Abstract
MicroRNAs (miRNAs) play critical roles in carcinogenesis and tumor progression. Recent research has revealed miR-101-3p as an important regulator in several cancers. Nevertheless, its function in salivary gland Adenoid cystic carcinoma (ACC), a relatively rare malignance with poor long-term survival rate arisen in head and neck region, remain unknown. In this study, down-regulated miR-101-3p expression was detected in ACC tissues and ACC cell lines with high potential for metastasis. Ectopic expression of miR-101-3p significantly repressed the invasion, proliferation, colony formation, and formation of nude mice xenografts and induced potent apoptosis in ACC cell lines. The provirus integration site for Moloney murine leukemia virus 1 (Pim-1) oncogene was subsequently confirmed as a direct target gene of miR-101-3p in ACC. Functional restoration assays revealed that miR-101-3p inhibits cell growth and invasion by directly decreasing Pim-1 expression. Protein levels of Survivin, Cyclin D1 and β-catenin were also down-regulated by miR-101-3p. miR-101-3p enhanced the sensitivity of cisplatin in ACC cell lines. Taken together, our results demonstrate that the novel miR-101-3p/Pim-1 axis provides excellent insights into the carcinogenesis and tumor progression of ACC and may be a promising therapeutic target for this type of cancer.
Keywords: miR-101-3p, Pim-1, ACC, chemotherapeutic sensitivity
Introduction
Salivary gland adenoid cystic carcinoma (ACC) is a relatively rare epithelial tumor characterized by neural and vessel invasion and a high incidence of distant metastasis [1]. Despite its slow growth, ACC exhibits high potential of recurrence. The long-term survival rate of patients with this cancer is fairly low, especially in patients with distant metastasis. In fact, 33% of all patients with distant metastasis are expected to die within 2 years [2,3]. Surgical resection followed by radiotherapy are suitable for treating the early stages of this malignancies in the absence of metastasis; chemotherapy is essential for management of advanced metastasis or local recurrence [4]. However, the overall response to single-agent is only 1% to 9%. Among these traditional agents currently available, cisplatin elicits the best results [5]. After exposure to a single chemotherapy agent, cancerous cells usually develop multidrug resistance, which is the leading factor influencing cancer-related deaths [6,7]. The precise underlying mechanisms of the ACC initiation and progression remain unclear. Therefore, a better understanding of molecular events during ACC progression is necessary; such knowledge may contribute to the development of a novel therapeutic strategy to improve the prognosis of ACC patients.
MicroRNAs (miRNAs) are a new class of regulatory endogenous small noncoding RNAs that are significantly involved in controlling gene expression. Mature miRNAs are composed of approximately 22 nucleotides. By binding with the 3’ un-translated region (3’UTR) imperfectly complementarily, miRNAs exert degradation, cleavage or inhibition effect on gene translation [8]. Substantial evidence indicates that miRNAs critically regulate tumor initiation and progression by targeting oncogenes, tumor suppressors, and genes regulating cell proliferation, angiogenesis, apoptosis or migration [9-12]. miRNAs expression profiling can be used as a tool for predicting the prognosis of cancer patients [13-15]. Among known miRNAs, miRNA-101 was suggested as a tumor suppressor because of its distinct down-regulation in numerous types of cancers including liver, breast, prostate cancer as well as head and neck cancer [16-20]. Emerging studies demonstrate that miR-101 affects the tumorigenicity, survival, invasion and migration of tumor cells in several types of cancer [16,17,21]. Moreover, notably, recently study revealed that miRNA-101 is a potential autophagy inhibitor by targeting STMN1, RAB5A and ATG4D [22] . Enforced miR-101-3p expression enhanced the drug sensitivity of hepatocellular carcinoma cells by inhibiting the protective autophagy induced by cisplatin [23]. However, no study has yet focused on miR-101-3p in salivary gland ACC.
We hypothesize that miR-101-3p plays may pivotal role in the initiation and progression of human salivary gland ACC. In the present study, we aim to identify miR-101-3p expression in human salivary gland ACC specimens. In vitro functional assay was used to confirm the anti-tumor effects of miR-101-3p in SACC-83 and its corresponding highly metastatic SACC-LM line by directly targeting Provirus integration site for Moloney murine leukemia virus 1 (Pim-1), a widely accepted oncogene that belongs to the Ser/Thr kinase family [24,25]. Our study also emphasizes the role of miR-101-3p in enhancing the drug sensitivity of cisplatin. In summary, miR-101-3p was found to be a novel potential therapeutic target for salivary adenoid cystic carcinoma.
Material and methods
Tissue specimens
Tissue samples comprising 30 histopathologically comformed salivary gland ACCs and 10 normal parotid glands were obtained from the Department of Oral and Maxillofacial-Head and Neck Oncology, School and Hospital of Stomatology, Wuhan University. The present study was approved by the ethics committee of Hospital of Stomatology, Wuhan University.
Cell lines and cell culture
The highly metastatic human salivary gland ACC cell line SACC-LM, and its corresponding parental cell line SACC-83 were obtained from the School and Hospital of Stomatology, Peking University as a gift. SACC-83 and SACC-LM cells were cultured in PRMI-1640 (HyClone, USA) with 10% fetal bovine serum (FBS) (Gibco, USA) at 37°C in a humidified atmosphere containing 5% CO2.
Vector construction
The human pri-miR-101-3p sequence was amplified by nested PCR through using Primer STAR Premix (TaKaRa, Japan) and cloned into the pWPXL lentivirus expression vector (addgene, USA) to construct pWPXL-miR-101-3p. The predicted binding sites in the 3’UTR of Pim-1, which is the potential gene of miR-101-3p, was amplified through nested PCR and then cloned into the region directly downstream of a CMV promoter-driven firefly luciferase cassette in a pcDNA3.0 vector (p-Luc). The mutant 3’UTR of Pim-1 with the mutated sequence, which is in the complementary site for the miR-101-3p seed region, was constructed through overlap-extension PCR according to the p-Luc-Pim-1 3’UTR-WT plasmid. The Open reading frame (ORF) of Pim-1 was amplified and then cloned into pWPXL vector.
Lentivirus production and transduction
After pWPLX-miR-101-3p or pWPXL-Pim-1 co-transfection with the packaging plasmid ps-PAX2 and the envelop plasmid pMD2G (addgene, USA) into HEK-293T cells by LipoFectamine 2000 (Invitrogen, USA) for 48 h, the virus particles were harvested. SACC-83 cells and SACC-LM cells were infected with recombinant lentivirus-transducing units and 6 μg/ml polybrene (Sigma, USA). The cells were validated by using quantitative real-time PCR (qRT-PCR) (for miR-101-3p) or Western blotting (for Pim-1).
Luciferase reporter assay
SACC-83 cells and SACC-LM cells were seeded in 96-well plates and co-transfected with 200 ng of pWPXL-miR-101-3p or pWPXL, 50 ng luciferase reporter as well as 10 ng pRL-CMV Renilla luciferase reporter by using Lipofectamine 2000 (Invitrogen. USA) for 48 h. The luciferase activities were examined by using a dual-luciferase reporter assay kit (Promega) according to the manufacturer’s instruction.
Oligonucleotide transfection
The specific siRNA sequence of Pim-1 (Qiagen, USA) was transfected into SACC-83 and SACC-LM cells at a final concentration of 5 nM by using LipoFectamine 2000 (Invitrogen. USA). All-star negative controls (Qiagen, USA) were used as negative controls to prove the absence of interference by other mRNAs. The miR-101-3p mimics and inhibitor (anti-miR-101-3p), which were purchased from Genepharma, China, were transfected into SACC-83 cells and SACC-LM cells at a final concentration of 50 nM by using LipoFectamine 2000 (Invitrogen. USA) according to the manufacturer’s instructions.
RNA extraction and qRT-PCR
Total RNA, including miRNA, was extracted by using a Qiagen miRNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. cDNA was synthesized from total RNA by using a PrimeScript RT reagent kit (TaKaRa, Japan). qRT-PCR analyses using SYBR Premix Ex Taq were carried out out to quantify relative mRNA expression. GAPHD was used as an internal loading control. Relative miR-101-3p expression was determine by using TaqMan microRNA assays (Applied Biosystems, USA). U6 small nuclear RNA was used as internal loading control. The relative miR-101-3p expression was normalized to U6 and Pim-1 was normalized to GAPDH by using the 2-ΔΔCt equation.
Cell proliferation assay and colony formation
Cell Counting Kit-8 (Dojindo Laboratories, Japan) was used for cell proliferation assays according to the manufacturer’s instruction. For the colony formation assay, SACC-83 or SACC-LM stably expressing miR-101-3p or the corresponding parental cells were seeded into flat-bottomed 24-well plated at a density of 300 cells per well. The plates were cultured in PRMI-1640 medium (HyClone, USA) with 10% fetal bovine serum (FBS) (Gibco, USA) at 37°C in a humidified atmosphere containing 5% CO2 for 10 d. After colonies bad become visible, they were fixed using methanol and stained with 0.1% crystal violet (Sigma, USA). The numbers of colonies formed were counted.
Transwell invasion assay
Costar Transwell inserts (#3422, pore size, 8 μm) (Coring, USA) were used for in vitro invasion assay. SACC-83 or SACC-LM stably expressing miR-101-3p or its corresponding parental cells were seeded into the upper chamber coated with Matrigel (BD Bioscience) at a density of 10000 cells per well with 100 µl FBS-free PRMI-1640 medium. Medium with 10% FBS was added into the bottom chamber of the insert for invasion stimulation. After incubation at 37% with 5% CO2 for 24 h, the cells in the upper chamber were carefully removed and the cells that invaded through the Matrigel were fixed using methanol and stained with 0.1% crystal violet. Cells that underwent invasion were photographed and quantified.
Flow cytometry
Cells were washed thrice by PBS and then lysed using 0.1% trypsin with 0.01% EDTA at pH 7.5. The collected cells were wash with normal medium and cold PBS (4°C) and then resuspended in 400 µl of 1 × binding buffer (BD-Pharmingen Biosciences, USA) per sample. The cells were incubated in the dark with 5 µl of Annexin V and 10 µl of propidium iodide (PI) (BD-Pharmingen Biosciences, USA) for 15 min. Afterward, their apoptotic rate was evaluated by using flow cytometry.
Nude mice xenografts formation assay
Female athymic BALB/c nude mice (18-20 g, 5-6 weeks old) were purchased form the Experimental Animal Laboratory of Wuhan University. All animals were kept in sterile laminar flow cabinets under appropriate pathogen-free conditions with a 12 h:12 h light:dark cycle. The mice used were kept and manipulated according to protocols approved by the Laboratory Animal Care and Use Committee of Wuhan University. SACC-LM cells and miR-101-3p expressing SACC-LM cells were harvested from culture plates and subcutaneously inoculated into the right flank of the mice at a density of 2 × 106 in 0.2 ml of medium (SACC-LM for control vector group, miR-101-3p expressing SACC-LM for miR-101-3p group). Tumors were measured by using a caliper and calculated according to the formula (width2 × length)/2. The mice were euthanized after 6 weeks for tumor harvest. Tumor tissues were weighed and then embedded in paraffin for immunohistochemical staining or frozen at -80°C for western blotting analysis.
Reagents, antibodies and Western blot analysis
Cisplatin was purchased from Selleck Chemicals (USA). The primary antibodies used in our study included rabbit anti-Pim-1 (1:1000, GeneTex, USA), rabbit anti-survivin (1:1000, Cell Signaling, USA), rabbit anti-Cyclin D1 (1:1000, Abcam, UK) and β-catenin (1:1000, cell signaling). Protein-lysed of cells or xenografts were separated on 10% SDS-PAGE gel and then transferred to a nitrocellulose membrane (Bio-Rad). The membrane was blocked with 5% non-fat milk and incubated overnight with appropriate antibodies at 4°C. Proteins were visualized by using enhanced chemiluminescense detection kit reagents (West Pico, Thermo Fisher Scientific, USA).
Immunohistochemistry
Nude mice xenograft tumor tissues were embedded in paraffin, cut into 4 µm sections, and dried at 60°C for 2 h. For antigen retrieval, sections were boiled in 0.01 M citric acid buffer solution (pH=6.0) at high pressure for 1.5 min. The sections were incubated with 3% hydrogen superoxide for 20 min to quench endogenous peroxidase activity. After incubation with 10% normal goat serum for non-specific binding blocking, the sections were incubated with rabbit anti-Ki67 antibody (1:200, GeneTex, USA) at 4°C overnight. Then the sections were incubated overnight with a secondary biotinylated immunoglobulin G antibody solution followed with an avidin-biotin-peroxidase solution for 20 min at 37°C. Protein expression was visualized by using 3,3’-diaminobenzidine tetrachloride. Mayer’s haematoxylin was used for counterstaining.
Statistical analysis
All experiments were independently replicated at least thrice. All Data values, which are expressed as means ± SD, were analyzed by using Graphpad Prism v5.0 software package (Graph Pad Software Inc, USA). Differences between groups were analyzed by using two-tailed Student’s t-test or one-way ANOVA followed by Turkey or Bonferroni multiple comparison tests. Correlation between the miR-101-3p expression and Pim-1 were determined by two-tailed Pearson’s statistics. P<0.05 was considered statistically significant (*P<0.05, **P<0.01, ***P<0.001).
Results
miR-101-3p expression in human salivary adenoid cystic carcinoma specimens
High-throughput miRNA profiling of head and neck cancer tissues revealed that miR-101-3p is down-regulated in these tissues [20]. To elucidate the expression of miR-101 in human salivary adenoid cystic carcinoma (ACC), qRT-PCR was performed in 10 samples of normal parotid glands and 30 samples of ACCs. Similar to the data obtained from several other cancer types, the expression of miR-101-3p in ACC tissues was significantly reduced compared with that in normal parotid glands (P<0.05, Figure 1A). Furthermore, different miR-101-3p expression levels in two ACC cell lines (SACC-83 and SACC-LM) were detected. The SACC-LM cell line, which is derived from the lung metastasis of the SACC-83 cell line, is considered more malignant because of its potential for metastasis [26]. miR-101-3p expression in the SACC-LM cell line was notably higher than this in the SACC-83 cell line (P<0.05, Figure 1C). These results demonstrate that miR-101-3p is probably a potential tumor suppressor in salivary ACC.
Figure 1.
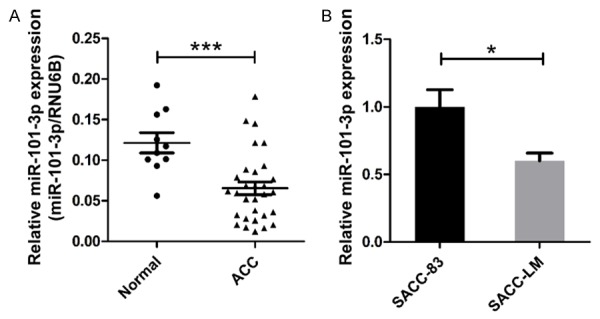
miR-101-3p expression in human salivary adenoid cystic carcinoma (ACC) specimens. A. Real-time PCR results showed that miR-101-3p is significantly decreased in human ACC tissues compared with normal parotid glands (t-test, P<0.001). B. miR-101-3p was down-regulated in the highly metastatic ACC cell line SACC-LM (t-test, P<0.05). *, P<0.05. **, P<0.01. ***, P<0.001. Error bars indicate mean ± S.D.
MiR-101-3p inhibits cell invasion, proliferation, and induces apoptosis of ACC cell lines
Decreased miR-101-3p expression in human salivary ACC tissues suggested that miR-101-3p may be associated with tumorigenesis. To elucidate miR-101-3p functions in ACC, a series of in vitro functional assays were performed on the SACC-83 and SACC-LM cell lines. SACC-83 and SACC-LM cell lines stably expressing miR-101-3p were constructed. Enforced miR-101-3p expression significantly decreased the Matrigel invasion of SACC-83 and SACC-LM cells; invasion could be partially or entirely rescued by anti-miR-101-3p (P<0.05, Figure 2A and with quantities in Figure 2B and 2C).
Figure 2.

miR-101-3p inhibits cell invasion, proliferation, and induces apoptosis of human salivary gland adenoid cystic carcinoma (ACC) cell lines. A. Ectopic miR-101-3p expression significantly inhibited the invasion of ACC cells, whereas miR-101-3p silencing (anti-miR-101-3p) enhanced the invasion of ACC cells. Scale bar=50 μm. B. Quantification of invaded SACC-83 cells (t-test, *, P<0.05. **, P<0.01. ***, P<0.001). C. Quantification of invaded SACC-LM cells (t-test, *, P<0.05. **, P<0.01. ***, P<0.001). D. Ectopic miR-101-3p expression induced apoptosis in SACC-83 and SACC-LM cells; PI indicates propidium iodide. E. Ectopic miR-101-3p expression significantly reduced the colony formation of SACC-83 and SACC-LM cells, with quantification on the right (t-test, P<0.001). F. Ectopic miR-101-3p expression significantly repressed the proliferation of SACC-83 and SACC-LM cells, whereas miR-101-3p silencing (anti-miR-101-3p) enhanced the proliferation of SACC-83 and SACC-LM cells (**, P<0.01. ***, P<0.001). Cell Counting Kit-8 was used for proliferation rate examination. Error bars indicate mean ± S.D.
Recent studies report that miR-101-3p induces apoptosis in hepatocellular carcinoma [17]. Thus, apoptosis rates between ACC cell lines expressing miR-101-3p and their corresponding parental cells were compared. As expected, flow cytometry results demonstrated increased apoptosis rates in both cell lines expressing miR-101-3p (Figure 2D). Moreover, miR-101-3p potently inhibited colony formation numbers in SACC-83 and SACC-LM cells (P<0.05, Figure 2E). To confirm the miR-101-3p effect on cell proliferation, an MTT cell proliferation assay was carried out. Ectopic miR-101-3p expression remarkably inhibited the growth of SACC-83 and SACC-LM cells whereas silencing significantly promoted the growth of both SACC-83 and SACC-LM cells (P<0.05, Figure 2F). These results establish the possibility that miR-101-3p probably plays a crucial role in inhibition of ACC progression.
MiR-101-3p attenuates ACC xenograft tumor growth in nude mice
To support in vitro experiments identifying the anti-tumor effect of miR-101-3p, the possible influence of miR-101-3p in tumorigenicity was examined in vivo. SACC-LM cells stably expressing miR-101-3p (n=5) or the vector (n=5) were subcutaneously injected into the right flank of nude mice. After 1 week, tumor volumes were measured by using a calliper. Notably, artificially overexpression of miR-101-3p significantly repressed the growth of tumors compared with the vector group (P<0.05, Figure 3A-C). Artificial overexpression of miR-101-3p significantly repressed tumor growth compared with the vector group (P<0.05, Figure 3A-C). After 5 weeks, the mice were sacrificed for tumor harvest. The weight of the tumors formed by miR-101-3p-expressing cells was significantly higher than that of the vector group (P<0.05, Figure 3D). In addition, the protein expression of Ki67, a gene related to proliferation, was detected through immunohistochemistry. The number of Ki67-positive cells in the miR-101-3p-expressing group was significantly lower than that in the vector group (Figure 3E and 3D).
Figure 3.
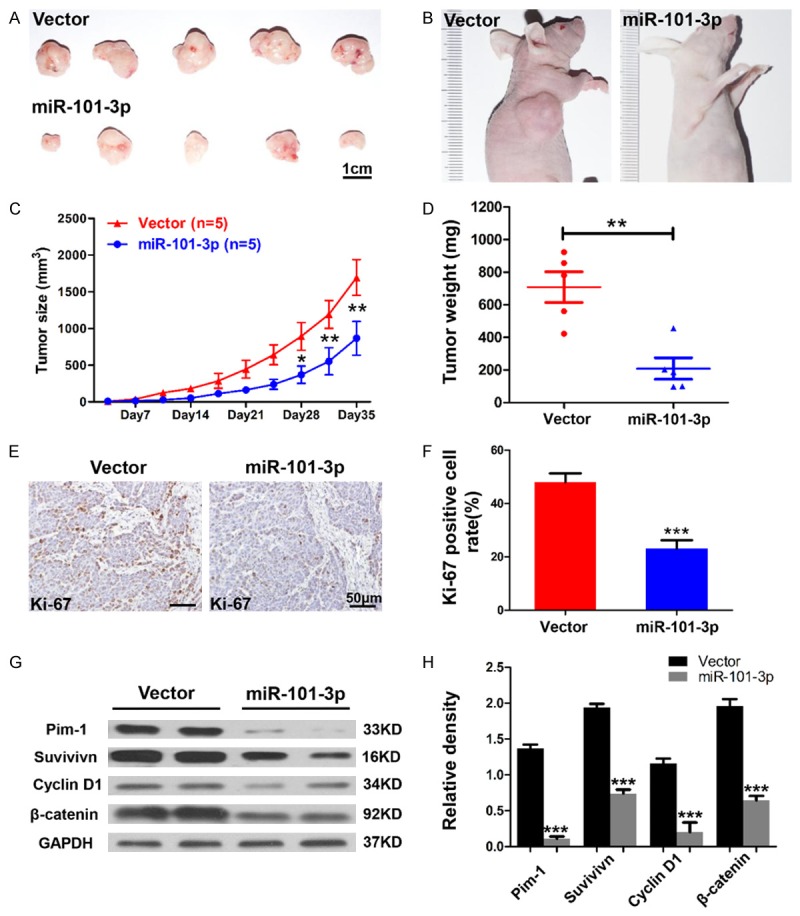
miR-101-3p attenuates ACC xenograft tumor growth in nude mice. A, B. Representative photographs of the tumors and nude mice. Scale bar=1 cm. C. SACC-LM cells expressing miR-101-3p (n=5) or vector (control, n=5) were subcutaneously injected into the right flanks of the nude mice. Tumor volume was measured twice a week for 5 weeks by using a caliper. Each data point represents the mean value of 5 tumors. The tumor volume of the miR-101-3p-expressing group was significantly higher than that of the vector group (*, P<0.05. **, P<0.01). D. The tumor weight of the miR-101-3p-expressing group was significantly higher than that of the vector group (t-test, **, P<0.01). E. Representative immunohistochemical staining results of Ki67. Scale bar=50 μm. F. Quantification of the rate of Ki67-positive cells (t-test, ***, P<0.001). G, H. Western blot analysis revealed that protein levels of Pim-1, survivin, cyclin D1, and β-catenin are significantly reduced in tumors formed by miR-101-3p-expressing SACC-LM cells. Quantification was carried out using Image J through pixel analysis of bands by normalization to GAPDH as a loading control. Error bars indicate mean ± S.D.
To elucidate the potential mechanism of the anti-tumor effects of miR-101-3p, protein lysis of the xenograft tumor was analyzed by using Western blot. Here, GAPDH was used as the loading control. As shown in Figure 3G, protein levels of Pim-1, survivin, cyclin D1, and β-catenin in the miR-101-3p group apparently decreased compared with the corresponding levels observed in the vector group. These results indicate that miR-101 exhibits anti-tumor effects by down regulating Pim-1, survivin, cyclin D1, and β-catenin in vivo.
Pim1 is a direct target of miR-101-3p
miRNAs are known to suppress several mRNA targets, thereby inducing complete changes in cell phenotypes [27]. To elucidate how miR-101-3p exerts its anti-tumor role in ACC, potential targets for miR-101-3p were searched by using TargetScan (www.targetScan.org) and miRanda (http://www.microrna.org). Pim1, the gene implicated in cancer cell proliferation, migration and invasion, was predicted as the potential target gene for miR-101-3p. To identify the correlation between miR-101-3p and Pim-1, we continuously detected that Pim-1 expression was significantly higher in ACC tissues than in normal parotid gland tissues (P<0.05, Figure 4A). The correlation between Pim-1 expression and miR-101-3p expression was then confirmed through Spearman rank correlation coefficient and linear tendency tests. Pim-1 expression exhibited more significant negative correlations with miR-101-3p expression in human salivary gland ACC specimens (P<0.05, r2=0.1132, Figure 4B) than in normal parotid gland specimens.
Figure 4.
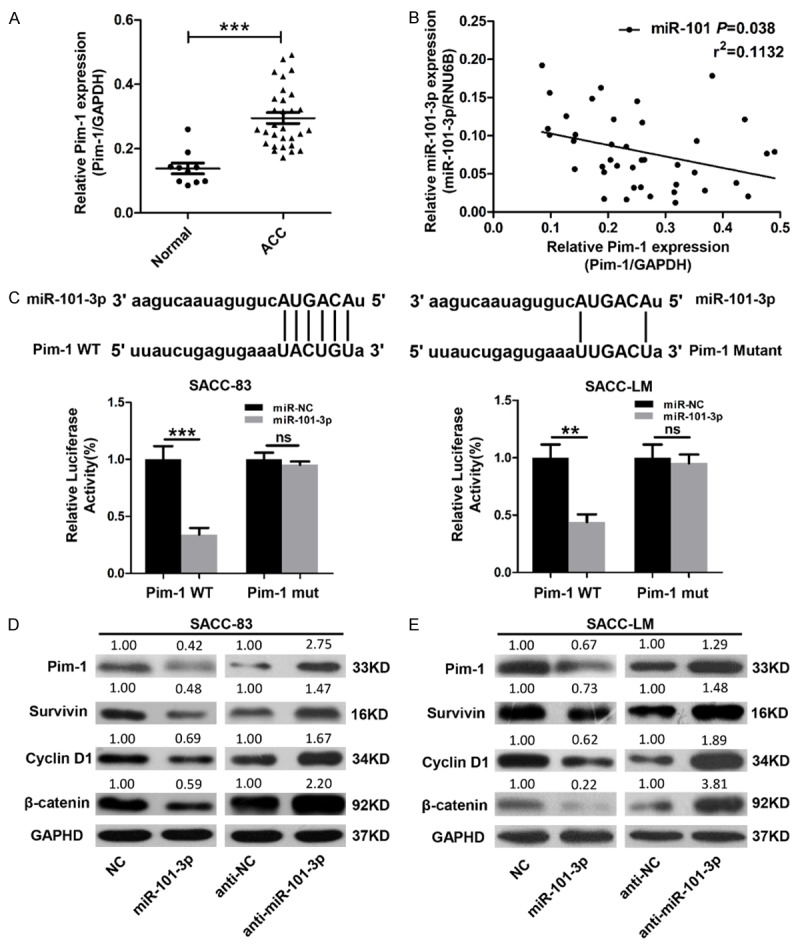
Pim1 is a direct target of miR-101-3p. A. Real-time PCR analysis showed that Pim-1 expression is significantly elevated in ACC tissues (t-test, ***, P<0.001). GAPDH was used as the internal loading control. B. Correlation between miR-101-3p expression and Pim-1 expression (P<0.038, r2=0.1132). C. Predicted miR-101-3p target sequences in 3’UTR of Pim-1 and its mutant (MUT) containing 4 mutated nucleotides. The wild type (WT) or mutant (MUT) reporter plasmid was co-transfected with miR-101-3p mimics or the negative control (NC) into SACC-LM or SACC-83 cells. Renilla luciferase vector was used as the internal control (t-test, **, P<0.01. ***, P<0.001, ns=no significance). D, E. Protein levels of Pim-1, survivin, cyclin D1, and β-catenin in SACC-83 and SACC-LM cells transfected with miR-101-3p mimics or miR-101-3p inhibitor (anti-miR-101-3p) were determined by using Western blot analysis. Quantification was carried out by using Image J through pixel analysis of bands with normalization to GAPDH as a loading control. Error bars indicate mean ± S.D.
To determine whether or not Pim-1 is a directly targeted gene of miR-101, Luc-Pim1-UTR-wild-type and its corresponding 3’-UTR mutant were constructed. Luciferase reporter assay demonstrated that miR-101-3p suppresses the transcriptional activity of the Luc-Pim-1-UTR-wide-type reporter more extensively than that of the 3’-UTR mutant (P<0.05, Figure 4C).
To identify the putative regulatory function of miR-101-3p on Pim-1, protein lysis of vector cells or cells stably expressing miR-101-3p were analyzed. The transfection efficiency of miR-101-3p was tested by qRT-PCR (Figure S1). miR-101-3p mimics observably down-regulated Pim-1 expression in the SACC-83 and SACC-LM cell lines. Moreover, the decrease in Pim-1 could be substantially rescued by anti-miR-101-3p. Interestingly, similar to out in vivo data, the expressions of survivin, cyclin D1, and β-catenin were also down-regulated (Figure 4D). These results suggest that Pim-1 is a direct target gene of miR-101-3p.
MiR-101-3p represses ACC cells proliferation and invasion by directly targeting Pim-1
To evaluate whether or not Pim-1 is a direct functional target of miR-101-3p in ACC cells, functional restoration assays were performed in SACC-83 and SACC-LM cell lines. By using a specific siRNA, Pim-1 was knocked down in both SACC-83 and SACC-LM cells. Obvious repression of proliferation that phenocopied the repressive effects of miR-101-3p was observed in Pim-1-depleted cells. miR-101-3p silencing by anti-miR-101-3p could not rescue the proliferation repression caused by Pim-1 siRNA (P<0.05, Figure 5A). Moreover, artificial over-expression of Pim-1 by using the Pim-1 plasmid ORF significantly promoted SACC-83 and SACC-LM proliferation. This effect could not be repressed through enforced miR-101-3p expression (P<0.05, Figure 5B). To investigate whether or not miR-101-3p inhibits invasion of ACC cells by directly targeting Pim-1, Matrigel transwell assays were carried out. Interestingly, Pim-1-expressing cells observably exhibited significantly enhanced invasion abilities that could be repressed by miR-101-3p (P<0.05, Figure 5C and 5D). These results reveal that miR-101-3p inhibits proliferation and invasion of ACC cells by directly down-regulating Pim-1.
Figure 5.
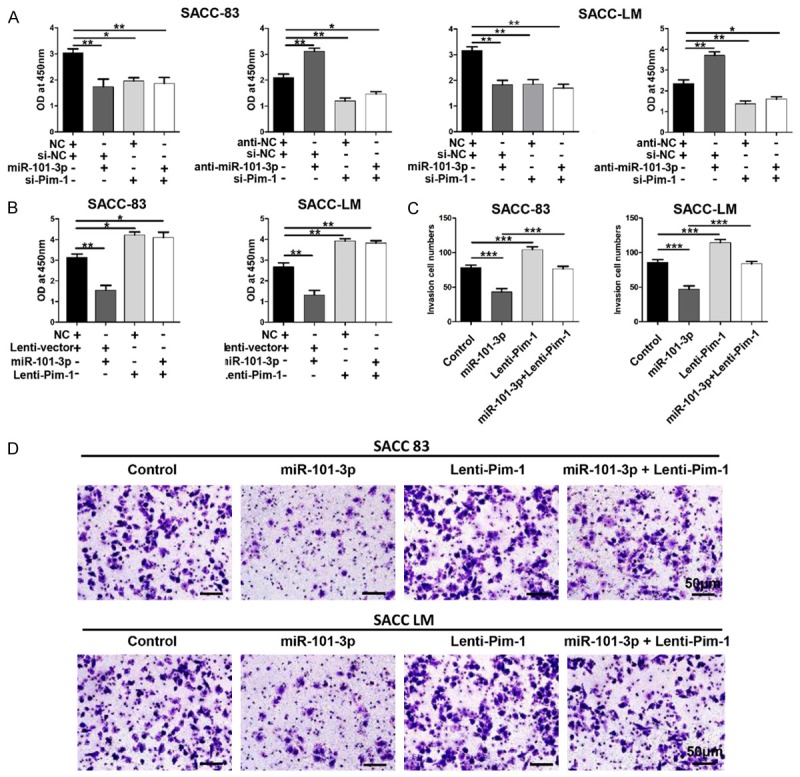
MiR-101-3p represses ACC cell proliferation and invasion by directly targeting Pim-1. A. Whereas Pim-1-specific siRNA significantly repressed the proliferation rate of SACC-83 and SACC-LM cells, ectopic miR-101-3p expression was unable to suppress cell proliferation in Pim-1-depleted SACC-83 and SACC-LM cells. miR-101-3p silencing (anti-miR-101-3p) accelerated cell proliferation but did not promote growth in Pim-1-depleted SACC-83 and SACC-LM (one-way ANOVA, *, P<0.05. **, P<0.01). B. Pim-1 up-regulation (ORF without 3’UTR) significantly promoted cell proliferation and rescued the cell growth inhibition induced by miR-101-3p in SACC-83 and SACC-LM cells (one-way ANOVA, *, P<0.05. **, P<0.01). C. Enforced expression of Pim-1 promoted cell invasion and abrogated the invasion inhibition induced by miR-101-3p in SACC-83 and SACC-LM cells (one-way ANOVA, *, P<0.05, **, P<0.01, ***, P<0.001). D. Representative photographs of invaded cells. Error bars indicate mean ± S.D. Scale bar=50 μm.
MiR-101-3p enhances drug sensitivity of ACC cells
Cisplatin can elicit better results than other types of traditional chemotherapy agents when used to treat ACC; nevertheless, the curative effect of cisplatin remains vague. To elucidate whether miR-101-3p can influence the drug sensitivity of ACC cells to cisplatin, miRNA levels of miR-101-3p and protein levels of Pim-1 in SACC-83 and SACC-LM cells were detected. The cells were treated with 10 μM cisplatin for 24 h. As expected, miR-101-3p expression was significantly down-regulated in cells treated with cisplatin (P<0.05, Figure 6A). Pim-1 expression also remarkably increased in cells treated with cisplatin (P<0.05, Figure 6B). SACC-83 and SACC-LM cell lines stably expressing miR-101-3p and their corresponding parental cell lines were subsequently treated with cisplatin for 24 h, and apoptosis rates were analyzed through flow cytometry. An apparent increase in apoptosis was observed in cells stably expressing miR-101-3p compared with their parental cells (Figure 6C). Proliferation repression induced by cisplatin in miR-101-3p expressing cells was also significantly enhanced (P<0.05, Figure 6D and 6E). These results indicate that miR-101-3p enhances the drug sensitivity of cisplatin in ACC cells.
Figure 6.
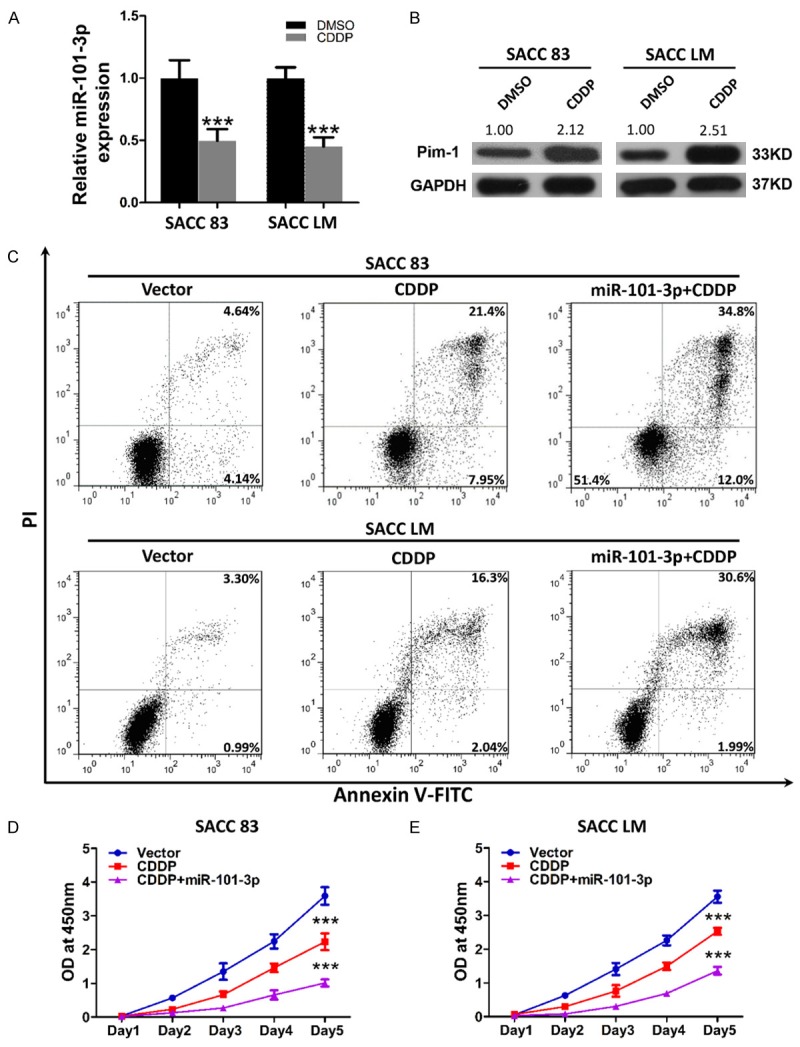
miR-101-3p enhances drug sensitivity of ACC cells. A. After exposure to 10 μM cisplatin for 24 h, the relative expression of miR-101-3p in SACC-83 (left) and SACC-LM (right) was significantly reduced (t-test, ***, P<0.001). B. After exposure to 10 μM cisplatin for 24 h, the relative protein expression of Pim-1 in SACC-83 (left) and SACC-LM (right) was significantly increased. Quantification was carried out using Image J through pixel analysis of band by normalization to GAPDH as a loading control. C. miR-101-3p increased cisplatin-induced apoptosis in SACC-83 and SACC-LM cells. PI indicates propidium iodide. D. miR-101-3p enhanced cisplatin-induced growth inhibition in SACC-83 and SACC-LM cells (***, P<0.001).
Discussion
ACC is a relatively rare malignance commonly appearing in the head and neck region, especially in salivary glands [1]. Because of variable patient histories, differences in neural and vessel invasion characteristics, and the high incidence of distant metastasis, the long-term survival rate of ACC has remained relatively unchanged [2,3]. To date, no definite systemic therapeutic strategy for ACC exists. Therefore, clarification of the molecular mechanism of ACC is urgently needed. Dysregulation of miRNAs in several cancers, including ACC, was recently reported [14,28]. Accumulating evidence confirms that miRNAs are involved in the tumorigenesis and tumor progression of several cancer types by manipulating cell proliferation, apoptosis, migration, or angiogenesis [9]. miRNAs have been regarded as promising prognostic markers, and miRNA expression profiling can be used to predict cancer patients’ outcomes [13-15,28]. However, studies on miRNAs in ACC are rare.
miR-101-3p is significantly down-regulated in various cancer types, including liver, breast, prostate, and head and neck cancers [16,17,20], and represses the proliferation, migration, and invasion of cancer cells. Thus, it is widely believed to be a tumor suppressor. These findings have motivated us to perform the present study to determine the expression and function of miR-101-3p in ACC.
miR-101-3p expression was evaluated for the first time in 30 cases of human salivary gland ACC specimens and 10 normal parotid gland specimens. As expected, miR-101-3p expression significantly decreased in ACC tissues compared with that in normal parotid glands. Moreover, various miR-101-3p expression levels were also defined in the highly metastatic cell line SACC-LM and its corresponding parental cell line SACC-83. SACC-LM, which revealed lower miR-101-3p expression level, is considered more malignant of the two cell lines. Our data reveal that miR-101-3p is a potential tumor suppressor of ACC. In vitro functional assays further confirmed the tumor suppressing role of miR-101-3p. Through construction of miR-101-3p-expressing cell lines, miR-101-3p was found to exhibit potent ability to suppress cell proliferation, invasion, and colony formation. Moreover, enforced expression of miR-101-3p induced apparent apoptosis in both SACC-83 and SACC-LM cell lines. These data are either completely or at least partially consistent with previous studies on liver, breast, and prostate cancers as well as in head and neck squamous cell carcinoma [29]. Xenograft tumor assay further confirmed the anti-tumor effects of miR-101-3p.
Over 5,300 human genes have been recognized as potential miRNA targets [30,31]. To understand the potential mechanism of the anti-tumor effect of miR-101-3p, TargetScan (http://www.targetScan.org) and miRanda (http://www.microrna.org) were used, and Pim-1 was selected as a potential target. As a member of the active serine/threonine kinase family, Pim-1 functions as a proto-oncogene by promoting tumor development in animal models [32]. Several Pim-1 target genes have been associated with cell cycle regulation, apoptosis, and invasion and the signaling pathway, all of which are linked to cancer cell survival [33]. Elevated Pim-1 expression is often found in human cancers, including hematological malignancies and solid tumors [34-38]. Interestingly, a recent study reported that Pim-1 is overexpressed in human salivary gland ACC and that a small molecular inhibitor of Pim-1 could attenuate the proliferation, cell cycle, and invasion of ACC cells [39]. In the present study, mRNA levels of Pim-1 were significantly elevated in human ACC tissues. These results are consistent with data from other studies. Data from the present study reveal that Pim-1 is a potential oncogene in human ACCs.
Some miRNAs have been reported to exhibit anti-tumor functions by targeting Pim-1. In lung cancer and breast cancer, ectopic miRNA-486-5p expression suppresses the proliferation, cell cycle, and migration of cancer cells [40-42]. Another research focused on miR-33a, a tumor suppressor targeting Pim-1 that substantially decelerates cell proliferation [43]. These studies have encouraged our group to clarify the association between miR-101-3p and Pim-1. miR-101-3p expression in human ACC tissues was hence detected, and correlations between miR-101-3p expression and of Pim-1 expression were identified.
Interestingly, Pim-1 expression was significantly negatively correlated with miR-101-3p expression. Luciferase reporter assays further confirmed that Pim-1 is a direct target of miR-101-3p. The present study reveals for the first time that Pim-1 is a direct target gene of miR-101-3p. Whereas miR-101-3p could significantly reduce protein levels of survivin, cyclin D1, and β-catenin in both ACC cell lines, miR-101-3p silencing elevated the expressions of these proteins. These results indicate that miR-101-3p is a novel tumor suppressor that functions by targeting Pim-1 in ACC.
During tumorigenesis, a single miRNA can affect biological behaviors by regulating numerous target genes. miR-101-3p reportedly inhibits breast cancer by targeting STMN1, RAB5A, and ATG4D through elimination of protective autophagy [22]. Moreover, EZH2, MCL-1, and FOS, which play essential roles in controlling cell proliferation, invasion, and maintaining stem cell-like phenotype, have also been confirmed as target genes of miR-101-3p [44]. Despite confirming that Pim-1 is a direct target gene of miR-101-3p, the anti-tumor effect of miR-101-3p could not be considered to be completely exhibited through Pim-1 down-regulation alone. Hence, functional restoration assays was performed, and miR-101-3p was found to regulate proliferation and invasion in ACC cells by targeting Pim-1.
Drug resistance is a leading factor influencing cancer-related deaths [6,7]. Traditional chemotherapeutic agents do not elicit promising results (only 1%-9%) in ACC [5]. In this regard, exploring new therapeutic targets or improve target sensitivity to traditional agents are necessary. Recent findings reveal that miRNAs play a role in drug resistance. By using miRNA expression profiles, Dai et al. identified several miRNAs, including miR-101-3p, that were significantly up-regulated in head and neck squamous cell carcinoma cell lines treated with docetaxel to establish drug resistance [45]. These data suggest that miR-101-3p promotes drug resistance in cancerous cells. By contrast, in breast and liver cancers, miR-101-3p enhances chemotherapeutic sensitivity by eliminating protective drug-induced autophagy through targeting of ATG4D, RAB5A, and STNM1 [22,46]. In the present study, miR-101-3p expression in ACC cells significantly decreased after exposure to cisplatin for 24 h, and Pim-1 protein levels increased. Such results indicate that miR-101-3p and Pim-1 are probably involved in ACC drug resistance. miR-101-3p-expressing cells and parental cells were treated with cisplatin for 24 h, and apparent increases in apoptosis rate were observed. Cisplatin exhibited significantly increased proliferation repression of miR-101-3p-expressing cells. These data are consistent with previous results of liver and breast cancer studies. However, the underlying mechanisms of the chemotherapeutic sensitivity induced by miR-101-3p require further study in future research.
Based on the present study’s results, miR-101-3p was determined to be frequently down-regulated in ACC. Moreover, miR-101-3p represses cell proliferation, invasion, and colony formation, induces apoptosis in ACC cell lines by directly targeting Pim-1, and enhances cisplatin sensitivity in ACC cells. In summary, miR-101-3p is a promising potential therapeutic target for human ACC.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81170991 and 30973357).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ellington CL, Goodman M, Kono SA, Grist W, Wadsworth T, Chen AY, Owonikoko T, Ramalingam S, Shin DM, Khuri FR, Beitler JJ, Saba NF. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973-2007 Surveillance, Epidemiology, and End Results data. Cancer. 2012;118:4444–4451. doi: 10.1002/cncr.27408. [DOI] [PubMed] [Google Scholar]
- 2.Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011;12:815–824. doi: 10.1016/S1470-2045(10)70245-X. [DOI] [PubMed] [Google Scholar]
- 3.Chau NG, Hotte SJ, Chen EX, Chin SF, Turner S, Wang L, Siu LL. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012;23:1562–1570. doi: 10.1093/annonc/mdr522. [DOI] [PubMed] [Google Scholar]
- 4.Hotte SJ, Winquist EW, Lamont E, MacKenzie M, Vokes E, Chen EX, Brown S, Pond GR, Murgo A, Siu LL. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23:585–590. doi: 10.1200/JCO.2005.06.125. [DOI] [PubMed] [Google Scholar]
- 5.Papaspyrou G, Hoch S, Rinaldo A, Rodrigo JP, Takes RP, van Herpen C, Werner JA, Ferlito A. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck. 2011;33:905–911. doi: 10.1002/hed.21458. [DOI] [PubMed] [Google Scholar]
- 6.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 8.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 9.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, Zhou L, Cheng Y, Sun L, Fan J, Liang J, Guo M, Liu N, Zhu L. MicroRNA-543 acts as an oncogene by targeting PAQR3 in hepatocellular carcinoma. Am J Cancer Res. 2014;4:897–906. [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C, Zhang L, Li H, Liu Z, Duan L, Lu C. MiRNA-1469 promotes lung cancer cells apoptosis through targeting STAT5a. Am J Cancer Res. 2015;5:1180–1189. [PMC free article] [PubMed] [Google Scholar]
- 12.Peng F, Jiang J, Yu Y, Tian R, Guo X, Li X, Shen M, Xu M, Zhu F, Shi C, Hu J, Wang M, Qin R. Direct targeting of SUZ12/ROCK2 by miR-200b/c inhibits cholangiocarcinoma tumourigenesis and metastasis. Br J Cancer. 2013;109:3092–3104. doi: 10.1038/bjc.2013.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–427. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 15.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varambally S, Cao Q, Mani RS, Shankar S, Wang XS, Ateeq B, Laxman B, Cao XH, Jing XJ, Ramnarayanan K, Brenner JC, Yu JD, Kim JH, Han B, Tan P, Kumar-Sinha C, Lonigro RJ, Palanisamy N, Maher CA, Chinnaiyan AM. Genomic Loss of microRNA-101 Leads to Overexpression of Histone Methyltransferase EZH2 in Cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su H, Yang JR, Xu T, Huang J, Xu L, Yuan YF, Zhuang SM. MicroRNA-101, Down-regulated in Hepatocellular Carcinoma, Promotes Apoptosis and Suppresses Tumorigenicity. Cancer Res. 2009;69:1135–1142. doi: 10.1158/0008-5472.CAN-08-2886. [DOI] [PubMed] [Google Scholar]
- 18.Chiang CW, Huang Y, Leong KW, Chen LC, Chen HC, Chen SJ, Chou CK. PKCalpha mediated induction of miR-101 in human hepatoma HepG2 cells. J Biomed Sci. 2010;17:35. doi: 10.1186/1423-0127-17-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buechner J, Tomte E, Haug BH, Henriksen JR, Lokke C, Flaegstad T, Einvik C. Tumoursuppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011;105:296–303. doi: 10.1038/bjc.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurul-Syakima AM, Yoke-Kqueen C, Sabariah AR, Shiran MS, Singh A, Learn-Han L. Differential microRNA expression and identification of putative miRNA targets and pathways in head and neck cancers. Int J Mol Med. 2011;28:327–336. doi: 10.3892/ijmm.2011.714. [DOI] [PubMed] [Google Scholar]
- 21.Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT, Xia YJ, Ye ZY, Tao HQ. MicroRNA-101 is downregulated in gastric cancer and involved in cell migration and invasion. Eur J Cancer. 2010;46:2295–2303. doi: 10.1016/j.ejca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Frankel LB, Wen J, Lees M, Hoyer-Hansen M, Farkas T, Krogh A, Jaattela M, Lund AH. microRNA-101 is a potent inhibitor of autophagy. EMBO J. 2011;30:4628–4641. doi: 10.1038/emboj.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu YH, An Y, Wang Y, Zhang CH, Zhang H, Huang CJ, Jiang H, Wang XH, Li XC. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep. 2013;29:2019–2024. doi: 10.3892/or.2013.2338. [DOI] [PubMed] [Google Scholar]
- 24.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer. 2011;11:23–34. doi: 10.1038/nrc2986. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson NS, Wang Z, Ding G, Reeves R. Why target PIM1 for cancer diagnosis and treatment? Future Oncol. 2010;6:1461–1478. doi: 10.2217/fon.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong L, Wang YX, Li SL, Yu GY, Gan YH, Li D, Wang CY. TGF-beta1 promotes migration and invasion of salivary adenoid cystic carcinoma. J Dent Res. 2011;90:804–809. doi: 10.1177/0022034511401407. [DOI] [PubMed] [Google Scholar]
- 27.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 28.Sun L, Liu B, Lin Z, Yao Y, Chen Y, Li Y, Chen J, Yu D, Tang Z, Wang B, Zeng S, Fan S, Wang Y, Li Y, Song E, Li J. MiR-320a acts as a prognostic factor and Inhibits metastasis of salivary adenoid cystic carcinoma by targeting ITGB3. Mol Cancer. 2015;14:96. doi: 10.1186/s12943-015-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng M, Jiang YP, Chen W, Li KD, Liu X, Gao SY, Feng H, Wang SS, Jiang J, Ma XR, Cen X, Tang YJ, Chen Y, Lin YF, Tang YL, Liang XH. Snail and Slug collaborate on EMT and tumor metastasis through miR-101-mediated EZH2 axis in oral tongue squamous cell carcinoma. Oncotarget. 2015;6:6797–6810. doi: 10.18632/oncotarget.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 31.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 32.van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T, Berns A. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989;56:673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 33.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer. 2011;11:23–34. doi: 10.1038/nrc2986. [DOI] [PubMed] [Google Scholar]
- 34.Amson R, Sigaux F, Przedborski S, Flandrin G, Givol D, Telerman A. The human protooncogene product p33pim is expressed during fetal hematopoiesis and in diverse leukemias. Proc Natl Acad Sci U S A. 1989;86:8857–8861. doi: 10.1073/pnas.86.22.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 36.Peltola K, Hollmen M, Maula SM, Rainio E, Ristamaki R, Luukkaa M, Sandholm J, Sundvall M, Elenius K, Koskinen PJ, Grenman R, Jalkanen S. Pim-1 kinase expression predicts radiation response in squamocellular carcinoma of head and neck and is under the control of epidermal growth factor receptor. Neoplasia. 2009;11:629–636. doi: 10.1593/neo.81038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weirauch U, Beckmann N, Thomas M, Grunweller A, Huber K, Bracher F, Hartmann RK, Aigner A. Functional role and therapeutic potential of the pim-1 kinase in colon carcinoma. Neoplasia. 2013;15:783–794. doi: 10.1593/neo.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu D, Allsop SA, Witherspoon SM, Snider JL, Yeh JJ, Fiordalisi JJ, White CD, Williams D, Cox AD, Baines AT. The oncogenic kinase Pim-1 is modulated by K-Ras signaling and mediates transformed growth and radioresistance in human pancreatic ductal adenocarcinoma cells. Carcinogenesis. 2011;32:488–495. doi: 10.1093/carcin/bgr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu X, Xu JJ, Hu SS, Feng JG, Jiang LH, Hou XX, Cao J, Han J, Ling ZQ, Ge MH. Pim-1 acts as an oncogene in human salivary gland adenoid cystic carcinoma. J Exp Clin Cancer Res. 2014;33:114. doi: 10.1186/s13046-014-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pang W, Tian X, Bai F, Han R, Wang J, Shen H, Zhang X, Liu Y, Yan X, Jiang F, Xing L. Pim-1 kinase is a target of miR-486-5p and eukaryotic translation initiation factor 4E, and plays a critical role in lung cancer. Mol Cancer. 2014;13:240. doi: 10.1186/1476-4598-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang G, Liu Z, Cui G, Wang X, Yang Z. MicroRNA-486-5p targeting PIM-1 suppresses cell proliferation in breast cancer cells. Tumour Biol. 2014;35:11137–11145. doi: 10.1007/s13277-014-2412-0. [DOI] [PubMed] [Google Scholar]
- 42.Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen J, Zhang Y, Lai P, Fan X, Zhou X, Lin J, Li M, Ma W, Luo S, Bai X. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014;74:3031–3042. doi: 10.1158/0008-5472.CAN-13-2193. [DOI] [PubMed] [Google Scholar]
- 43.Thomas M, Lange-Grunweller K, Weirauch U, Gutsch D, Aigner A, Grunweller A, Hartmann RK. The proto-oncogene Pim-1 is a target of miR-33a. Oncogene. 2012;31:918–928. doi: 10.1038/onc.2011.278. [DOI] [PubMed] [Google Scholar]
- 44.Konno Y, Dong P, Xiong Y, Suzuki F, Lu J, Cai M, Watari H, Mitamura T, Hosaka M, Hanley SJ, Kudo M, Sakuragi N. MicroRNA-101 targets EZH2, MCL-1 and FOS to suppress proliferation, invasion and stem cell-like phenotype of aggressive endometrial cancer cells. Oncotarget. 2014;5:6049–6062. doi: 10.18632/oncotarget.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai Y, Xie CH, Neis JP, Fan CY, Vural E, Spring PM. MicroRNA expression profiles of head and neck squamous cell carcinoma with docetaxel-induced multidrug resistance. Head Neck. 2011;33:786–791. doi: 10.1002/hed.21540. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, An Y, Wang Y, Zhang C, Zhang H, Huang C, Jiang H, Wang X, Li X. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep. 2013;29:2019–2024. doi: 10.3892/or.2013.2338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


