Abstract
AIM: To study the correlation between pancreatic phase CT enhancement, intratumor microvessel density (MVD) and pathologic grading of pancreatic carcinoma and to evaluate the relationship between the degrees of CT enhancement and malignancy of pancreatic carcinoma.
METHODS: Thirty four patients with pancreatic carcinoma underwent CT scanning before resection. The enhancement degrees and forms of tumor were observed in pancreatic phase. The operative sample was stained with HE and CD34 marked by immunohistochemistry. MVD and histopathological grades of pancreatic carcinoma were examined. CT enhancement of the tumor, MVD counting in hot spot areas of neoplastic parenchymal cells and pathological grades of pancreatic carcinoma were comparatively analyzed.
RESULTS: Highly differentiated pancreatic adenocarcinoma was identified in 16 patients, moderately-differentiated tumor in 7 and poorly-differentiated in 11. Isodensity CT enhancement was demonstrated in 13 cases, slightly low density enhancement in 9, slightly low density enhancement with small cystic lesions in 9 and slightly low density enhancement with large cystic lesions in 3. The counting of MVD with CD34 marked by immunohistochemistry in hot spot areas of neoplastic parenchyma cells was small in 10 cases, medium in 16 and large in 8. The pathological grades correlated well with CT enhancement of the tumor (r = 0.7857, P < 0.001) and with MVD counting of tumor (r = 0.3613, P < 0.05). The CT enhancement of tumor correlated with MVD(r = 0.6768, P < 0.001).
CONCLUSION: There is an obvious and significant correlation between CT enhancement, pathological grades and MVD number in the hot spot areas of tumor. The extent of CT enhancement is inversely proportional to the malignant degree of pancreatic carcinoma, and to the MVD number in the hot spot areas of neoplastic parenchyma. The MVD in the hot spot areas of neoplastic parenchyma cells can also reflect the prognosis of the patients, and is directly proportional to the malignant degree of pancreatic carcinoma.
INTRODUCTION
Pancreatic carcinoma is a common malignant tumor. Patients with pancreatic carcinoma have a high mortality rate and short survival time[1-10]. The resection rate of the tumor is only 10%-25%[1,3,6,7]. Early diagnosis is of great clinical significance for pancreatic carcinoma. Some authors reported[11-17,23-32] that tumor angiogenesis was closely correlated with the growth, metastasis and malignancy degree of the tumor. CT enhancement of pancreatic carcinoma in pancreatic phase, intratumor MVD and tumor malignancy degree were assessed in this article.
MATERIALS AND METHODS
Patients
Thirty four patients with pancreatic carcinoma (26 men, 8 women; age range 31-76 years, mean age 55.5 years) were evaluated retrospectively. All the patients underwent CT enhancement and surgery from June 1997 to May 2002. The tissue samples available were studied with CD34 marker by immunohistochemistry in our hospital.
CT scaning
Somatom plus spiral CT scanner (Siemens Company, Germany) and electron beam CT (EBCT) (Imatron Co, America.) were used. Before examination, the patients took 1000 ml water containing a few iodinated contrast materials (Iodinated degree less than 5%). The section thickness was usually 10 mm in liver and 5 mm in pancreas. The scanning time was 1 s. Iodinated non-ionic contrast material (iopamidol 300; Bracco, Milan, Italy) was injected at a rate of 2.5-3 ml/S for a total of 100 ml by a high pressure autoinjector. General scanning was done at first. CT enhancement was performed in the pancreatic phase, and the scanning time was 35 s after injection of the contrast agent.
Pathologic study
Tumor specimens obtained from resected pancreatic carcinoma were fixed with 10% formalin and embedded in paraffin. The specimens were stained with hematoxylin-eosin. Four-micron-thick sections were mounted on silanized slides. The histopathologic grades of pancreatic carcinoma were observed by microscopy. The neoplastic parenchyma cells, fibrotic stroma, necrotic tissuse and residual pancreatic tissue were also examined. The sections were then incubated with mouse monoclonal antibody of CD34 for 24 h at room temperature. After being washed with phosphate-buffered solution (PBS), the sections were incubated with avidin-biotin-peroxidase complex (Histofine) for 30 min and washed once more with PBS. The sections were finally incubated with 3,3-diaminobenzidine (Simplestain DAB; Nichirei). To assess the intratumor MVD, we used the counting methods of intratumor MVD reported by Weidenr[17]. We assessed delineated CD34-positive cells as microvessels (size 0.02-0.10). Stained vessels were counted in a × 200 microscopic field in hot spot areas of neoplastic parenchyma cells. To count the immunohistochemical anti-CD34 antibody stained vessels in hot spot areas of tumor parenchymal cells, the average MVDs were calculated in three hot spot areas. The tumor MVD was categorized into four grades: “+” the average number of MVD < 10, “++” between 10 and 20, “+++” between 20 and 40, “++++” > 40.
Statistical analysis
Methods of evaluating extent of CT enhancement The enhancement effects of pancreatic parenchymal tissue were the same in the pancreatic-phase and dual-phase enhanced scanning[18-20,33-36]. Therefore, the extent of CT enhancement of pancreatic carcinoma was only assessed in the pancreatic phase. The degree and form of the tumor in CT enhancement compared to normal pancreatic tissue could be classified as follows: I-isodense enhancement, indicating the degree of tumor CT enhancement was the same as that of the surrounding normal pancreatic tissues; II-slightly low density enhancement, indicating the degree of tumor CT enhancement was slightly lower than that of the surrounding normal pancreatic tissue, with few blood vessels in the tumor; III-slightly low density enhancement with small hypodense cystic lesions, indicating the tumor enhancement was the same as II, but with small cystic density regions in the tumor; IV-cystic low density lesion with slight enhancement of surrounding tumor tissue, indicating large necrotic lesions in the central area and slight enhancement in the surrounding tumor tissue.
Statistical methods The degree of CT enhancement of the tumor, pathological grading of pancreatic carcinoma and the average number of MVD in hot spot areas of neoplastic parenchymal cells were assessed by SPSS software. We used Spearman correlation test with two unpaired samples, a level of significance was set when P value < 0.05.
RESULTS
The results of the patients’ CT scanning, HE staining and immunohistochemistry were calculated. Well-differentiated pancreatic carcinoma was identified in 16 cases, one with solid cystic-papillary tumor. Moderately-differentiated pancreatic carcinoma was identified in 7, among which one was highly moderately-differentiated. Poorly differentiated pancreatic carcinoma was identified in 11, among which one was highly poorly-differentiated and 2 were moderately poorly-differentiated. The tumor was localized in pancreatic head in 19 cases, pancreatic head and body in 2, pancreatic body in 6, pancreatic body and tail in 1, pancreatic tail in 4, upper edge of pancreas with involvement of ligamentum hepatoduodenale in 1, pancreatic groove with involvement of second part of horizontal duodenum in 1. Immunohistochemical study of tumor specimens’ showed MVD count in hot spot areas of tumor parenchymal cells was least in 10 cases, medium in 16 and most in 8. Its routine histopathological results, CT enhancement, MVD and pathological grading are shown in Table 1 and Table 2 and Figure 1, Figure 2, Figure 3, Figure 4.
Table 1.
CT enhancement, pathologic grades and MVD number in hot spot areas of neoplastic parenchymal cells of pancreatic carcinoma
| Pathologic grades | No of cases |
CT enhancement degree of tumor |
MVD in hot spot area in neoplastic parenchymal cells |
||||||
| I | II | III | IV | + | ++ | +++ | +++ | ||
| Well differentiated | 16 | 12 | 3 | 1 | 0 | 8 | 5 | 3 | 0 |
| Moderately differentiated | 7 | 1 | 4 | 2 | 0 | 1 | 5 | 1 | 0 |
| Poorly differentiated | 11 | 0 | 2 | 6 | 3 | 1 | 6 | 4 | 0 |
| Total | 34 | 13 | 9 | 9 | 3 | 10 | 16 | 8 | 0 |
Table 2.
CT enhancement degree in pancreatic phase and MVDs in hot spot areas of tumor parenchyma in pancreatic carcinoma
|
CT enhancement degree |
MVDs in hot spot areas in tumor parenchyma |
||||
| Enhanced type | n | + | ++ | +++ | ++++ |
| I | 13 | 9 | 4 | 0 | 0 |
| II | 9 | 1 | 5 | 3 | 0 |
| III | 9 | 0 | 6 | 3 | 0 |
| IV | 3 | 0 | 1 | 2 | 0 |
Figure 1.

A 52-year-old man with well-differentiated pancreatic head carcinoma. A: EBCT imaging revealed that bulging pancreatic head (white arrow), density of mass in head and neck were the same as that of pancreatic body; B: EBCT enhancement imaging was 35 s after administration of contrast agent revealed that enhancement of mass in pancreatic head (white arrow) was the same as that of normal pancreatic tissue; C: Hematoxylin-eosin-stained specimen (100 ×) demonstrated the irregularity around tumor cell adeno-tubula structure and residual pancreatic tissue; D: Showing immunohistochemical staining of anti-CD34 antibody (200 ×). CD34-positive cells were counted as microvessels (tan color), MVDs in hot spot area of the neoplastic cells in well-differentiated pancreatic carcinomal; E: High MVDs of residual pancreatic tissue in well differenti-ated pancreatic carcinoma (200 ×).
Figure 2.
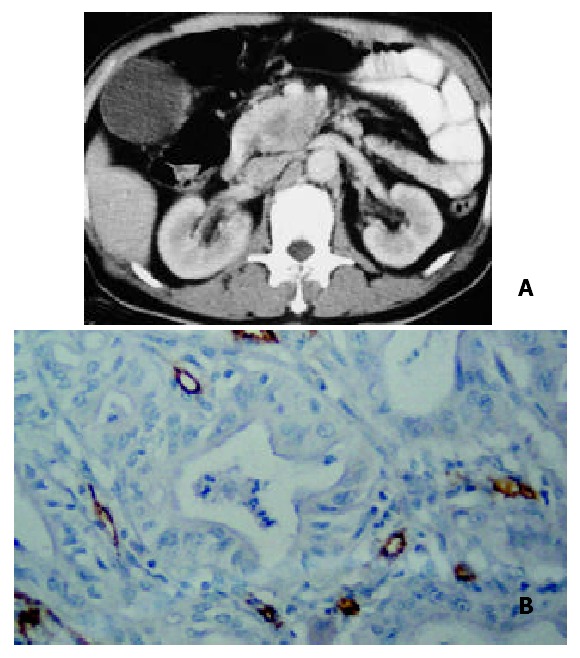
A 52-year-old man with moderately-differentiated pancreatic head carcinoma. A: Pancreatic-phase helical CT enhancement imaging 35 s after administration of contrast agent revealed that CT enhancement of mass in pancreatic head was slightly lower than that of normal pancreatic tissues. Scarce blood vessel area appeared in the central region of the mass; B: Showing immunohistochemical staining of anti-CD34 antibody (200 ×). CD34-positive cells were counted as microvessels (tan color), moderate MVDs in hot spot area of the neoplastic cells in moderately-differentiated pancreatic carcinoma.
Figure 3.
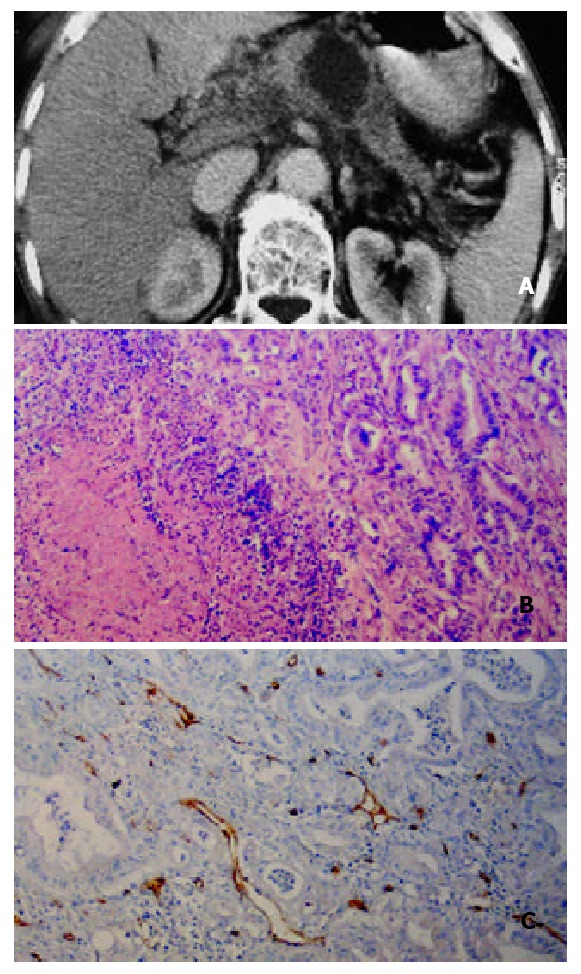
A 59-year-old man with poorly differentiated pancreatic body carcinoma. A: Helical CT enhancement imag-ing 35 s after administration of contrast agent revealed that pancreatic-phase CT enhancement of mass in pancreatic body was lower than that of surrunding normal pancreatic tissues. Necrotic tissue in the center of the mass was not enhanced. Tumor tissue around the necrotic tissue was slightly enhanced; B:Hematoxylin-eosin-stained specimen (100 ×) demonstrated massive necrosis and decreased re-sidual pancreatic tissue; C: Showing immunohistochemical staining of anti-CD34 antibody (200 ×). CD34-positive cells were seen as microvessels (brown yellow color), more MVDs in hot spot area of the neoplastic cells in poorly-differenti-ated pancreatic carcinoma.
Figure 4.

A 70-year-old woman with solid-cystic-papillary well-differentiated pancreatic carcinoma with involvement of ligamentum hepatoduodenale. A: Helical CT enhancement imaging 35 s after administration of contrast agent revealed that CT enhancement of mass was heterogenously slight low density with low density necrotic region; B: Hematoxylin-eosin-stained specimen (100 ×) demonstrated massive necrosis tissuse and irregular tubula structure in well-differentiated pancreatic carcinoma.
CT enhancement degree of tumor, pathological grading of pancreatic carcinoma and the average count of MVD in hot spot areas of neoplastic parenchyma cells were comparatively assessed by Spearman correlation test. The pathological grades correlated well with CT enhancement of tumor (r = 0.7857, t = 7.1851, P < 0.001), and with MVD count of tumor(r = 0.3613, t = 2.1917, P < 0.05).
There was a significant correlation between CT enhancement degree in pancreatic phase and MVDs in hot spot areas of neoplastic parenchyma in pancreatic carcinoma (r = 0.6768, t = 5.2006, P < 0.001).
DISCUSSION
The grading of the tumor, its occurrence, progression and metastasis, were all dependent on tumor angiogenesis[13,17,23-25]. Tumor angiogenesis was first proposed by Folkman[11,13]. In recent years, study of intratumor angiogenesis has become a hot spot in neoplastic research[11-17,23-25]. Tumor angiogenesis mainly includes an increase of newly grown microvessels and their permeability change. Histopathology usually reflects an increase or decrease of intratumour MVDs. CT enhancement can also indirectly reflect the situation of intratumor angiogenesis because the enhancement degree of tumor is dependent upon the number of blood vessels within the tumor[12,21,22].
The result of our study showed that the pathological grades of pancreatic carcinoma and CT enhancement of the tumor were significantly correlated (r = 0.7857, P < 0.001). Table 1 shows isodensity in 75% (12/16) of well-differentiated pancreatic cancer, 14.3% (1/7) in moderately-differentiated, and 0% (0/11) in poorly differentiated. Slightly low density or low density was 25% (4/16) in well-differentiated tumour, 85.7% (6/7) in moderately-differentiated tumour, 100% (11/11) in poorly differentiated tumour. This indicated that the malignant degree of pancreatic carcinoma was inversely proportional to its CT enhancement degree, i.e., the higher the malignant degree, the lower the CT enhancement.
It is agreed that the pathological grades of pancreatic carcinoma are directly proportional to the number of intratumor MVD[21], but Table 2 shows that CT enhancement was inversely proportional to the number of MVD in hot spot areas of neoplastic parenchymal cells (P < 0.001). The reason was that the microvessel and its tissue composition of pancreatic carcinoma were different from malignant tumors of other organs such as lung cancer and renal cancer. Pancreatic carcinoma has its own distinct characteristics, usually diffuse fibrosis[23]. Therefore, the CT enhancement is decided by the combination of the total number of intratumor MVD, the fibrous stroma and residual normal pancreatic tissue. A small number of tumor parenchymal cells, a medium number of fibrosis and a large number of residual normal pancreatic tissue constitute well-differentiated pancreatic carcinoma shown in its histopathology. The MVD of the residual pancreatic tissue stained CD34 by immunohistochemistry was abundant (Figure 1D), and the MVD in hot spot areas of neoplastic parenchymal cells was scarce in well-differentiated pancreatic cancer. So the total number of intratumor MVD was mainly decided by the number of MVD within the residual pancreatic tissue (Figure 1E). As we could see that the total number of MVD within the well-differentiated pancreatic cancer was almost the same as that of the surrounding normal pancreatic tissue, hence, the CT enhancement degree of well-differentiated pancreatic carcinoma in pancreatic phase was similar to that of the surrounding normal pancreatic tissue. Whereas in the moderately and poorly-differentiated pancreatic carcinoma, the volume of tumor parenchymal cells increased and the residual pancreatic tissue was relatively decreased. Therefore, CT enhancement degree in these pancreatic carcinoma was lower than that in the well-differentiated pancreatic carcinoma. One could see the scarce microvessel showed slightly-low density enhancement (Figure 2A). Some small cyst and large cystic or low density regions within the tumor were necrosis lesions (Figure 3A).
Linder and others belivered that MVD count of tumor cells was larger than that of the tumor matrix, the former was closely related to the degree of malignancy[23]. According to our data, the pathological grades of pancreatic carcinoma were to some extent correlated with the intratumor MVD count (P < 0.05), but significantly correlated with the CT enhancement degree in the pancreatic-phase. This indicated that CT enhancement could effectively evaluate the malignant degree of pancreatic carcinoma before operation. However, pancreatic-phase CT enhancement of pancreatic carcinoma has its own particular features (Figure 4).
To sum up, if CT enhancement degree of pancreatic carcinoma is as high as that of the surrounding normal pancreatic tissues, it would reflect a high MVD in the residual pancreatic tissue and a small count of intratumoral MVD indicating pancreatic carcinoma is well-differentiated. If CT enhancement degree is slightly lower than that of the normal pancreatic tissue with a vessel voided area, with or without low-density cystic areas, it would reflect a moderately-differentiated pancreatic cancer. The appearance of heterogeneously slight-low density areas with a large low density necrotic lesion might reflect a highly malignant and poorly-differentiated pancreatic carcinoma.
ACKNOWLEDGEMENTS
I wish to express our sincere and hearty thanks to Professor Li Jieshou, academician of the Chinese Academy of Engineering. We are deeply indebted to his careful and enlightening advice.
Footnotes
Edited by Wu XN and Wang XL
References
- 1.Ghaneh P, Slavin J, Sutton R, Hartley M, Neoptolemos JP. Adjuvant therapy in pancreatic cancer. World J Gastroenterol. 2001;7:482–489. doi: 10.3748/wjg.v7.i4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin LX. Chromosomal aberrations related to metastasis of human solid tumors. World J Gastroenterol. 2002;8:769–776. doi: 10.3748/wjg.v8.i5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar A, Russell RC. Recent advances in the surgical treatment of pancreatic cancer. World J Gastroenterol. 2001;7:622–626. doi: 10.3748/wjg.v7.i5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo XZ, Friess H, Shao XD, Liu MP, Xia YT, Xu JH, Buchler MW. KAI1 gene is differently expressed in papillary and pancreatic cancer: influence on metastasis. World J Gastroenterol. 2000;6:866–871. doi: 10.3748/wjg.v6.i6.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu B, Staren E, Iwamura T, Appert H, Howard J. Effects of Taxotere on invasive potential and multidrug resistance phenotype in pancreatic carcinoma cell line SUIT-2. World J Gastroenterol. 2001;7:143–148. doi: 10.3748/wjg.v7.i1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidler IJ. Angiogenesis and cancer metastasis. Cancer J. 2000;6 Suppl 2:S134–S141. [PubMed] [Google Scholar]
- 7.Ruf W, Mueller BM. Tissue factor in cancer angiogenesis and metastasis. Curr Opin Hematol. 1996;3:379–384. doi: 10.1097/00062752-199603050-00008. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Staren E, Iwamura T, Appert H, Howard J. Taxotere resistance in SUIT Taxotere resistance in pancreatic carcinoma cell line SUIT 2 and its sublines. World J Gastroenterol. 2001;7:855–859. doi: 10.3748/wjg.v7.i6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou ZG, Chen YD, Sun W, Chen Z. Pancreatic microcirculatory impairment in experimental acute pancreatitis in rats. World J Gastroenterol. 2002;8:933–936. doi: 10.3748/wjg.v8.i5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demachi H, Matsui O, Kobayashi S, Akakura Y, Konishi K, Tsuji M, Miwa A, Miyata S. Histological influence on contrast-enhanced CT of pancreatic ductal adenocarcinoma. J Comput Assist Tomogr. 1997;21:980–985. doi: 10.1097/00004728-199711000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. What is the evidence that tumors are angiogenesis dependent. J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 12.Passe TJ, Bluemke DA, Siegelman SS. Tumor angiogenesis: tutorial on implications for imaging. Radiology. 1997;203:593–600. doi: 10.1148/radiology.203.3.9169673. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Fighting cancer by attacking its blood supply. Sci Am. 1996;275:150–154. doi: 10.1038/scientificamerican0996-150. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 15.Jiang WG, Puntis MC, Hallett MB. Molecular and cellular basis of cancer invasion and metastasis: implications for treatment. Br J Surg. 1994;81:1576–1590. doi: 10.1002/bjs.1800811107. [DOI] [PubMed] [Google Scholar]
- 16.Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56:4264–4266. [PubMed] [Google Scholar]
- 17.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 18.Boland GW, O'Malley ME, Saez M, Fernandez-del-Castillo C, Warshaw AL, Mueller PR. Pancreatic-phase versus portal vein-phase helical CT of the pancreas: optimal temporal window for evaluation of pancreatic adenocarcinoma. AJR Am J Roentgenol. 1999;172:605–608. doi: 10.2214/ajr.172.3.10063844. [DOI] [PubMed] [Google Scholar]
- 19.Sheridan MB, Ward J, Guthrie JA, Spencer JA, Craven CM, Wilson D, Guillou PJ, Robinson PJ. Dynamic contrast-enhanced MR imaging and dual-phase helical CT in the preoperative assessment of suspected pancreatic cancer: a comparative study with receiver operating characteristic analysis. AJR Am J Roentgenol. 1999;173:583–590. doi: 10.2214/ajr.173.3.10470884. [DOI] [PubMed] [Google Scholar]
- 20.McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebremariam A. Multi--detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97–102. doi: 10.1148/radiology.220.1.r01jl1897. [DOI] [PubMed] [Google Scholar]
- 21.Miles KA. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: a review. Eur J Radiol. 1999;30:198–205. doi: 10.1016/s0720-048x(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 22.Tateishi U, Nishihara H, Watanabe S, Morikawa T, Abe K, Miyasaka K. Tumor angiogenesis and dynamic CT in lung adenocarcinoma: radiologic-pathologic correlation. J Comput Assist Tomogr. 2001;25:23–27. doi: 10.1097/00004728-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Linder S, Blåsjö M, von Rosen A, Parrado C, Falkmer UG, Falkmer S. Pattern of distribution and prognostic value of angiogenesis in pancreatic duct carcinoma: a semiquantitative immunohistochemical study of 45 patients. Pancreas. 2001;22:240–247. doi: 10.1097/00006676-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kasper HU, Ebert M, Malfertheiner P, Roessner A, Kirkpatrick CJ, Wolf HK. Expression of thrombospondin-1 in pancreatic carcinoma: correlation with microvessel density. Virchows Arch. 2001;438:116–120. doi: 10.1007/s004280000302. [DOI] [PubMed] [Google Scholar]
- 25.Hotz HG, Reber HA, Hotz B, Sanghavi PC, Yu T, Foitzik T, Buhr HJ, Hines OJ. Angiogenesis inhibitor TNP-470 reduces human pancreatic cancer growth. J Gastrointest Surg. 2001;5:131–138. doi: 10.1016/s1091-255x(01)80024-x. [DOI] [PubMed] [Google Scholar]
- 26.Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, Evans DB, Abbruzzese JL, Hicklin DJ, Radinsky R. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6:1936–1948. [PubMed] [Google Scholar]
- 27.Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 28.Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239–2245. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee SK, Zoubine MN, Mullick M, Weston AP, Cherian R, Campbell DR. Tumor angiogenesis in chronic pancreatitis and pancreatic adenocarcinoma: impact of K-ras mutations. Pancreas. 2000;20:248–255. doi: 10.1097/00006676-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S, Doi R, Imamura M. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer. 1998;34:1439–1447. doi: 10.1016/s0959-8049(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 31.Terris B, Scoazec JY, Rubbia L, Bregeaud L, Pepper MS, Ruszniewski P, Belghiti J, Fléjou J, Degott C. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology. 1998;32:133–138. doi: 10.1046/j.1365-2559.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, et al. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553–1563. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coley SC, Strickland NH, Walker JD, Williamson RC. Spiral CT and the pre-operative assessment of pancreatic adenocarcinoma. Clin Radiol. 1997;52:24–30. doi: 10.1016/s0009-9260(97)80301-7. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko K, Honda H, Hayashi T, Fukuya T, Ro T, Irie H, Masuda K. Helical CT evaluation of arterial invasion in pancreatic tumors: comparison with angiography. Abdom Imaging. 1997;22:204–207. doi: 10.1007/s002619900173. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa H, Iwata R, Moriyama N, Kosuge T. Selective intraarterial contrast-enhanced CT of pancreaticoduodenal tumors: early clinical experience in evaluating blood supply and detectability. AJR Am J Roentgenol. 2000;175:91–97. doi: 10.2214/ajr.175.1.1750091. [DOI] [PubMed] [Google Scholar]
- 36.O'Malley ME, Boland GW, Wood BJ, Fernandez-del Castillo C, Warshaw AL, Mueller PR. Adenocarcinoma of the head of the pancreas: determination of surgical unresectability with thin-section pancreatic-phase helical CT. AJR Am J Roentgenol. 1999;173:1513–1518. doi: 10.2214/ajr.173.6.10584794. [DOI] [PubMed] [Google Scholar]


