Abstract
AIM: To investigate the clinical pathologic features of gastrointestinal leiomyoma and the diagnostic value of endoscopic ultrasonography (EUS) on gastrointestinal leiomyoma.
METHODS: A total of 106 patients with gastrointestinal leiomyoma diagnosed with EUS were studied. The location, size and layer origin of gastric and esophageal leiomyomas were analyzed and compared. The histological diagnosis of the resected specimens by endoscopy or surgery in some patients was compared with their results of EUS.
RESULTS: The majority of esophageal leiomyomas were located in the middle and lower part of the esophagus and their size was smaller than 1.0 cm, and 62.1% of esophageal leiomyomas originated from the muscularis mucosae. Most of the gastric leiomyomas were located in the body and fundus of the stomach with a size of 1-2 cm. Almost all gastric leiomyomas (94.2%) originated from the muscularis propria. The postoperative histological results of 54 patients treated by endoscopic resection or surgical excision were completely consistent with the preoperative diagnosis of EUS, and the diagnostic specificity of EUS to gastrointestinal leiomyoma was 94.7%.
CONCLUSION: The size and layer origin of esophageal leiomyomas are different from that of gastric leiomyomas. Being safe and accurate, EUS is the best method not only for gastrointestinal leiomyoma diagnosis but also for the follow-up of patients.
INTRODUCTION
With the development and popularization of endoscopic ultrasonography (EUS) in clinical diagnosis, great progress has been made in diagnosis and treatment of gastrointestinal leiomyoma[1-3]. We collected 106 patients with gastrointestinal leiomyoma diagnosed by EUS from the First Affiliated Hospital, School of Medicine, Zhejiang University, in China from August 2000 to September 2002. This report is to summarize and analyze the clinical pathologic features and results of diagnosis and treatment of gastrointestinal leiomyoma and to evaluate the clinical diagnostic value of EUS for gastrointestinal leiomyoma.
MATERIALS AND METHODS
Patients
The patients with submucosal protruding lesions in gastrointestine by conventional endoscopy were examinated by EUS. Before making EUS, physical examinations were performed. One hundred and six patients (63.8%) were diagnosed having gastrointestinal leiomyoma by EUS among 166 patients with true submucosal lesions, their mean age was 51 years, ranging from 2 to 88 years. There were 52 men and 54 women. Including 66 cases of esophageal leiomyoma, 35 cases of gastric leiomyoma, 2 cases of duodenum leiomyoma and 3 cases of colon leiomyoma.
Instrument
Instruments of EUS included Fujino EG-410D double-cavity electronic gastroscope, Olympus CF-VL electronic colonoscope and Fujino SP-70 high-frequency echoprobe system. The frequency of probe is between 7.5 MHz to 20 MHz.
Methods
According to the information of the location and size of lesion in gastrointestine shown by the conventional endoscopy examination, we chose different frequency microprobes and examination methods (water-ballon method, water-soak method or water-pour method) to scan the lesion[4]. Then a diagnosis was made for the size, origin, invasion field and nature of the lesion. Some patients were treated by endoscopic resection or surgical excision after EUS, the postoperative histological results were compared with the preoperative diagnoses of EUS. In addition, a follow-up with EUS was made for a few patients without endoscopic or surgical resection because of different reasons.
RESULTS
Ninety-eight patients (92.5%) showed no related symptoms and were found by conventional endoscopic examination occasionally among 106 patients with gastrointestinal leiomyoma diagnosed by EUS. Only 8 patients (7.5%) had fixed symptoms, of them, 6 cases had esophageal leiomyoma and 2 cases had gastric leiomyoma. The size of tumor was > 2.0 cm, and major symptoms were dysphagia, feeling of foreign body, pain behind chest bone, upper abdominal indisposition, etc. The distribution, layer of origin, size and number of esophageal or gastric leiomyoma were summarized in Table 1 and Table 2. The majority of esophageal leiomyoma were located in the middle and lower part of the esophagus, and their size was < 1.0 cm, and 62.1% of the esophageal leiomyomas originated from the muscularis mucosae. Most of the gastric leiomyomas were located in the body and fundus of stomach, their size was 1-2 cm. Almost all gastric leiomyomas (94.2%) originated from the muscularis propria. Duodenal leiomyomas in two patients were derived from the muscularis propria, being 0.5-0.8 cm in size. Colon leiomyomas in three patients were located in cecum, transverse colon and sigmoid colon, respectively. The lesions were originated from the muscularis propria, 1.1-1.6 cm in size. One hundred and one of 106 patients just had single leiomyoma. After EUS examination, 35 patients with leiomyoma originating from muscularis mucosa were treated with endoscopic resection (Figure 1A, Figure 1B, Figure 1C). The other group of 22 patients received surgical excision because their lesions appeared to be in the proper muscle layer. The size, number and layer-origin of the lesions in 57 patients treated with endoscopic or surgical resection were completely consistent with the preoperative diagnosis of EUS. However, postoperative histological results of only 3 patients were carcinoid, esophageal cyst gland hyperplasia and tubercle, respectively, which were not in agreement with the preoperative diagnosis of EUS. The diagnostic accuracy of EUS for leiomyoma was 94.1% (54/57). The remaining 49 patients were not treated with endoscopic or surgical resection due to various reasons. They were observed and followed up. Fifteen of 49 patients were examined with EUS at three, six and twelve months later, the results of examination showed that the position, shape and structure of their lesions were unchanged. In our study, all the patients could well tolerate EUS without serious complications such as bleeding, perforation, shock and asphyxia except a few patients who felt disorder in throat, and abdominal distension. No complication occurred in 35 patients treated by endoscopic resection.
Table 1.
Clinical pathological characteristics of gastric leiomyoma (n = 35)
| Location (n) | Origin (n) | Size (n) | Number (n) |
| Antumn (6) | Muscularis mucosae (3) | ≤ 1.0 cm (4) | Single (34) |
| Body (11) | Muscularis propria (32) | > 1.0, ≤ 2.0 cm (20) | Multiple (1) |
| Fundus (11) | > 2.0 cm (11) | ||
| Cardia (7) |
Table 2.
Clinical pathological characteristics of esophgeal leiomyoma (n = 66)
| Location (n) | Origin (n) | Size (n) | Number (n) |
| Upper part (11) | Muscularis mucosae (41) | ≤ 1.0 cm (32) | Single (62) |
| Middle part (28) | Muscularis propria (25) | > 1.0, ≤ 2.0 cm (24) | Multiple (4) |
| Lower part (27) | > 2.0 cm (10) |
Figure 1.
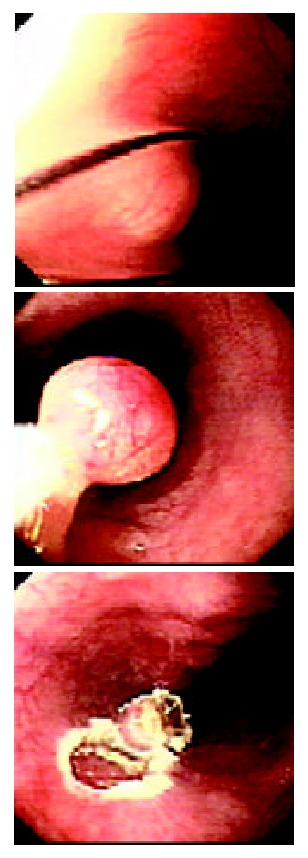
Endoscopic resection of esophgeal leiomyoma.
DISCUSSION
Gastrointestinal leiomyoma is a common kind of benign submucosal tumor in gastrointestine[1,5], because it originates from muscularis mucosa or muscularis propria, the conventional endoscopy can not diagnose it accurately. Since EUS was used in clinical diagnosis, the diagnostic situation of gastrointestinal leiomyoma has changed greatly[6-9]. The five-layered structure of gastrointestinal wall can be shown clearly, and gastrointestinal leiomyoma presents homogeneous and hypoechoic lesion with clear margin, and the lesion is around the hyperechoic wrapping area under endosonography (Figure 2, Figure 3). According to these features of ultrasonography, leiomyoma is easy to be distinguished from hemoangioma, cyst and lipoma in digestive tract wall[10-15]. Thus we can define not only the nature of leiomyoma, but also its size, number and the layer of origin by EUS. Our clinical study showed that gastrointestinal leiomyoma mainly occurred in esophagus and stomach. The incidence in duodenum and colon is markedly lower than that in esophagus and stomach. The partial reason of the lower incidence of colon leiomyoma may be that the number of patients undergoing colonoscopic examination was significantly less than that of gastroscopic examination (the ratio of gastroscopy to colonoscopy was 3:1 in this study). The incidence of esophageal leiomyoma was higher than that of stomach leiomyoma, the size and layer origin of esophageal leiomyoma were different from gastric leiomyoma. The reason is still unknown. Although leiomyoma was located in different positions of gastrointestine, almost all the patients (101/106) only had single lesion, which conformed with other reports[2,16]. With regard to the diagnosis of gastrointestinal leiomyoma, our clinical data indicated that most cases (92.5%) were occasionally found by endoscopic examination, these patients showed no related symptoms and signs, and no positive change in blood examination. The diagnosis of leiomyoma mainly depends on EUS, which combines the function of endoscope and ultrasonic, by which we can not only inspect the surface shape of gastrointestinal lesion, but also gain the image of the layer of origin, the invasive scope and the structure of the lesion. According to the literature[17-19], the diagnostic specificity of EUS to gastrointestinal leiomyoma is superior to other imaging techniques such as B type ultrosonophy, gastrointestinal radiography and computed tomography. In our clinical study, the size, number and the layer of origin of the resected lesions were completely consistent with the diagnosis of EUS in 57 patients treated by endoscopic resection or surgical excision, The nature of the lesions in 54 of 57 patients was in agreement with the diagnosis of EUS, the diagnostic accuracy of EUS for leiomyoma was 94.7%. Our study indicated that EUS had a very important diagnostic value for gastrointestinal leiomyoma[20]. However, we are still possible to make a mistake in the diagnosis of gastrointestinal leiomyoma, because the image of ultrasonophy of a few other lesions is the same as that of gastrointestinal leiomyoma, e.g, the gastrointestinal carcinoid and tubercle. For these diseases, we should depend on other clinical information to differentiate them. Furthermore, when EUS finds that the size of leiomyoma is bigger than 4 cm, or the surface of leiomyoma has erosion or ulceration, or internal echo being unhomogeneous, we should consider the possibility of leiomyosarcoma[21]. Presently, EUS is considered the best method for the diagnosis of submucosal lesion[7,8,22], Which can not only diagnose leiomyoma correctly, but also help us work out scientific and rational therapeutic strategies. EUS can clearly show the origin of gastrointestinal leiomyoma, either from the muscularis mucosae or the muscularis propria. Usually, leiomyoma originating from the muscularis mucosae can be treated by endoscopic resection[23-26], whereas leiomyoma originating from the proper muscle layer contraindicates endoscopic resection. Unwell-planned resection will bring about perforation of gastrointestine. Thirty-five patients with leiomyoma originating from muscularis mucosae were treated by endoscopic resection in our study. No complications such as bleeding, perforation occurred, showing that EUS has a very important value to the selection of therapeutic methods for gastrointestinal leiomyoma[27-30]. It makes the therapy of gastrointestinal leiomyoma more rational, safe and economic. In addition, for those patients with gastrointestinal leiomyoma who refused to receive or could not be treated by endoscopic resection or surgical excision, we followed up them by EUS periodically. The results showed that gastrointestinal leiomyoma grew slowly and showed no marked change in a short time. Thus, we can choose observation and follow-up for the patients with small lesions, and lesions originating from the muscularis propria, or the special position of lesion. In conclusion, EUS is a safe and effective diagnostic method for gastrointestinal leiomyoma.
Figure 2.
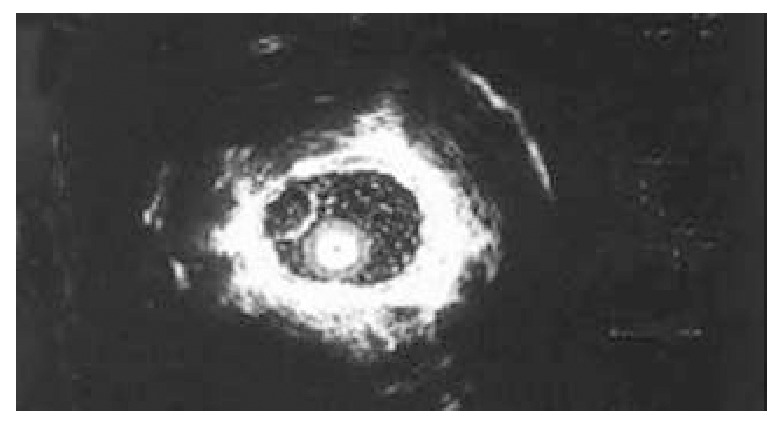
Esophageal leiomyoma, originating from muscularis mucosa.
Figure 3.
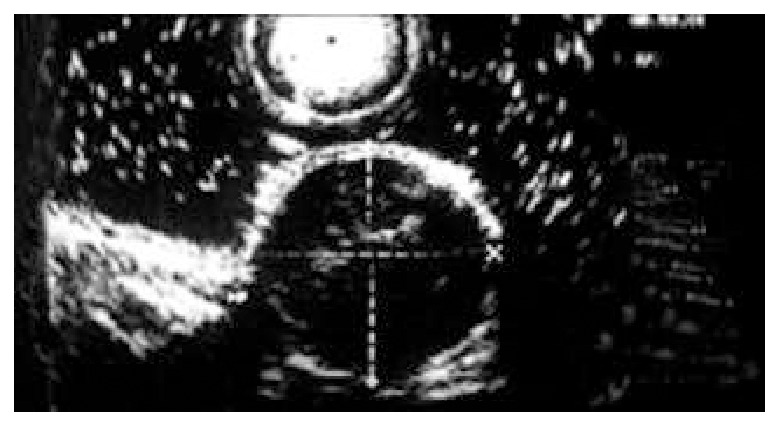
Gastric leiomyoma, originating from muscularis propria.
Footnotes
Supported by the Initiative Fund of Ministry of Education for Returned Overseas Scholars, No. 491010-G50040
Edited by Ma JY and Wang XL
References
- 1.Chak A. EUS in submucosal tumors. Gastrointest Endosc. 2002;56:S43–S48. doi: 10.1016/s0016-5107(02)70085-0. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Sun Y, Liu Y, Li Y, Wang Z. [Transesophageal intraluminal ultrasonography in diagnosis and differential diagnosis of esophageal leiomyoma] Zhonghua Yixue Zazhi. 2002;82:456–458. [PubMed] [Google Scholar]
- 3.Nomura N, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Usefulness of contrast-enhanced EUS in the diagnosis of upper GI tract diseases. Gastrointest Endosc. 1999;50:555–560. doi: 10.1016/s0016-5107(99)70083-0. [DOI] [PubMed] [Google Scholar]
- 4.Xu GM, Niu YL, Zou XP, Jin ZD, Li ZS. The diagnostic value of transendoscopic miniature ultrasonic probe for esophageal diseases. Endoscopy. 1998;30 Suppl 1:A28–A32. [PubMed] [Google Scholar]
- 5.Xu GQ, Li YM, Chen WX, Ji F, Huang HD. Diagnostic value of transendoscopic miniature ultrasonic probes on esophageal and gastric submucosal lesions. Zhonghua Chaosheng Yingxiangxue Zazhi. 2002;11:188–189. [Google Scholar]
- 6.Shen EF, Arnott ID, Plevris J, Penman ID. Endoscopic ultrasonography in the diagnosis and management of suspected upper gastrointestinal submucosal tumours. Br J Surg. 2002;89:231–235. doi: 10.1046/j.0007-1323.2001.02002.x. [DOI] [PubMed] [Google Scholar]
- 7.Gress F, Schmitt C, Savides T, Faigel DO, Catalano M, Wassef W, Roubein L, Nickl N, Ciaccia D, Bhutani M, et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest Endosc. 2001;53:71–76. doi: 10.1067/mge.2001.111384. [DOI] [PubMed] [Google Scholar]
- 8.Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856–862. [PubMed] [Google Scholar]
- 9.Kameyama H, Niwa Y, Arisawa T, Goto H, Hayakawa T. Endoscopic ultrasonography in the diagnosis of submucosal lesions of the large intestine. Gastrointest Endosc. 1997;46:406–411. doi: 10.1016/s0016-5107(97)70032-4. [DOI] [PubMed] [Google Scholar]
- 10.Massari M, De Simone M, Cioffi U, Gabrielli F, Boccasanta P, Bonavina L. Endoscopic ultrasonography in the evaluation of leiomyoma and extramucosal cysts of the esophagus. Hepatogastroenterology. 1998;45:938–943. [PubMed] [Google Scholar]
- 11.Varas Lorenzo MJ, Maluenda MD, Pou JM, Abad R, Turró J, Espinós JC. [The value of endoscopic ultrasonography in the study of submucosal tumors of the digestive tract] Gastroenterol Hepatol. 1998;21:121–124. [PubMed] [Google Scholar]
- 12.Araki K, Ohno S, Egashira A, Saeki H, Kawaguchi H, Ikeda Y, Kitamura K, Sugimachi K. Esophageal hemangioma: a case report and review of the literature. Hepatogastroenterology. 1999;46:3148–3154. [PubMed] [Google Scholar]
- 13.Lu ZC, Jing ZD. Submucosal tumors of the esophagus. Modern Intralumen Ultrasonics. Beijing: Science Press. 2000:174–182. [Google Scholar]
- 14.Buscarini E, Stasi MD, Rossi S, Silva M, Giangregorio F, Adriano Z, Buscarini L. Endosonographic diagnosis of submucosal upper gastrointestinal tract lesions and large fold gastropathies by catheter ultrasound probe. Gastrointest Endosc. 1999;49:184–191. doi: 10.1016/s0016-5107(99)70484-0. [DOI] [PubMed] [Google Scholar]
- 15.Hizawa K, Matsumoto T, Kouzuki T, Suekane H, Esaki M, Fujishima M. Cystic submucosal tumors in the gastrointestinal tract: endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712–714. doi: 10.1055/s-2000-9025. [DOI] [PubMed] [Google Scholar]
- 16.Zou XP. Gastric leiomyoma. Modern Intralumen Ultrasonics. Beijing: Science Press. 2000:202–205. [Google Scholar]
- 17.Koch J, Halvorsen RA, Levenson SD, Cello JP. Prospective comparison of catheter-based endoscopic sonography versus standard endoscopic sonography: evaluation of gastrointestinal-wall abnormalities and staging of gastrointestinal malignancies. J Clin Ultrasound. 2001;29:117–124. doi: 10.1002/1097-0096(200103/04)29:3<117::aid-jcu1010>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Catalano MF. Endoscopic ultrasonography for esophageal and gastric mass lesions. Gastroenterologist. 1997;5:3–9. [PubMed] [Google Scholar]
- 19.Xu GQ. Benign tumors of the esophagus. Modern Esopgagology. Shanghai: Shanghai Science And Technique Press. 1999:268–273. [Google Scholar]
- 20.Futagami K, Hata J, Haruma K, Yamashita N, Yoshida S, Tanaka S, Chayama K. Extracorporeal ultrasound is an effective diagnostic alternative to endoscopic ultrasound for gastric submucosal tumours. Scand J Gastroenterol. 2001;36:1222–1226. doi: 10.1080/00365520152584888. [DOI] [PubMed] [Google Scholar]
- 21.Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92. doi: 10.1136/gut.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massari M, Lattuada E, Zappa MA, Pieri G, Cioffi U, De Simone M, Segalin A, Bonavina L. Evaluation of leiomyoma of the esophagus with endoscopic ultrasonography. Hepatogastroenterology. 1997;44:727–731. [PubMed] [Google Scholar]
- 23.Kawamoto K, Yamada Y, Furukawa N, Utsunomiya T, Haraguchi Y, Mizuguchi M, Oiwa T, Takano H, Masuda K. Endoscopic submucosal tumorectomy for gastrointestinal submucosal tumors restricted to the submucosa: a new form of endoscopic minimal surgery. Gastrointest Endosc. 1997;46:311–317. doi: 10.1016/s0016-5107(97)70116-0. [DOI] [PubMed] [Google Scholar]
- 24.Waxman I, Saitoh Y, Raju GS, Watari J, Yokota K, Reeves AL, Kohgo Y. High-frequency probe EUS-assisted endoscopic mucosal resection: a therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc. 2002;55:44–49. doi: 10.1067/mge.2002.119871. [DOI] [PubMed] [Google Scholar]
- 25.Waxman I, Saitoh Y. Clinical outcome of endoscopic mucosal resection for superficial GI lesions and the role of high-frequency US probe sonography in an American population. Gastrointest Endosc. 2000;52:322–327. doi: 10.1067/mge.2000.105723. [DOI] [PubMed] [Google Scholar]
- 26.Kajiyama T, Sakai M, Torii A, Kishimoto H, Kin G, Uose S, Ueda S, Okuma M, Inoue K. Endoscopic aspiration lumpectomy of esophageal leiomyomas derived from the muscularis mucosae. Am J Gastroenterol. 1995;90:417–422. [PubMed] [Google Scholar]
- 27.Giovannini M, Bernardini D, Moutardier V, Monges G, Houvenaeghel G, Seitz JF, Derlpero JR. Endoscopic mucosal resection (EMR): results and prognostic factors in 21 patients. Endoscopy. 1999;31:698–701. doi: 10.1055/s-1999-79. [DOI] [PubMed] [Google Scholar]
- 28.Izumi Y, Inoue H, Kawano T, Tani M, Tada M, Okabe S, Takeshita K, Endo M. Endosonography during endoscopic mucosal resection to enhance its safety: a new technique. Surg Endosc. 1999;13:358–360. doi: 10.1007/s004649900989. [DOI] [PubMed] [Google Scholar]
- 29.Takada N, Higashino M, Osugi H, Tokuhara T, Kinoshita H. Utility of endoscopic ultrasonography in assessing the indications for endoscopic surgery of submucosal esophageal tumors. Surg Endosc. 1999;13:228–230. doi: 10.1007/s004649900950. [DOI] [PubMed] [Google Scholar]
- 30.Sun S, Wang M, Sun S. Use of endoscopic ultrasound-guided injection in endoscopic resection of solid submucosal tumors. Endoscopy. 2002;34:82–85. doi: 10.1055/s-2002-19386. [DOI] [PubMed] [Google Scholar]


