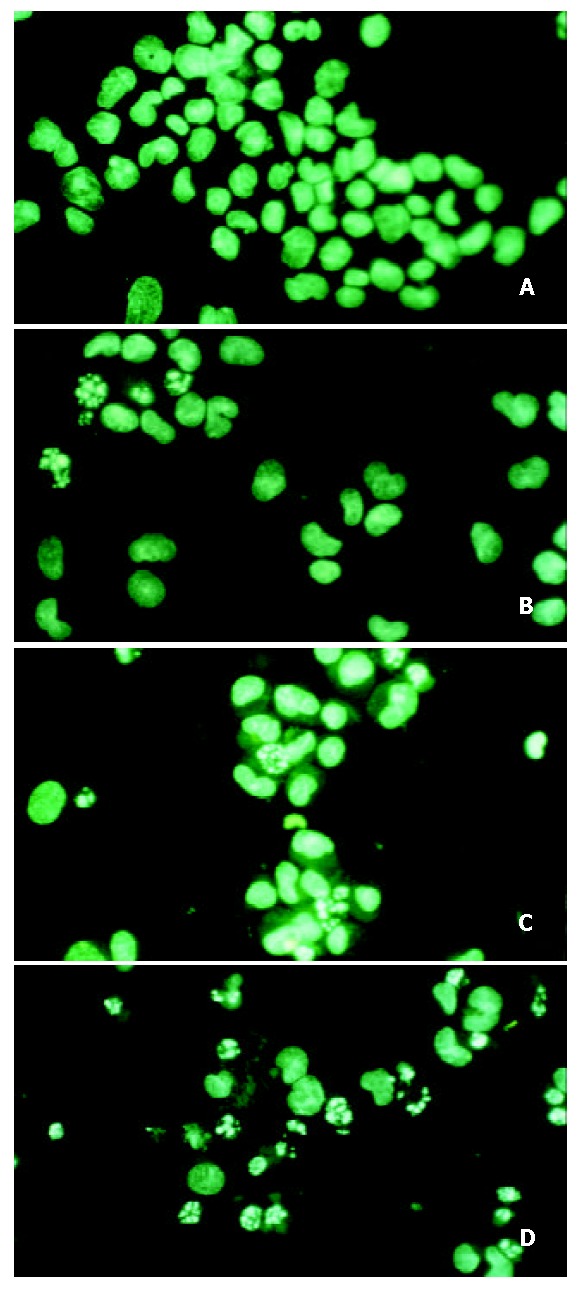
Fluorescence microscopy for apoptosis induced by HAP nanoparticles for 48 h (× 200). A: Control; B: 50 mg/L; C: 100 mg/L; D: 200 mg/L.
Transmission electron microscopic observation
Under transmission electron microscopy, control cells were big and round, with intact nuclear membrane and low density in nuclear chromatin (Figure 2A). However, the cells treated with HAP nanoparticles exhibited characteristics of apoptosis including cell membrane shrinkage, cytoplasm budding, condensation and fragmentation of nuclear chromatin adjacent to nuclear membrane (Figure 2B).
Figure 2.

TEM ultrastructural changes of apoptosis induced by HAP nanoparticles for 48 h. A: Control × 15000; B: 10 mg/L × 10000.
Flow cytometry analysis
The hypodiploid peak appearing before the G1 phase on histogram was called apoptotic peak, which indicated reduced DNA content in apoptotic cells in FCM analysis. Table 2 shows that apoptotic rates increased with increasing concentrations of HAP nanoparticles. Correlation analysis showed a significantly positive correlation between concentration of HAP nanoparticles and apoptotic rate (r = 0.994, P < 0.01).
Table 2.
Apoptotic rates of HAP nanoparticles on hepatoma cell line BEL-7402
| Concentrations (mg/L) | Apoptotic rate (%) | t vs control |
| 0(control) | 2.23 ± 0.14 | |
| 50 | 20.35 ± 2.23b | 13.32 |
| 75 | 25.35 ± 1.92b | 22.55 |
| 100 | 29.34 ± 4.61b | 10.50 |
| 150 | 44.92 ± 3.78bc | 20.30 |
| 200 | 53.64 ± 3.49bd | 24.51 |
aP < 0.05,
P < 0.01 vs 0 mg/L group;
P < 0.05,
P < 0.01 vs 50 mg/L group.
DISCUSSION
Hydroxyapatite nanoparticles used in this study was synthesized by sol-gel method, with uniform particle size of 50 nm and was well dispread. After human hepatoma BEL-7402 cells treated at various concentrations of hydroxyapatite nanoparticles for 48 h, MTT colorimetric analysis showed that nanoparticles could significantly inhibit the proliferation of BEL-7402 cells, with IC50 values of 29.30 mg/L. Moreover, the inhibitory rates at 25, 50, 75 and 100 mg/L concentration of HAP nanoparticles were all more than 50%, but there was no statistical difference among the four treatment groups, which indicated the tolerance of human hepatoma BEL-7402 cells subjected to HAP nanoparticles. This would provide some experimental basis for its clinical use.
With cytochemical staining and under transmission electron microscopy, typical apoptotic alterations such as cells shrinkage, nuclear chromatin condensation and fragmentation, cytoplasmic budding and formation of apoptotic bodies were observed after BEL-7402 cells were treated by HAP nanoparticles for 48 h. Quantified by FCM, the higher the concentration of HAP nanoparticles, the more apoptotic cells there were. The apoptotic rates were in accordance with the results counted under fluorescence microscopy. At the same time, no statistical difference was found among the 50, 75 and 100 mg/L groups, in which the tolerance of BEL-7402 cells subjected to HAP nanoparticles was identified. Therefore, the present study demonstrated that HAP nanoparticles could induce human hepatoma cell apoptosis in vitro. This might be the first report regarding the antineoploastic mechanism of HAP nanoparticles on human hepatoma cells through apoptotic induction.
Kerr et al[1-19] first described the concept of apoptosis. It is the programmed death of cells by fragmentation of DNA, cell shrinkage, followed by cell fragmentation and formation of membrane vesicles called apoptosis bodies. A variety of studies have revealed that the uncontrolled growth of neoplasm is not only due to the over proliferation but also due to the loss of natural apoptosis. Therefore, exploring new medicine that could induce cancer cell apoptosis would be helpful for neoplasm treatment[20-23]. To date, chemotherapeutic medicines, growth inhibitor analogs, arsenic and various Chinese herbal medicines have been testified to suppress tumor cell growth by inducing cell apoptosis[24-38].
In this study, hydroxyapatite nanoparticles inhibited human hepatoma BEL-7402 cell growth and induced apoptosis, which may add a new way to use nanopaticles for tumor treatment. Further studies are needed to clarify the mechanism of apoptosis induced by hydroxyapatite nanoparticles.
Footnotes
Edited by Ma JY and Wang XL
References
- 1.He SW, Shen KQ, He YJ, Xie B, Zhao YM. Regulatory effect and mechanism of gastrin and its antagonists on colorectal carcinoma. World J Gastroenterol. 1999;5:408–416. doi: 10.3748/wjg.v5.i5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483–487. doi: 10.3748/wjg.v8.i3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao HQ, Zou SC. Effect of preoperative regional artery chemotherapy on proliferation and apoptosis of gastric carcinoma cells. World J Gastroenterol. 2002;8:451–454. doi: 10.3748/wjg.v8.i3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, Su WJ. Induction of apoptosis by TPA and VP-16 is through translocation of TR3. World J Gastroenterol. 2002;8:446–450. doi: 10.3748/wjg.v8.i3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka S, Akaike T, Fang J, Beppu T, Ogawa M, Tamura F, Miyamoto Y, Maeda H. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer. 2003;88:902–909. doi: 10.1038/sj.bjc.6600830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shigeno M, Nakao K, Ichikawa T, Suzuki K, Kawakami A, Abiru S, Miyazoe S, Nakagawa Y, Ishikawa H, Hamasaki K, et al. Interferon-alpha sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NF-kappa B inactivation. Oncogene. 2003;22:1653–1662. doi: 10.1038/sj.onc.1206139. [DOI] [PubMed] [Google Scholar]
- 8.Perez EA, Gandara DR, Edelman MJ, O'Donnell R, Lauder IJ, DeGregorio M. Phase I trial of high-dose tamoxifen in combination with cisplatin in patients with lung cancer and other advanced malignancies. Cancer Invest. 2003;21:1–6. doi: 10.1081/cnv-120016397. [DOI] [PubMed] [Google Scholar]
- 9.Lin HL, Lui WY, Liu TY, Chi CW. Reversal of Taxol resistance in hepatoma by cyclosporin A: involvement of the PI-3 kinase-AKT 1 pathway. Br J Cancer. 2003;88:973–980. doi: 10.1038/sj.bjc.6600788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita H, Iwase H, Toyama T, Fujii Y. Naturally occurring dominant-negative Stat5 suppresses transcriptional activity of estrogen receptors and induces apoptosis in T47D breast cancer cells. Oncogene. 2003;22:1638–1652. doi: 10.1038/sj.onc.1206277. [DOI] [PubMed] [Google Scholar]
- 11.Shen ZY, Shen J, Li QS, Chen CY, Chen JY, Yi Z. Morphological and functional changes of mitochondria in apoptotic esophageal carcinoma cells induced by arsenic trioxide. World J Gastroenterol. 2002;8:31–35. doi: 10.3748/wjg.v8.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen ZY, Shen WY, Chen MH, Shen J, Cai WJ, Yi Z. Nitric oxide and calcium ions in apoptotic esophageal carcinoma cells induced by arsenite. World J Gastroenterol. 2002;8:40–43. doi: 10.3748/wjg.v8.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun ZJ, Pan CE, Liu HS, Wang GJ. Anti-hepatoma activity of resveratrol in vitro. World J Gastroenterol. 2002;8:79–81. doi: 10.3748/wjg.v8.i1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Yang XK, Yu XX, Ge ML, Wang WL, Zhang J, Hou YD. Overexpression of p27(KIP1) induced cell cycle arrest in G(1) phase and subsequent apoptosis in HCC-9204 cell line. World J Gastroenterol. 2000;6:513–521. doi: 10.3748/wjg.v6.i4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YN, Chen JC, Yin SC, Wang GS, Tsauer W, Hsu SF, Hsu SL. Effector mechanisms of norcantharidin-induced mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer. 2002;100:158–165. doi: 10.1002/ijc.10479. [DOI] [PubMed] [Google Scholar]
- 17.Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223–227. doi: 10.3748/wjg.v6.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plas DR, Thompson CB. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol Metab. 2002;13:75–78. doi: 10.1016/s1043-2760(01)00528-8. [DOI] [PubMed] [Google Scholar]
- 19.Shan CM, Li J. Study of apoptosis in human liver cancers. World J Gastroenterol. 2002;8:247–252. doi: 10.3748/wjg.v8.i2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misawa M, Tauchi T, Sashida G, Nakajima A, Abe K, Ohyashiki JH, Ohyashiki K. Inhibition of human telomerase enhances the effect of chemotherapeutic agents in lung cancer cells. Int J Oncol. 2002;21:1087–1092. [PubMed] [Google Scholar]
- 21.Shoieb AM, Elgayyar M, Dudrick PS, Bell JL, Tithof PK. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int J Oncol. 2003;22:107–113. [PubMed] [Google Scholar]
- 22.Satomi D, Takiguchi N, Koda K, Oda K, Suzuki H, Yasutomi J, Ishikura H, Miyazaki M. Apoptosis and apoptosis-associated gene products related to the response to neoadjuvant chemotherapy for gastric cancer. Int J Oncol. 2002;20:1167–1171. [PubMed] [Google Scholar]
- 23.Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532–539. doi: 10.3748/wjg.v6.i4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792–796. doi: 10.3748/wjg.v8.i5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JM, Zhou H, Cai Q, Xiao GX. Role of mitochondrial dysfunction in hydrogen peroxide-induced apoptosis of intestinal epithelial cells. World J Gastroenterol. 2003;9:562–567. doi: 10.3748/wjg.v9.i3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L, Tai H, Li C, Zhang Y, Wang ZH, Ji WZ. Photodynamic inhibitory effects of three perylenequinones on human colorectal carcinoma cell line and primate embryonic stem cell line. World J Gastroenterol. 2003;9:485–490. doi: 10.3748/wjg.v9.i3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JK, Li J, Zhang J, Chen HB, Chen SB. Antitumor immunopreventive and immunotherapeutic effect in mice induced by hybrid vaccine of dendritic cells and hepatocarcinoma in vivo. World J Gastroenterol. 2003;9:479–484. doi: 10.3748/wjg.v9.i3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li QF, Ou-Yang GL, Peng XX, Hong SG. Effects of tachyplesin on the regulation of cell cycle in human hepatocarcinoma SMMC-7721 cells. World J Gastroenterol. 2003;9:454–458. doi: 10.3748/wjg.v9.i3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou HB, Zhu JR. Paclitaxel induces apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2003;9:442–445. doi: 10.3748/wjg.v9.i3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou HB, Yan Y, Sun YN, Zhu JR. Resveratrol induces apoptosis in human esophageal carcinoma cells. World J Gastroenterol. 2003;9:408–411. doi: 10.3748/wjg.v9.i3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu WB, Yang CQ, Jiang W, Wang YQ, Guo JS, He BM, Wang JY. Inhibition on the production of collagen type I, III of activated hepatic stellate cells by antisense TIMP-1 recombinant plasmid. World J Gastroenterol. 2003;9:316–319. doi: 10.3748/wjg.v9.i2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang ZH, Fan YF, Xia H, Feng HM, Tang FX. Effects of TNP-470 on proliferation and apoptosis in human colon cancer xenografts in nude mice. World J Gastroenterol. 2003;9:281–283. doi: 10.3748/wjg.v9.i2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu BW, Wu Y, Wang JL, Lin JS, Yuan SY, Li A, Cui WR. Study on the mechanism of epidermal growth factor-induced proliferation of hepatoma cells. World J Gastroenterol. 2003;9:271–275. doi: 10.3748/wjg.v9.i2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Liu FK, Qi XP, Li JS. The study of chemiluminescence in gastric and colonic carcinoma cell lines treated by anti-tumor drugs. World J Gastroenterol. 2003;9:242–245. doi: 10.3748/wjg.v9.i2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie DP, Chen LB, Liu CY, Liu JZ, Liu KJ. Effect of oxytocin on contraction of rabbit proximal colon in vitro. World J Gastroenterol. 2003;9:165–168. doi: 10.3748/wjg.v9.i1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi M, Wang FS, Wu ZZ. Synergetic anticancer effect of combined quercetin and recombinant adenoviral vector expressing human wild-type p53, GM-CSF and B7-1 genes on hepatocellular carcinoma cells in vitro. World J Gastroenterol. 2003;9:73–78. doi: 10.3748/wjg.v9.i1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu JW, Tang Y, Shen Y, Zhong XY. Synergistic effect of cell differential agent-II and arsenic trioxide on induction of cell cycle arrest and apoptosis in hepatoma cells. World J Gastroenterol. 2003;9:65–68. doi: 10.3748/wjg.v9.i1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Röcken C, Carl-McGrath S. Pathology and pathogenesis of hepatocellular carcinoma. Dig Dis. 2001;19:269–278. doi: 10.1159/000050693. [DOI] [PubMed] [Google Scholar]


