Abstract
Lung cancer is the most frequent cancer among men in many countries. It is the result of interactions between genetic and environmental factors, among which tobacco smoking is a key environmental factor. CHRNA5, Cholinergic Receptor, Neuronal Nicotinic, Alpha Polypeptide-5, was previously reported to be associated with lung cancer risk. To identify the genetic susceptibility and tobacco smoking that influence lung cancer risk in Han population, we performed a case-control study in 228 patients and 301 controls. These data were compared using the χ2-test, genetic model analysis, and haplotype analysis. rs495956, rs680244, rs601079, rs555018, 588765 and rs11637635 showed an increased risk of lung cancer in both allelic model and genetic mode analysis. The genotype G/A-A/A of rs11637635 was most strongly associated with a 2.17-fold increased risk of lung cancer in dominant model (p = 0.018). One SNP, rs684513, was associated with a 0.645-fold decreased risk (p = 0.033) in allelic model analysis. By haplotype association analysis, haplotype sequences CTTATCAAAGA and GA of CHRNA5 were found to be associated with a 2.03-fold and 1.91-fold increased lung cancer risk (p < 0.05). Our results, combined with those from previous studies, suggest that genetic variation in CHRNA5 may influence susceptibility to lung cancer among Han smokers.
Keywords: Lung cancer, smoking behavior, SNP, CHRNA5, case-control studies
Introduction
Lung cancer is one of the most common malignant tumors, and it causes the highest number of cancer-related deaths [1]. It appears to result from interactions between genetic susceptibility of the individual and risk factors in the environment. Among the latter, tobacco smoking is the primary risk factor for developing lung cancer [1-3], and this has prompted a search for susceptibility genes linking smoking behavior and nicotine dependence [4].
In recent genome-wide association studies, several genetic variants at chromosomal locus 15q25, including the cholinergic receptor nicotinic α5-encoding gene (CHRNA5), were found to be associated with lung cancer risk and nicotine dependence [5-7]. Most of the earlier studies had focused solely on European and American populations [8,9]. The genetic structures of these loci differ between Asians and whites, however, indicating the need for additional studies in Asian populations. To examine whether CHRNA5 may also contribute to lung cancer risk in a defined Eastern population, the Han population of northwest China was studied. We selected 22 single nucleotide polymorphisms (SNPs) in CHRNA5 that were previously reported to be associated with lung cancer susceptibility [10-12] for a case-control study in this population.
Materials and methods
Study participants
Samples from cases of lung cancer (n = 228) were seen between January 2010 and February 2013 at the First Affiliated Hospital of the Medical College of Xi’an Jiaotong University. All patients were newly diagnosed with lung cancer and were characterized histologically. None of the patients had previous history of other cancers, chemotherapy, or radiotherapy. All healthy individuals were received physical examination at First Affiliated Hospital were selected for a control group. The control group comprised 301 unrelated healthy individuals who had no known medical illness or hereditary disorders and who were not taking any medications. Participants were chosen without restrictions of age, gender, or disease stage. Basic characteristics of the participants, e.g., gender, age, and pathology, are listed in Table 1.
Table 1.
Basic characteristics of case and control patients
| Lung cancer (n = 228) | Control (n = 301) | p | |
|---|---|---|---|
| Age (yr) | 58.7 ± 10 | 50.2 ± 8.1 | < 0.001a |
| Sex | < 0.001b | ||
| Male | 178 (78.1%) | 188 (62.5%) | |
| Female | 50 (21.9%) | 113 (37.5%) | |
| Smoking status | < 0.001a | ||
| Smoker | 165 (72.4%) | 91 (30.2%) | |
| Nonsmoker | 63 (27.6%) | 210 (69.8%) | |
| Drinking Status | 0.101a | ||
| Drinking | 37 (16.2) | 66 (21.9) | |
| Nondrinking | 191 (83.8) | 235 (78.1) | |
| Histology type | |||
| SCLC | 37 (16.2%) | ||
| LAC | 73 (32%) | ||
| LSCC | 80 (35.1%) | ||
| LASC | 11 (4.8%) | ||
| Others | 27 (32%) |
Abbreviations: SCLC, small-cell lung cancer; LAC, lung adenocarcinoma; LSCC, lung squamous cell carcinoma; LASC, lung adenosquamous carcinoma.
p values were calculated by Student t tests;
p values were calculated from two-sided chi-square tests.
Clinical data and demographic information
We used a standard epidemiological questionnaire and in-person interview to collect personal data, including residential region, age, smoking status, gender, education status, and family history of cancer. Never smokers were defined as those who smoked less than 100 cigarettes in their lifetime (or before diagnosis for cases) and former smokers as those who quit smoking at least 1 year before the time of the survey. All of the smokers had at least a 10-pack-year history of smoking. The case information was collected through consultation with treating physicians or from medical chart review. All of the participants signed an informed consent agreement. The Human Research Committee for Approval of Research Involving Human Subjects, First Affiliated Hospital of Xi’an Jiaotong University, approved the use of human blood in this study.
Selection of SNPs and methods of genotyping
All of the 22 selected SNPs had been previously reported to be associated with lung cancer, with minor allele frequencies of > 5%, in the HapMap of the Chinese Han Beijing (CHB) population. Extraction of DNA from whole-blood samples was done with GoldMag-Mini Whole Blood Genomic DNA Purification Kits (GoldMag Co., Ltd.; Xi’an City, China), and DNA concentration was measured with a NanoDrop 2000 spectrophotometer. The multiplexed SNP MassEXTENDED assay was designed using SequenomMassARRAY Assay Design 3.0 Software [24]. Genotyping was done with the Sequenom MassARRAY RS1000 system using the standard protocol recommended by the manufacturer. Data management and analysis was done using SequenomTyper 4.0 Software [24,25]. The software and kits for SNP analyses were obtained from Sequenom (Sequenom. San Diego, California, USA).
Statistical analysis
The SPSS17.0 statistical software and Microsoft Excel were used for statistical analysis. All p values presented in this study are two sided, and we used p ≤ 0.05 as the cutoff value for statistical significance. An exact test was used to assess the variation of each SNP frequency from Hardy-Weinberg equilibrium (HWE) in the control subjects. Student t test was used to determine the differences in age at diagnosis, smoking and drinking status. Chi-square (Pearson’s χ2) test or Fisher’s exact test was used where necessary to calculate the P values and corresponding odds ratios (ORs) with 95% confidence intervals (CIs) to determine the associations between genotypes and lung cancer risk [26]. Odds ratios (ORs) and 95% confidence intervals (CIs) were determined using unconditional logistic regression analysis with adjustments for age, gender and drinking status [27].
Associations between CHRNA5 and risk of lung cancer were tested in genetic models (co-dominant, dominant, recessive, over-dominant, and log-additive) by analysis with SNP Stats software, obtained from http://bioinfo.iconcologia.net. Values of OR and 95% CI were calculated as above. Akaike’s Information Criterion and Bayesian Information Criterion were applied to estimate the best-fit model for each SNP.
Haploview software version 4.2 was used to analyze the association between haplotype and lung cancer. Linkage disequilibrium (LD) analysis was done using genotype data from all the subjects. The pattern of LD was analyzed using two parameters, r2 and D0. Statistical significance was set at p ≤ 0.05.
Results
A total of 529 participants, including 228 lung cancer cases (178 males, 50 females; mean age at diagnosis 58.7 ± 10 yr) and 301 controls (188 males, 113 females; mean age 50.8 ± 8.1 yr) were successfully genotyped for further analysis (Table 1). More smokers were observed in cases compared with subjects in the control group (p < 0.001). This result was expected because most lung cancers can be attributed to smoking. A total of 22 SNPs in CHRNA5 were genotypedin lung cancer patients (smoker and nonsmoker) and healthy controls. The average SNP call rate was 98.5% in cases and controls. Table 2 summarizes the basic information of CHRNA5 SNPs in the smoker subpopulation. One SNP (rs692780) was excluded at the 5%-HWE p level.
Table 2.
Frequency distributions of CHRNA5 alleles and their associations with lung cancer risk in smokers
| SNP ID | Position | Alleles | MAF | H-W p-value | Without adjustment | With adjustment | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Aa/B | case | control | ORs | 95% CI | Allele p b | ORs | 95% CI | Allele p c | |||
| rs10519205 | 78878791 | C/T | 0.061 | 0.038 | 0.703 | 1.623 | 0.673-3.916 | 0.277 | 1.371 | 0.510-3.687 | 0.532 |
| rs12903839 | 78867042 | G/C | 0.009 | 0.005 | 0.958 | 1.671 | 0.172-16.180 | 0.939 | 0.725 | 0.104-5.070 | 0.746 |
| rs16969948 | 78864786 | G/A | 0.064 | 0.038 | 0.703 | 1.699 | 0.708-4.077 | 0.230 | 1.384 | 0.516-3.713 | 0.519 |
| rs17408276 | 78881618 | C/T | 0.235 | 0.167 | 0.255 | 1.532 | 0.959-2.448 | 0.073 | 1.741 | 1.013-2.991 | 0.045* |
| rs17486278 | 78867482 | C/A | 0.282 | 0.306 | 0.233 | 0.892 | 0.599-1.327 | 0.572 | 0.837 | 0.532-1.316 | 0.440 |
| rs495956 | 78869930 | G/A | 0.280 | 0.187 | 0.208 | 1.697 | 1.089-2.645 | 0.019* | 1.856 | 1.116-3.086 | 0.017* |
| rs601079 | 78869579 | A/T | 0.291 | 0.198 | 0.342 | 1.664 | 1.077-2.571 | 0.021* | 1.787 | 1.086-2.940 | 0.022* |
| rs680244 | 78871288 | A/G | 0.291 | 0.194 | 0.282 | 1.700 | 1.096-2.636 | 0.017* | 1.834 | 1.110-3.030 | 0.018* |
| rs7180002 | 78873993 | T/A | 0.033 | 0.044 | 0.661 | 0.750 | 0.296-1.900 | 0.543 | 0.687 | 0.238-1.985 | 0.488 |
| rs951266 | 78878541 | T/C | 0.033 | 0.049 | 0.620 | 0.663 | 0.269-1.631 | 0.368 | 0.629 | 0.225-1.759 | 0.377 |
| rs16969968 | 78882925 | A/G | 0.042 | 0.044 | 0.661 | 0.964 | 0.396-2.342 | 0.935 | 1.022 | 0.370-2.820 | 0.967 |
| rs1875869 | 78868157 | T/A | 0.064 | 0.038 | 0.703 | 1.699 | 0.708-4.077 | 0.230 | 1.384 | 0.516-3.713 | 0.519 |
| rs667282 | 78863472 | C/T | 0.430 | 0.500 | 0.753 | 0.755 | 0.525-1.086 | 0.130 | 0.759 | 0.502-1.147 | 0.190 |
| rs11637635 | 78877150 | A/G | 0.215 | 0.143 | 0.328 | 1.645 | 1.006-2.688 | 0.046* | 1.975 | 1.120-3.485 | 0.019* |
| rs12903575 | 78881087 | A/G | 0.009 | 0.011 | 0.915 | 0.817 | 0.135-4.932 | 0.803 | 1.213 | 0.113-13.012 | 0.873 |
| rs16969949 | 78866964 | T/C | 0.064 | 0.038 | 0.703 | 1.699 | 0.708-4.077 | 0.230 | 1.384 | 0.516-3.713 | 0.519 |
| rs684513 | 78858400 | G/C | 0.227 | 0.313 | 0.971 | 0.645 | 0.430-0.968 | 0.033* | 0.652 | 0.411-1.035 | 0.070 |
| rs2277550 | 78858263 | A/C | 0.015 | 0.022 | 0.830 | 0.685 | 0.181-2.582 | 0.833 | 0.473 | 0.100-2.231 | 0.344 |
| rs555018 | 78879242 | C/T | 0.227 | 0.154 | 0.496 | 1.618 | 1.003-2.609 | 0.047* | 1.883 | 1.086-3.263 | 0.024* |
| rs588765 | 78865425 | T/C | 0.227 | 0.154 | 0.496 | 1.618 | 1.004-2.609 | 0.047* | 1.883 | 1.086-3.263 | 0.024* |
| rs7178897 | 78862975 | C/T | 0.061 | 0.038 | 0.845 | 1.623 | 1.005-3.916 | 0.277 | 1.266 | 0.466-3.442 | 0.644 |
| rs692780 | 78876505 | G/C | 0.236 | 0.159 | 0.035# | – | – | – | – | – | – |
Minor allele.
p values were calculated from two-sided chi-square tests or Fisher’s exact tests for either allele frequency.
p values were calculated by unconditional logistic regression adjusted for age and sex.
p value ≤ 0.05 indicates statistical significance.
site with HWE p ≤ 0.05 is excluded.
CI, confidence interval; HWE, Hardy-Weinberg Equilibrium; MAF, minor allele frequency; ORs, odds ratios; SNP, single nucleotide polymorphism.
We compared the differences in frequency distributions of alleles between cases and controls by χ2-test or Fisher’s exact test and found seven significant SNPs in the smoker subpopulation. The polymorphisms detected by rs495956 (OR = 1.70; 95% CI, 1.09-2.65, p = 0.019), rs680244 (OR = 1.70; 95% CI, 1.10-2.64, p = 0.017), rs601079 (OR = 1.66; 95% CI, 1.08-2.57, p = 0.021), rs11637635 (OR = 1.64; 95% CI, 1.01-2.69, p = 0.046), rs555018 (OR = 1.62; 95% CI, 1.00-2.61, p = 0.047), and rs588765 (OR = 1.62; 95% CI, 1.00-2.61, p = 0.047) showed an increased risk of lung cancer. Only the rs684513 (OR = 0.64; 95% CI, 0.43-40.91, p = 0.033) was associated with a decreased risk. After adjusted by age, gender and drinking status, rs684513 was not statistically significant anymore (p = 0.07).
As summarized in Supplemental Table 3, we analyzed the SNPs in the context of various genetic models. In the smoker subpopulation, the genotypes G/A-G/G of rs495956 (OR = 2.07; 95% CI, 1.12-3.82, p = 0.018), A/T-A/A of rs601079 (OR = 1.99; 95% CI, 1.09-3.64, p = 0.024), G/A-A/A of rs680244 (OR = 2.07; 95% CI, 1.12-3.81, p = 0.018), G/A-A/A of rs11637635 (OR = 2.17; 95% CI, 1.12-4.19, p = 0.018), C/T-C/C of rs555018 (OR = 2.07; 95% CI, 1.09-3.93, p = 0.023), and T/C-T/T of rs588765 (OR = 2.07; 95% CI, 1.09-3.93, p = 0.023) in CHRNA5 showed an increased risk of lung cancer in the dominant model (Table 3). However, no statistically significant evidence suggested that the polymorphisms tested were associated with lung cancer risk in the nonsmoker subpopulation.
Table 3.
Dominant model analysis of relationship between SNPs and lung cancer risk in the smoking versus nonsmoking subpopulations
| SNP | Genotype | Smoking subpopulation | Nonsmoking subpopulation | ||
|---|---|---|---|---|---|
|
| |||||
| ORa (95% CI) | p-value | ORa (95% CI) | p-value | ||
| rs495956 | A/A | 1.00 | 1.00 | ||
| G/A-G/G | 2.07 (1.12-3.82) | 0.018* | 0.90 (0.48-1.70) | 0.75 | |
| rs601079 | T/T | 1.00 | 1.00 | ||
| A/T-A/A | 1.99 (1.09-3.64) | 0.024* | 0.82 (0.44-1.56) | 0.55 | |
| rs680244 | G/G | 1.00 | 1.00 | ||
| G/A-A/A | 2.07 (1.12-3.81) | 0.018* | 0.82 (0.44-1.56) | 0.55 | |
| rs588765 | C/C | 1.00 | 1.00 | ||
| T/C-T/T | 2.07 (1.09-3.93) | 0.023* | 0.89 (0.46-1.73) | 0.74 | |
| rs555018 | T/T | 1.00 | 1.00 | ||
| C/T-C/C | 2.07 (1.09-3.93) | 0.023* | 1.00 (0.52-1.92) | 0.99 | |
| rs11637635 | G/G | 1.00 | 1.00 | ||
| G/A-A/A | 2.17 (1.12-4.19) | 0.018* | 0.88 (0.45-1.72) | 0.71 | |
A p value ≤ 0.05 indicates statistical significance;
adjusted by sex, age, and drinking status.
Abbreviations: OR, odds ratio; CI, confidence interval.
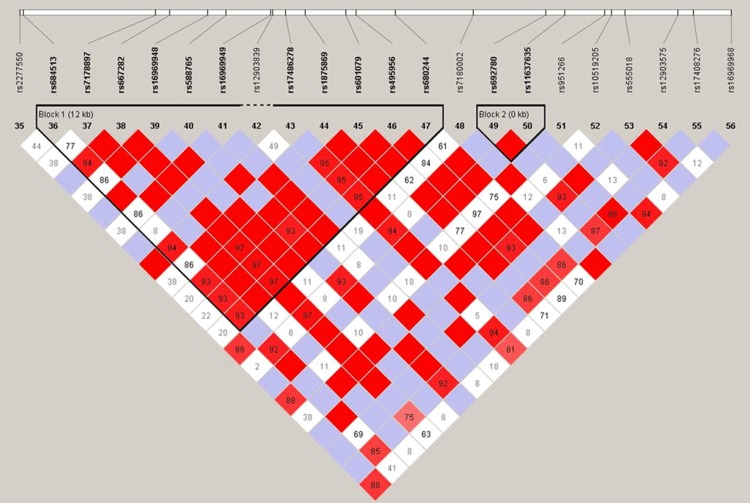
The LD and haplotype analyses of the SNPs in the case and control samples for the smoker subpopulation were further studied. The LD of 22 SNPs distributed throughout CHRNA5 sequences was analyzed (D0 and r2). Thirteen SNPs were found to exist in 2 LD blocks: 11 SNPs (rs684513, rs7178897, rs667282, rs16969948, rs588765, rs16969949, rs17486278, rs1875869, rs601079, rs495956, and rs680244) in block 1, and 2 completely linked SNPs (rs692780 and rs11637635) in block 2 (Figure 1). The SNPs located in the same LD block were used for a haplotype-based association study among the individuals with a history of tobacco smoking. Results showing association between CHRNA5 haplotype and risk of lung cancer are summarized in Table 4. We found that the haplotype CTTATCAAAGA, composed of the variant alleles of rs16969948, rs588765, rs601079, rs495956, and rs680244 and the reference allele of rs684513, rs7178897, rs667282, rs16969949, rs17486278 and rs1875869 were found to be increased lung cancer risk by 2.03-fold (OR = 2.03; 95% CI, 1.07-3.88, p = 0.032); the haplotype GA of variant alleles rs692780 and rs11637635 also increased lung cancer risk, by 1.91-fold (OR = 2.03; 95% CI, 1.07-3.88, p = 0.032). Haplotype analysis for the CHRNA5 polymorphisms showed an association with lung cancer risk.
Figure 1.
D′ linkage map of 22 SNPs in CHRNA5. A standard color scheme is used to display LD with bright red for very strong LD (LOD = 2, D′ = 1), white for no LD (LOD < 2, D′ < 1), pink red (LOD = 2, D′ < 1), and blue (LOD < 2, D′ = 1) for intermediate LD.
Table 4.
CHRNA5 haplotype frequencies and associations with the lung cancer risk in individuals who smokedin the cases and controls
| Block | Haplotype | Frequency | OR | p-value |
|---|---|---|---|---|
| 1 | CTTACCCATAG | 0.2834 | 1.00 | --- |
| GTCACCAATAG | 0.2482 | 0.84 (0.48-1.50) | 0.57 | |
| CTCACCAATAG | 0.2050 | 1.39 (0.75-2.59) | 0.3 | |
| CTTATCAAAGA | 0.1914 | 2.03 (1.07-3.88) | 0.032* | |
| CCTGCTATAGA | 0.0508 | 1.82 (0.59-5.66) | 0.3 | |
| rare | 0.0214 | 1.21 (0.26-5.60) | 0.81 | |
| 2 | CG | 0.7910 | 1.00 | --- |
| GA | 0.1895 | 1.91 (1.10-3.33) | 0.023* | |
| GG | 0.0195 | 0.95 (0.24-3.72) | 0.94 |
A p-value of ≤ 0.05 indicates statistical significance.
Abbreviations: OR, odds ratio; CI, confidence interval. Block 1: rs684513, rs7178897, rs667282, rs16969948, rs588765, rs16969949, rs17486278, rs1875869, rs601079, rs495956, rs680244; Block 2: rs692780, rs11637635.
Discussion
In this study we investigated the associations between 22 selected CHRNA5 SNPs and risk of lung cancer in the Han population from the northwest China. We did not find significant association with lung cancer in the whole population (Supplemental Table 1). However, 6 SNPs showed a significant association with increased risk of lung cancer, and 1 SNP contributed to a decreased risk of lung cancer, by individual SNP analysis in the smoker subpopulation. The results suggest that the polymorphisms of CHRNA5 may play an important role in the risk of lung cancer in the subpopulation of Han individuals who smoke.
Nicotine acetylcholine receptor genes are expressed in key regions of the brain and play an important role in controlling smoking behavior. Acetylcholine receptors can bind to tobacco carcinogens, such as nicotine and nitrosamines, in the body. Subsequently, activated nicotinic acetylcholine receptors can promote tumorigenic conversion of cells, angiogenesis, and cell growth, thereby contributing to tumor development [6]. The corresponding genes are located on chromosome 15q25 [10,13], and the encoded receptors are the primary targets for nicotine in the brain, leading to strong downstream responses in that tissue [14,15]. Smoking is the major environmental risk factor for lung cancer because tobacco smoke contains strong carcinogens, such as empyreumatic oil and benzo(a)pyrene [16]. In earlier studies, sequence variants in CHRNA SNPs have been associated with increased (self-reported) cigarette dose and nicotine dependence, and with increased risk of lung cancer in smokers [7,8], whereas, no association in nonsmokers was reported [8].
In the present study, CHRNA5 rs684513 polymorphisms were found to contribute to reduced risk of lung cancer. In contrast, the rs495956, rs601079, rs11637635, rs555018, rs588765, and rs680244 showed an increased risk in the smoker subpopulation. However, none of these CHRNA5 polymorphisms were associated with lung cancer in Han individuals in our study who had never smoked (Supplemental Table 2). This result may be because CHRNA5 encodes a polypeptide of the nicotinic acetylcholine receptor, and smoking behavior a priori will appear as an environmental risk factor for lung cancer most strongly in smokers. Previous findings generally suggested that that the aetiology, clinical characteristics, and prognosis of lung cancer in never smokers are substantially different to those in smokers [17]. In our study, rs684513, located in the first intron of CHRNA5, was the only SNP that show a decreased risk of lung cancer. This results show an consistency with previous studies in other population [11]. A study before showed that rs601079, rs11637635, rs555018, rs588765, and rs680244 were associated with mRNA expression level of CHRNA5 [18]. Other study before showed that rs11637635 and rs588765 is associated with smokers’ total puff volume per cigarette [19]. This may be the reason why these SNPs associated with an increased risk of lung cancer.
Some studies before showed that rs16969968 was associated with lung cancer risk in European-ancestry populations and Japanese population [15,20-22]. In a study of African-Americans, CHRNA5 rs17486278 was found to be associated with increased lung cancer risk [23]. However, in our study, the same SNPs were not associated with lung cancer risk. These differences may be primarily attributed to distinct genetic backgrounds and differences in specific environmental factors of the Han people compared to other populations.
In conclusion, our study has described the association between SNPs in CHRNA5 and lung cancer risk in a group composed of the Han individuals of northwestern China for the first time. Our findings suggested that genetic variants and environmental factors especially tobacco smoking may play an important role in occurrence of lung cancer in this population.
Acknowledgements
This work was supported by the National 863 High-Technology Research and Development Program (No. 2012AA02A519).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M Comparative Risk Assessment collaborating group (Cancers) Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 3.Parkin D, Pisani P, Lopez A, Masuyer E. At least one in seven cases of cancer is caused by smoking. Global estimates for 1985. Int J Cancer. 1994;59:494–504. doi: 10.1002/ijc.2910590411. [DOI] [PubMed] [Google Scholar]
- 4.Shields PG. Molecular epidemiology of smoking and lung cancer. Oncogene. 2002;21:6870–6876. doi: 10.1038/sj.onc.1205832. [DOI] [PubMed] [Google Scholar]
- 5.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, Sullivan K, Matakidou A, Wang Y, Mills G, Doheny K, Tsai YY, Chen WV, Shete S, Spitz MR, Houlston RS. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G, Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F, Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F, Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S, Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P, Trichopoulos D, Holcátová I, Merletti F, Kjaerheim K, Agudo A, Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A, Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 7.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsäter A, Flex A, Aben KK, de Vegt F, Mulders PF, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Marchand L, Derby KS, Murphy SE, Hecht SS, Hatsukami D, Carmella SG, Tiirikainen M, Wang H. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008;68:9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan P, Hainaut P, Boffetta P. Genetics of lung-cancer susceptibility. Lancet Oncol. 2011;12:399–408. doi: 10.1016/S1470-2045(10)70126-1. [DOI] [PubMed] [Google Scholar]
- 11.Chen LS, Saccone NL, Culverhouse RC, Bracci PM, Chen CH, Dueker N, Han Y, Huang H, Jin G, Kohno T, Ma JZ, Przybeck TR, Sanders AR, Smith JA, Sung YJ, Wenzlaff AS, Wu C, Yoon D, Chen YT, Cheng YC, Cho YS, David SP, Duan J, Eaton CB, Furberg H, Goate AM, Gu D, Hansen HM, Hartz S, Hu Z, Kim YJ, Kittner SJ, Levinson DF, Mosley TH, Payne TJ, Rao DC, Rice JP, Rice TK, Schwantes-An TH, Shete SS, Shi J, Spitz MR, Sun YV, Tsai FJ, Wang JC, Wrensch MR, Xian H, Gejman PV, He J, Hunt SC, Kardia SL, Li MD, Lin D, Mitchell BD, Park T, Schwartz AG, Shen H, Wiencke JK, Wu JY, Yokota J, Amos CI, Bierut LJ. Smoking and genetic risk variation across populations of European, Asian, and African American ancestry--a meta-analysis of chromosome 15q25. Genet Epidemiol. 2012;36:340–351. doi: 10.1002/gepi.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lips EH, Gaborieau V, McKay JD, Chabrier A, Hung RJ, Boffetta P, Hashibe M, Zaridze D, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Field JK, Liloglou T, Xinarianos G, McLaughlin J, Liu G, Skorpen F, Elvestad MB, Hveem K, Vatten L, Study E, Benhamou S, Lagiou P, Holcátová I, Merletti F, Kjaerheim K, Agudo A, Castellsagué X, Macfarlane TV, Barzan L, Canova C, Lowry R, Conway DI, Znaor A, Healy C, Curado MP, Koifman S, Eluf-Neto J, Matos E, Menezes A, Fernandez L, Metspalu A, Heath S, Lathrop M, Brennan P. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol. 2010;39:563–577. doi: 10.1093/ije/dyp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J Jr, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minna JD. Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer. J Clin Invest. 2003;111:31–33. doi: 10.1172/JCI17492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starek A, Podolak I. [Carcinogenic effect of tobacco smoke] . Rocz Panstw Zakl Hig. 2008;60:299–310. [PubMed] [Google Scholar]
- 17.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J. Clin. Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 18.Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP, Duan W, Fox L, Grucza RA, Kern J, Mayo K, Reyes O, Rice J, Saccone SF, Spiegel N, Steinbach JH, Stitzel JA, Anderson MW, You M, Stevens VL, Bierut LJ, Goate AM COGEND collaborators and GELCC collaborators. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macqueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Park JY, Drobes DJ, Evans DE. Variation in the α 5 nicotinic acetylcholine receptor subunit gene predicts cigarette smoking intensity as a function of nicotine content. Pharmacogenomics J. 2014;14:70–76. doi: 10.1038/tpj.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiraishi K, Kohno T, Kunitoh H, Watanabe S, Goto K, Nishiwaki Y, Shimada Y, Hirose H, Saito I, Kuchiba A, Yamamoto S, Yokota J. Contribution of nicotine acetylcholine receptor polymorphisms to lung cancer risk in a smoking-independent manner in the Japanese. Carcinogenesis. 2009;30:65–70. doi: 10.1093/carcin/bgn257. [DOI] [PubMed] [Google Scholar]
- 21.Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, Bierut LJ, Neuman RJ, Pomerleau OF. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson EO, Chen LS, Breslau N, Hatsukami D, Robbins T, Saccone NL, Grucza RA, Bierut LJ. Peer smoking and the nicotinic receptor genes: an examination of genetic and environmental risks for nicotine dependence. Addiction. 2010;105:2014–2022. doi: 10.1111/j.1360-0443.2010.03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen HM, Xiao Y, Rice T, Bracci PM, Wrensch MR, Sison JD, Chang JS, Smirnov IV, Patoka J, Seldin MF, Quesenberry CP, Kelsey KT, Wiencke JK. Fine mapping of chromosome 15q25. 1 lung cancer susceptibility in African-Americans. Hum Mol Genet. 2010;19:3652–3661. doi: 10.1093/hmg/ddq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. 2009 doi: 10.1002/0471142905.hg0212s60. Chapter 2: Unit 2.12. [DOI] [PubMed] [Google Scholar]
- 25.Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM, Wang M, Feng W, Zander T, MacConaill L, Lee JC, Nicoletti R, Hatton C, Goyette M, Girard L, Majmudar K, Ziaugra L, Wong KK, Gabriel S, Beroukhim R, Peyton M, Barretina J, Dutt A, Emery C, Greulich H, Shah K, Sasaki H, Gazdar A, Minna J, Armstrong SA, Mellinghoff IK, Hodi FS, Dranoff G, Mischel PS, Cloughesy TF, Nelson SF, Liau LM, Mertz K, Rubin MA, Moch H, Loda M, Catalona W, Fletcher J, Signoretti S, Kaye F, Anderson KC, Demetri GD, Dummer R, Wagner S, Herlyn M, Sellers WR, Meyerson M, Garraway LA. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 26.Adamec C. Example of the use of the nonparametric test. Test X2 for comparison of 2 independent examples. Cesk Zdrav. 1964;12:613. [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistics notes. The odds ratio. BMJ. 2000;320:1468. doi: 10.1136/bmj.320.7247.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.