Abstract
AIM: Polymorphonuclear neutrophil (PMN) plays a major role in liver ischemia/reperfusion injury. Protective effect of ischemic preconditioning (IP) has been confirmed in liver ischemia/reperfusion injury. The purpose of this study was to investigate the effect of IP on C-X-C chemokine expression and PMNs recruitment early after liver transplantation.
METHODS: Male Sprague-Dawley rats were used as donors and recipients of orthotopic liver transplantation (OLT). The donor liver was stored 24 h in University of Wisconsin (UW) solution at 4 °C pre-implantation. IP was done by clamp of the portal vein and hepatic artery of the donor liver for 10 minutes followed by reperfusion for 10 minutes before harvesting. The neutrophilic infiltration in liver was quantified using a myeloperoxidase (MPO) assay. Intragraft expression of macrophage inflammatory protein-2 (MIP-2) mRNA was investigated with in situ hybridization. The serum levels of MIP-2 and tumor necrosis factor (TNF)-α were also monitored.
RESULTS: After liver transplantation without IP, the hepatic MPO increased significantly compared with sham operated group. In IP group, PMN in liver indicated by MPO was reduced significantly. In situ hybridization showed no MIP-2 mRNA in sham group but dramatic expression in hepatocytes in non-IP group. In IP group, MIP-2 mRNA was significantly down-regulated. Similarly, serum MIP-2 and TNF-α levels were significantly elevated in non-IP group and both were reduced in IP group.
CONCLUSION: IP might protect graft liver from preservation-reperfusion injury after OLT through down-regulating C-X-C chemokine expression of hepatocytes, and alleviating PMNs recruitment after reperfusion.
INTRODUCTION
Liver transplantation as an effective therapy for end-stage liver diseases has been accepted. Though preservation techniques have been greatly improved, ischemia/reperfusion injury resulting in primary liver nonfunction still poses significant clinical problems and contributes to mortality[1-2]. Jaeschke et al[3-6] established that there were two distinct phases of liver injury after warm ischemia and reperfusion. The initial phase of injury which is far less than that observed at later time points is characterized by Kupffer cell-induced oxidant stress. Events occurred during the initial phase including activation of Kupffer cells, initiate a complex inflammatory pathway that culminates in hepatic accumulation of neutrophils[7]. Recruited neutrophils directly damage hepatocytes by releasing oxidants and proteases and are responsible for the later phase of liver injury induced by ischemia/reperfusion. Activated PMN has also been implicated as a vital factor in the development of ischemia/reperfusion injury in both experimental and clinical liver transplantations[8-11].
C-X-C chemokines are a group of molecules that have both inflammatory and repairable properties and are best known for their neutrophil chemotactic properties[12,13]. MIP-2, belonging to C-X-C chemokines has been shown not only to regulate PMN recruitment from vascular compartment to the tissues[14] but also to cause PMN activation[15]. Kataoka et al demonstrated that MIP-2 played a crucial role in PMN recruitment and activation after liver transplantation[10].
IP is a process of a short period of ischemia and reperfusion, which leads to an unexpected resistance to a long-term ischemia/reperfusion injury. It has been documented in several organs, including the liver[16-19]. In experimental liver transplantation, IP has been confirmed as an effective strategy for protecting the grafts from ischemia/reperfusion injury[20]. But few studies have been performed on whether and how IP effects PMNs accumulation and activation in protecting grafted liver from ischemia/reperfusion injury after liver transplantation. In this study, we therefore investigated the effect of IP on PMNs recruitment, as well as MIP-2 expression in grafted livers, to determine the role of C-X-C chemokine expression and PMNs recruitment in protecting grafted liver from prolonged preservation/reperfusion injury early after OLT.
MATERIALS AND METHODS
Animals
Male Spraque Dawley rats weighing 200 to 250 g were used as donors and recipients. They were housed in pathogen-free conditions with a 12-hr light-dark cycle and were allowed to drink water and fasted for 14 h before operation. All experiments were performed in compliance with the standards for animal use and care set by Institutional Animal Care Committee.
Surgical procedures
OLT. Liver transplantation was performed according to Kamada’s cuff-technique[21] with minor modifications. Before liver harvesting, 1 mL saline containing 50 units of heparin was given intravenously, and the donor liver was perfused in situ via the portal vein with 20 mL of cold physiological saline solution to which 50 units of heparin was added. Following cuff preparation, the liver was stored in a beaker containing University of Wisconsin solution at 4 °C for 24 h. At the end of storage, the liver was slowly flushed with 20 mL of cold (4 °C) Ringer’s lactate and transplanted orthotopically into a recipient animal. The hepatic artery was not reconstructed.
IP. Before harvesting donor liver, the portal vein and hepatic artery were interrupted by placing a bulldog clamp for 10 min. Reflow was initiated by removing of the clamp for another 10 min.
In sham group, the left phrenic vein and the right suprarenal vein were ligated and the hepatic artery was freed by ligating and dividing.
Experimental design
All rats were randomly divided into: sham groups, non-IP group and IP group. To obtain blood and tissue samples, six animals were killed in non-IP and IP group after 1, 2, 4 and 6 hr of reperfusion and four animals at each time point in sham group. Plasma samples were collected from inferior vena cava, and separated by centrifugation, and median lobe of the liver was carefully excised and stored at -80 °C for analysis. Serum levels of alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) were measured using standard clinical automated analysis. Serum levels of MIP-2 (IBL Immuno-Biological Laboratories, Hamburg) and TNF-α (R & D Systems, Inc) were measured using commercial enzyme-linked immunosorbent assay kits, respectively.
MPO assay
MPO was used as a marker of pulmonary and hepatic neutrophil infiltration[22]. MPO activity was measured photometrically employing 3, 3’, 5, 5’-tetramethylbenzidine as a substrate. Frozen liver tissues were macerated, homogenized, sonicated, and centrifuged at 4000 g for 12 minutes at 4 °C as described previously[22]. MPO activity was measured in the supernatant, with calculations based on the absorbance change at 460 nm. All values were normalized to tissue weight.
Hybridization
Chunks (1 cm3) from fresh rat liver were immediately fixed in 4% paraformaldehyde at 4 °C for 8 h and dehydrated through graded ethanol, then embedded in paraffin and sectioned. The sections were layered onto glass slides by standard procedures. Five μm thick sections were cut for analysis. They were deparaffinized with xylene and quickly rehydrated through graded ethanol. The deparaffinized sections were quenched from endogenous peroxidase activity in 3% H2O2 diluted in methanol. Then, the expression of MIP-2 mRNA was detected in rat liver tissue sections using a commercial ISH kit (TBD biotech Co.). All solutions of it were RNase free. The sections were digested using solution I (0.4% pepsin, 0.1 M Hcl, TBD biotech Co.), then washed in 0.5 M PBS twice for 5 minutes each. The non-specific IgG binding sites were blocked with blocking solution I (1.5% normal blocking serum, TBD biotech Co.) at RT and excess serum was blotted from the sections. Then, the sections covered with coverslips were prehybridized with a prehybridized solution (formamide, standard saline citrate, 100 × denhardt’s, SDS, sperm DNA, TBD biotech Co.) at 37 °C for 4 h in a sealed humidity chamber. The sections were washed twice in 0.5 M PBS and hybridized with digitoxin-labeled oligonucleotide probe synthesized by TBD cooperation Lab, (5’-CCACTCGCCAGCTCCTCAATGCTGTACTGGTCCTGCTCCT-3’). Hybridization was performed at 38 °C overnight in a humidity chamber, and washed in 2 × SSC, 0.5 × SSC, 0.2 × SSC for 5 minutes each. In the following step, the sections were blocked with blocking solution II (25 μg/ml avidin, TBD biotech Co.) for 15 minutes at RT, then excess solution was blotted. The sections covered by coverslips were incubated with biotinylated mouse anti-digitoxin antibody (TBD Biotech Co.) for 1 h at 37 °C in a sealed humidity chamber and washed three times in 0.01 M PBS for 5 minutes each. The sections were incubated with streptavidin-biotin-peroxidase complex (TBD Biotech Co.) at 37 °C for 1 h and washed three times in 0.01 M PBS for 5 minutes each. The complex was detected with DAB (0.1% diaminonbenzidine, 0.02% hydrogen peroxide, TBD Biotech Co.) in sections and counterstained with hematoxylin (Sigma). The first negative control group was performed in which the mouse anti-digitoxin antibody was substituted with normal mouse serum and the second was performed with RNase (20 μg/ml, Sigma) before hybridization. The last one was hybridized without any oligonucleotide probe.
Histology study
Liver samples were fixed in 4% neutral buffered formalin, paraplast-embedded and cut into 4 μm thick sections, and stained with hematoxylin-eosin according to the standard procedures.
Statistical analysis
The data were expressed as mean ± SEM. Means of different groups were compared using a one-way ANOVA. The Student t test was performed to evaluate the significant differences between groups. Significance was determined at P < 0.05.
RESULTS
Serum levels of ALT, LDH
Both ALT (A) and LDH (B) were significantly elevated after OLT without IP, compared with sham-operated group. When the liver grafts were pretreated with IP, the increases in ALT and LDH were relatively reduced to the non-IP group in each time point respectively (Figure 1).
Figure 1.
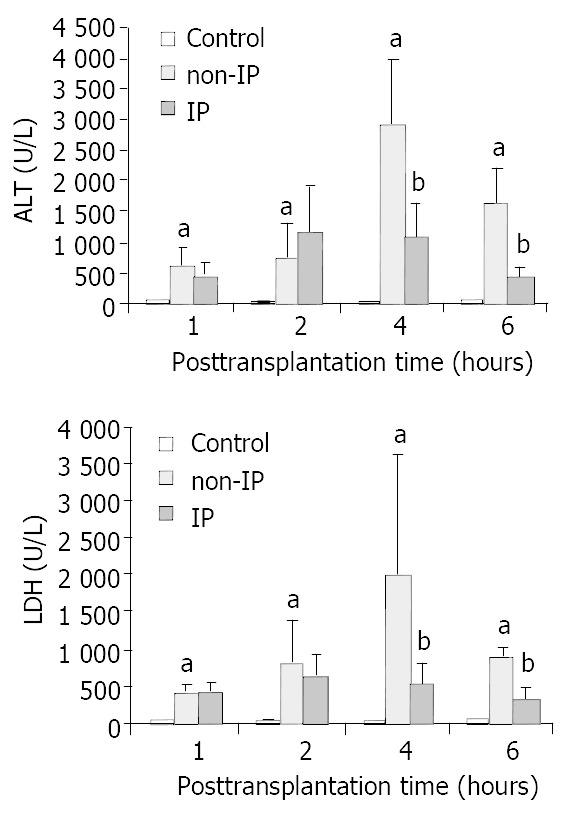
Changes in serum ALT (A) and LDH (B) levels. The serum ALT and LDH levels were significantly elevated in non-IP groups compared with sham-operated group (aP < 0.01, non-IP group vs sham-operated group). After IP, the increase was significantly reduced at 4 and 6 h points after reperfusion (bP < 0.05, IP group vs non-IP group).
Hepatic PMNs accumulation
PMNs accumulation in the grafted livers was assessed by investigating the levels of MPO. Figure 2 shows changes in PMNs accumulation in three groups. The increases in non-IP groups were significantly reduced in IP group, especially at 4 and 6 h points after reperfusion.
Figure 2.
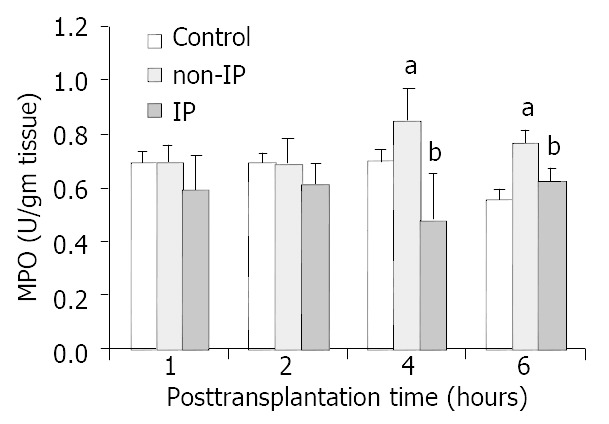
Changes of PMNs accumulation in grafted liver. PMNs accumulation was assessed by investigating the levels of MPO. The increase in non-IP groups was significantly re-duced in IP groups, especially at 4 and 6 h points after reperfusion (aP < 0.05, non-IP group vs sham-operated group. bP < 0.05, IP group vs non-IP group).
Serum levels of MIP-2, TNF-α
In non-IP group, MIP-2 increased significantly in each time point and peaked at 4 h after reperfusion compared with the sham operated group. In IP group, the increases were reduced in each time point respectively (Figure 3). Similar results of TNF-α were obtained. But interestingly, the levels of TNF-α peaked at 2 h in all three groups in this study (Figure 4).
Figure 3.
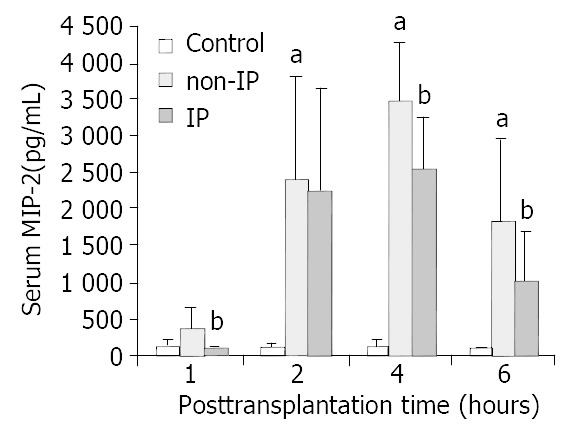
Changes in serum MIP-2 levels. MIP-2 was signifi-cantly increased in non-IP group compared with sham-oper-ated group (aP < 0.01, non-IP group vs sham-operated group). After IP, the increase was significantly reduced at 1, 4 and 6 h after reperfusion (bP < 0.05, IP group vs non-IP group).
Figure 4.
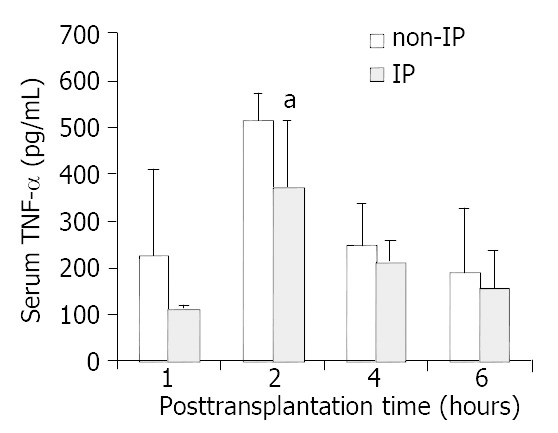
Changes in serum TNF-α levels. The levels of serum TNF-α were decreased to an extent in each time point respectively in IP group compared with non-IP group, especially at 2 h after reperfusion. The decrease was significant (aP < 0.05, IP group vs non-IP group).
Intragraft MIP-2 mRNA expression
In situ hybridization was performed at 4 h point after reperfusion in all three groups (Figure 5). MIP-2 mRNA was not detectable in sham operated group (A). MIP-2 mRNA in the non-IP group (B) was remarkably up-regulated at 4 h point and after IP (C), the expression was detectable at a lower level. It was emphasized that the MIP-2 mRNA was mostly expressed in hepatocytes in grafted livers.
Figure 5.
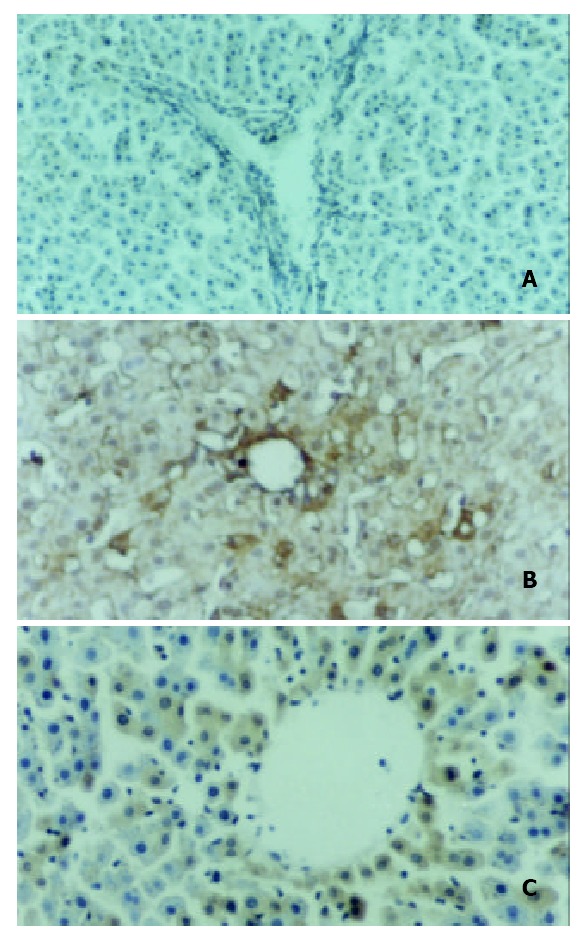
Intragraft MIP-2 mRNA expression. In situ hybrid-ization was performed at 4 h point after reperfusion in all three groups. MIP-2 mRNA was not detectable in sham oper-ated group (A), and remarkably up-regulated in non-IP group (B), and after IP, the expression was detectable at a lower level (C). MIP-2 mRNA was mostly expressed in hepatocytes in grafted livers.
Histological changes of grafts
Similar to serum transferase indicating liver function, histological change of the graft showed that 24 h cold storage in UW solution and reperfusion resulted in grafted liver damage. After IP and OLT, the damage was ameliated.
DISCUSSION
In this study, we demonstrated that 24 h cold storage in UW solution and reperfusion (OLT) resulted in grafted liver injury indicated by serum transferase and histological changes. In this process, TNF-α and C-X-C chemokines (i.e., MIP-2) were stimulated, and induced PMN-mediated tissue damage. Also we demonstrated that IP protected the grafted liver from prolonged cold preservation-reperfusion injury through down-regulation of TNF-α and MIP-2 and attenuation of PMNs accumulation. In situ hybridization revealed that MIP-2 mRNA was mostly expressed in hepatocytes.
IP is a phenomenon in which a short period of ischemia leads to unexpected resistance of subsequently prolonged preservation-reperfusion injury. It was found in the heart and named by Murry et al[16] in 1986. Recently it was reported that the phenomenon of preconditioning of the heart could be extended to the models of liver resection[19] and liver transplantation[20]. The role of IP in reducing myocardial and liver ischemic injury has been widely documented[23-26]. However, the mechanism of its protective effect remains unclear.
It is well known that accumulated PMNs in the grafted liver sinusoids might be important effector cells in the pathogenosis of liver preservation-reperfusion injury after OLT[8-11]. In this study, an increased serum level of MPO in non-IP group was synchronized with elevated serum ALT and LDH levels. The reactive oxygen and proteases released by activated PMNs might promote progressive hepatocellular damage[27]. This study demonstrated that IP decreased PMNs accumulation to protect the grafted liver from prolonged ischemia/reperfusion injury after OLT.
As the functions of cellular adhesion molecules were concerned, C-X-C chemokines (i.e., MIP-2) were intricately involved in the process of PMNs recruitment[28]. During warm hepatic ischemia/reperfusion injury, early production of proinflammatory mediators such as TNF-α has been documented[29-32]. Kataoka et al revealed that TNF-α could stimulate C-X-C chemokine production in hepatocytes of prolonged ischemia-reperfusion injury after OLT[10]. Kupffer cell stimulation during extended cold preservation might result in overresponse and excessive production of TNF-α in the initial phase of reperfusion[33]. Then, TNF-α released from Kupffer cells might act locally on hepatocytes and other cells, resulting in increased C-X-C chemokine expression. This study demonstrated IP could interrupt this complex cascade of inflammatory mediators and ameliate the grafted liver damage after OLT. MIP-2 mRNA of hepatocytes performed by in situ hybridization was expressed in a lower level in IP group compared with non-IP group. And serum levels of TNF-α and MIP-2 in recipients also decreased significantly after IP.
In conclusion, IP can protect grafted livers from prolonged preservation-reperfusion injury in a rat liver transplantation model. One possible mechanism of its protective effect in the initial phase after reperfusion may be that IP interrupts the chain reaction of preservation-reperfusion injury in which excessive TNF-α released from activated Kupffer cells induces local expression of C-X-C chemokines of hepatocytes and accumulation of PMNs which play an important role in mediating the liver graft injury. Obviously, the preservation-reperfusion injury leading to primary nonfunction of grafted liver after OLT is multifactorial, and multi-mediators take part in it and intercross one another. Further experiments to define the protective role of IP in preservation-reperfusion injury should be performed.
ACKNOWLEDGEMENTS
We are grateful to Dr. Xin-Hua Zhu for his technical assistance in establishing rat liver transplantation models, Ms. Ru-Tian Li for her help with the statistical analysis, Dr. Qing-Xiang Xu and Dr. Xi-Tai Sun for their helpful discussions.
Footnotes
Supported by the Chinese Medical Administration Bureau of Jiangsu Province, No. SZ9902
Edited by Bo XN and Wang XL
References
- 1.Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957–978. doi: 10.1097/00007890-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Smith CV, Mitchell JR. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988;81:1240–1246. doi: 10.1172/JCI113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–284. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- 6.Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115–136. doi: 10.1016/0009-2797(91)90077-k. [DOI] [PubMed] [Google Scholar]
- 7.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 8.Takei Y, Marzi I, Gao WS, Gores GJ, Lemasters JJ, Thurman RG. Leukocyte adhesion and cell death following orthotopic liver transplantation in the rat. Transplantation. 1991;51:959–965. doi: 10.1097/00007890-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Marzi I, Knee J, Bühren V, Menger M, Trentz O. Reduction by superoxide dismutase of leukocyte-endothelial adherence after liver transplantation. Surgery. 1992;111:90–97. [PubMed] [Google Scholar]
- 10.Kataoka M, Shimizu H, Mitsuhashi N, Ohtsuka M, Wakabayashi Y, Ito H, Kimura F, Nakagawa K, Yoshidome H, Shimizu Y, et al. Effect of cold-ischemia time on C-X-C chemokine expression and neutrophil accumulation in the graft liver after orthotopic liver transplantation in rats. Transplantation. 2002;73:1730–1735. doi: 10.1097/00007890-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 11.Pesonen EJ, Höckerstedt K, Mäkisalo H, Vuorte J, Jansson SE, Orpana A, Karonen SL, Repo H. Transhepatic neutrophil and monocyte activation during clinical liver transplantation. Transplantation. 2000;69:1458–1464. doi: 10.1097/00007890-200004150-00042. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 13.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 14.Watanabe K, Konishi K, Fujioka M, Kinoshita S, Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989;264:19559–19563. [PubMed] [Google Scholar]
- 15.Frevert CW, Farone A, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of rat chemokine macrophage inflammatory protein-2. Inflammation. 1995;19:133–142. doi: 10.1007/BF01534386. [DOI] [PubMed] [Google Scholar]
- 16.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 17.Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotter G, Closa D, Prados M, Fernández-Cruz L, Prats N, Gelpí E, Roselló-Catafau J. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun. 1996;222:27–32. doi: 10.1006/bbrc.1996.0692. [DOI] [PubMed] [Google Scholar]
- 19.Peralta C, Hotter G, Closa D, Gelpí E, Bulbena O, Roselló-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934–937. doi: 10.1002/hep.510250424. [DOI] [PubMed] [Google Scholar]
- 20.Yin DP, Sankary HN, Chong AS, Ma LL, Shen J, Foster P, Williams JW. Protective effect of ischemic preconditioning on liver preservation-reperfusion injury in rats. Transplantation. 1998;66:152–157. doi: 10.1097/00007890-199807270-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 22.Peralta C, Fernández L, Panés J, Prats N, Sans M, Piqué JM, Gelpí E, Roselló-Catafau J. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100–113. doi: 10.1053/jhep.2001.20529. [DOI] [PubMed] [Google Scholar]
- 23.Koeppel TA, Thies JC, Schemmer P, Trauner M, Gebhard MM, Otto G, Post S. Inhibition of nitric oxide synthesis in ischemia/reperfusion of the rat liver is followed by impairment of hepatic microvascular blood flow. J Hepatol. 1997;27:163–169. doi: 10.1016/s0168-8278(97)80297-8. [DOI] [PubMed] [Google Scholar]
- 24.Gao W, Washington MK, Bentley RC, Clavien PA. Antiangiogenic agents protect liver sinusoidal lining cells from cold preservation injury in rat liver transplantation. Gastroenterology. 1997;113:1692–1700. doi: 10.1053/gast.1997.v113.pm9352874. [DOI] [PubMed] [Google Scholar]
- 25.Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK. Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-alpha-induced apoptosis and toxicity in the liver. J Med Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]
- 26.Kim YM, de Vera ME, Watkins SC, Billiar TR. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997;272:1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- 27.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology. 1993;17:915–923. [PubMed] [Google Scholar]
- 28.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 29.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest. 1995;95:134–141. doi: 10.1172/JCI117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and Kupffer cells. Hepatology. 1998;27:507–512. doi: 10.1002/hep.510270226. [DOI] [PubMed] [Google Scholar]
- 31.Yoshidome H, Lentsch AB, Cheadle WG, Miller FN, Edwards MJ. Enhanced pulmonary expression of CXC chemokines during hepatic ischemia/reperfusion-induced lung injury in mice. J Surg Res. 1999;81:33–37. doi: 10.1006/jsre.1998.5490. [DOI] [PubMed] [Google Scholar]
- 32.Hisama N, Yamaguchi Y, Miyanari N, Ichiguchi O, Goto M, Mori K, Ogawa M. Ischemia-reperfusion injury: the role of Kupffer cells in the production of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family. Transplant Proc. 1995;27:1604–1606. [PubMed] [Google Scholar]
- 33.Arii S, Monden K, Adachi Y, Zhang W, Higashitsuji H, Furutani M, Mise M, Fujita S, Nakamura T, Imamura M. Pathogenic role of Kupffer cell activation in the reperfusion injury of cold-preserved liver. Transplantation. 1994;58:1072–1077. [PubMed] [Google Scholar]


