Abstract
Purpose: This study examined the efficacy and safety of using nintedanib as single-regimen in 2nd-line chemotherapy for Chinese patients with advanced (beyond stage IIIB) non-small-cell lung cancer (NSCLC). Methods: Chinese patients were those with stage IIIB or IV NSCLC and had unsuccessful 1st-line platinum based chemotherapy. Patients received two oral intakes of 200 mg nintedanib everyday from day 1 to day 21, on every 4-week cycle. Primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS) and disease control rate. Results: There were 62 eligible patients enrolled in the study. Half of the patients were male (n = 31, 50.0%). The median age was 64.2 years with youngest age of 33 years and oldest age of 83 years. Median PFS was 3.9 months (95% CI, 2.7-6.4 months). Median OS was 6.7 months (95% CI, 4.8-10.1 months). No patients (0.0%) had complete response. Thirty-one patients (50.0%) had stable disease and 23 patients (37.1%) had partial response. The most common severe adverse events (AEs), graded as 3 or 4, were heart failure (n = 12, 19.4%), hypertension (n = 7, 11.8%) and diarrhea (n = 6, 9.8%). Conclusion: NSCLC Patients in 2nd-line chemotherapy reached similar PFS, as compared with other FDA-approved second-line regimens. Also, the toxicity of nintedanib was well tolerated. Thus, nintedanib may be used as a standard regimen for 2nd-line chemotherapy for patients with advanced NSCLC.
Keywords: Non-small-cell lung cancer, nintedanib, second-line chemotherapy, PFS, OS
Introduction
Lung cancer is one of the most common cancers to be occurred in both men and women [1]. Globally, lung cancer accounts for more than 25% of all cancer deaths [1]. Any type of epithelial lung cancer other than small cell lung carcinoma is considered as Non-small-cell lung cancer (NSCLC), including quamous cell carcinoma, large cell carcinoma, and adenocarcinoma. Every year, NSCLC may account for more than 70% of total new cases of lung cancers [2]. For patients with NSCLC, platinum-based chemotherapy is the standard treatment, yet most of them would still experience disease progression within 6 months from 1st-line therapy, therefore needing 2nd-line chemotherapy [3].
In spite of endless efforts to seeking optimal strategies to treat advanced NSCLC patients who had disease progression beyond 1st-line therapy, only three regimens, permetrexed, erlotinib, and docetaxel had been approved by FDA to be used in 2nd-line chemotherapy [4-6]. Thus, there is a great demand for efficient 2nd-line chemotherapy regimens to treat NSCLC patients.
In the new era of targeted therapy, human monoclonal antibodies such as bevacizumab (inhibiting vascular endothelial growth factor, anti-VEGF), or Sorafenib, (VEGF receptor tyrosine kinase inhibitor, VEGFR-TKI) have shown great promises in improving prognosis among NSCLC patients [7]. Recently, a new regimen, nintedanib or BIBF 1120 (Boehringer Ingelheim, Germany) was shown to be a triple angiokinase-inhibitor that binds not only VEGFRs, but also fibroblast growth factor receptors (FGFRs) and platelet-derived growth factor receptors (PDGFRs) [8]. In lung cancer, nintedanib was shown to inhibit tumor growth and induce hypoxia in animal models [9]. Most importantly, a phase III clinical trial LUME-Lung 1 demonstrated that, in combination with docetaxel in 2nd-line chemotherapy, nintedanib significantly increased NSCLC patients’ progression-free survival (PFS) and overall survival (OS) in patients with adenocarcinoma [10]. Ongoing clinical trials also suggested that better prognosis in 2nd-line chemotherapy may be achievable while nintedanib was combined with pemetrexed [11]. Therefore, it would be very important to learn whether using nintedanib alone as a single-regimen in 2nd-line chemotherapy may yield clinically equivalent efficacy as other FDA-approved 2nd-line regimens including permetrexed, erlotinib, and docetaxel.
Thus, in our work of phase II clinical study, we used nintedanib as a single-regimen in 2nd-line chemotherapy for patients with advanced NSCLC and previous unsuccessful 1st-line platinum-based chemotherapy. We assessed PFS, OS and response rates for efficacy and adverse events (AEs) for safety.
Methods
Patients
Between January 2011 and February 2013, 62 eligible patients with advanced NSCLC were enrolled at the Department of thoracic surgery, Daqing Oilfield General Hospital in Daqing, China. The eligibility criteria included, patients were adult (≥ 18 years, ≤ 83 years), had recurrent stage IIIB or IV NSCLC confirmed by histology, had 1st-line platinum-based chemotherapy but still developed disease progression, had Eastern Cooperative Oncology Group performance statuses (ECOG PSs) 0 or 1, had at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 3.0. Patients were excluded if, patients had life expectances of 12 weeks or less; more than one targeted chemotherapy regimens were used in 1st-line chemotherapy, including bevacizumab, cediranib, sunitinib, sorafenib or pazopanib; patients underwent other 2nd-line chemotherapy, immunotherapy, radiotherapy or major surgeries within 8 weeks of the onset of current study; patients had radiological evidence of brain tumors, significant thrombosis or other cardiovascular conditions.
Written consent forms were signed by all patients. Our study was in compliance with the Declaration of Helsinki. All clinical procedures were conducted in accordance with Good Clinical Practice Guidelines and any related municipal or federal regulations. All clinical protocols were reviewed independently and approved unanimously by both the principal investigators of current study and the primary care physicians/radiologists of patients. In addition, all protocols and procedures were reviewed and approved by the participating institutes.
Treatment procedure and study design
Patients received two oral intakes of 200 mg nintedanib everyday from day 1 to day 21, on every 4-week cycle. Treatments were continuously conducted until patients experienced severe AEs (grade 5) or disease progression. If patients experienced significant AEs (grade 3 or 4), a 25% dose reduction was allowed, given the permissions from both the principal investigators of our study and the primary physicians of participating patients.
Cancer lesions were evaluated by an independent review panel outside the participating institutes every 4 weeks according to RECIST (version 3.0). AEs (grade 1 to 5) were evaluated by principal investigators according to the guideline of Common Terminology Criteria for Adverse Events version 3.0 [12], every 2 weeks during the study and follow-ups.
The primary endpoint was progression-free survival (PFS), assessed from the onset of study till disease progression or death, whichever occurs first. The major secondly endpoint was overall survival (OS), assessed from the onset of study till death. Other secondly endpoints were disease control rates, including complete response, partial response and stable disease.
Statistical analysis
In our single-arm phase II study, a Kaplan-Meier model with unstratified log-rank test and 95% confidence interval (CI) was used to evaluate PFS and OS. The primary endpoint is PFS. The goal of primary endpoint was set to be 3.6 months, based on a null hypothesis of 1.8 months reported in previous study [5]. The median follow-up after treatment was 12.4 months (95% CI, 9.4-15.7 months). Therefore, using a single proportion log-rank test with 0.95 power and 0.05 significance level, we will need a sample size of 55 patients to reject the null hypothesis.
Results
Patients
Between January 2011 and February 2013, a total of 62 eligible Chinese patients with advanced NSCLC were enrolled in the study. The demographics and baseline disease characteristics were presented in Table 1. Half of the patients were male (n = 31, 50.0%). The censored median age was 64.2 years (33~83 years). The majority of the patients had Eastern Cooperative Oncology Group (PCOG) performance status 1 (n = 39, 62.9%), were current or ex-smokers (n = 39, 62.9%), were histologically diagnosed with adenocarcinoma (n = 35, 56.5%), enrolled in the current study within 2 months from 1st-line chemotherapy (n = 42, 67.7%) and had stable disease from 1st-line chemotherapy (n = 38, 61.3%).
Table 1.
The baseline demographics and disease characteristics of 62 Chinese patients with advanced NSCLC and 2nd-line chemotherapy of nintedanib
| Number | Percentage | |
|---|---|---|
| Sex | ||
| Male | 31 | 50.0% |
| Female | 31 | 50.0% |
| Age (years) | ||
| Median | 64.2 | |
| Range | 33-83 | |
| ≥ 60 years | 45 | 72.6% |
| ECOG Performance Status | ||
| 0 | 11 | 17.7% |
| 1 | 39 | 62.9% |
| 2 | 12 | 19.4% |
| Smoking History | ||
| Current or ex-smoker | 39 | 62.9% |
| Never | 23 | 37.1% |
| NSCLC sub-type | ||
| Adenocarcinoma | 35 | 56.5% |
| Squamous carcinoma | 17 | 27.4% |
| Large carcinoma | 6 | 9.7% |
| Other | 4 | 6.5% |
| Time since 1st-line chemotherapy (months) | ||
| ≤ 2 | 42 | 67.7% |
| > 2 | 20 | 32.3% |
| Response to 1st-line chemotherapy | ||
| Complete response | 0 | 0.0% |
| Partial response | 11 | 17.7% |
| Stable disease | 38 | 61.3% |
| Progressive disease | 13 | 21.0% |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Efficacy
The cut-off date of our study is March 2014. The median treatment duration was 11.2 months (95% CI, 8.2-13.1 months), and the median follow-up after treatment was 12.4 months (95% CI, 9.4-15.7 months). The primary endpoint, PFS was evaluated by Kaplan-Meier method (Figure 1A). The secondary endpoint OS was also evaluated by Kaplan-Meier method (Figure 1B). Our results demonstrated that, among 62 Chinese patients with advanced NSCLC, the median PFS was 3.9 months (95% CI, 2.7-6.4 months) and the median OS was 6.7 months (95% CI, 4.8-10.1 months).
Figure 1.
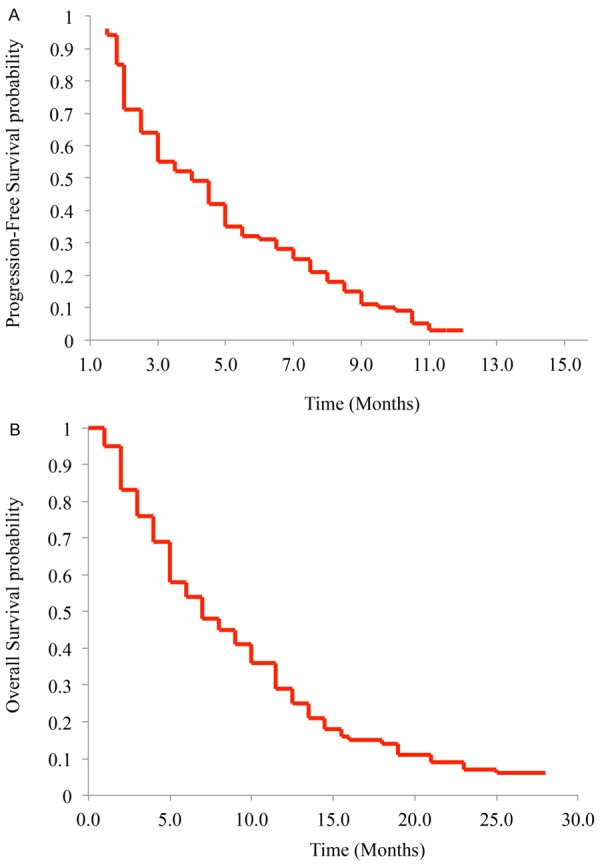
Progression-free survival (A) and overall survival (B) were evaluated by Kaplan-Meier model.
Other secondary endpoints of disease control rates, including complete response (C.R.), partial response (P.R.), stable disease (S.D.) and disease progression (D.P.) were also evaluated (Table 2). There have been no patients with C.R. However, over 20% of total patients had P.R. (n = 23, 37.1%). Half of total patients reached S.D. (n = 31, 50.0%). The remaining patients had D.P. (n = 8, 8.1%).
Table 2.
Disease control rates of 62 Chinese patients with advanced NSCLC and 2nd-line chemotherapy of nintedanib
| Disease control rates | Number | Percentage |
|---|---|---|
| C.R. | 0 | 0.0% |
| P.R. | 23 | 37.1% |
| S.D. | 31 | 50.0% |
| D.P. | 8 | 8.1% |
Abbreviations: C.R., complete response; P.R., partial response; S.D., stable disease; D.P., disease progression.
Efficacy
Treatment-associated grade 1-4 AEs were evaluated (Table 3). Most of the AEs were tolerated by patients with optimized supportive care. Eight patients had reduced dose. The most common moderate AEs (grade 1-2) were diarrhea (n = 44, 71.0%), heart failure (n = 31, 50.0%), nausea (n = 26, 43.5%), chest pain (n = 17, 27.4%) and hypertension (n = 13, 21.0%). The most common severe AEs (grade 3-4) were heart failure (n = 12, 19.4%), hypertension (n = 7, 11.8%) and diarrhea (n = 6, 9.8%).
Table 3.
Adverse events of 62 Chinese patients with advanced NSCLC and 2nd-line chemotherapy of nintedanib
| Adverse Events | Grade 1 or 2 | Grade 3 or 4 | ||
|---|---|---|---|---|
|
| ||||
| Number | Percentage | Number | Percentage | |
| Diarrhea | 44 | 71.0% | 6 | 9.8% |
| Nausea | 26 | 43.5% | 4 | 6.5% |
| Chest pain | 17 | 27.4% | 3 | 4.8% |
| Fatigue | 10 | 16.1% | 2 | 3.2% |
| Nausea | 13 | 21.0% | 2 | 3.2% |
| Asthenia | 8 | 12.9% | 0 | 0% |
| Pneumonia | 9 | 14.5% | 5 | 8.1% |
| Hypertension | 13 | 21.0% | 7 | 11.3% |
| Vomiting | 7 | 11.3% | 3 | 4.8% |
| Hyperglycaemia | 8 | 12.9% | 1 | 1.6% |
| Bleeding | 6 | 9.7% | 1 | 1.6% |
| Anemia | 4 | 6.5% | 2 | 3.2% |
| Heart failure | 31 | 50.0% | 12 | 19.4% |
Discussions
In this phase II clinical study, we evaluated the efficacy and safety of nintedanib as single 2nd-line chemotherapy regimen for Chinese patients with advanced NSCLC. The primary endpoint of our study was PFS, with a pre-designed aim of 3.8 months, based on a null hypothesis of 1.8 months reported in previous study [5]. As shown in the results section of our study, the median PFS was 3.9 months (95% CI, 2.7-6.4 months) for Chinese patients with advanced NSCLC. In the previous double-blinded placebo-controlled phase III clinic trial, erlotinib, one of the three FDA-approved 2nd-line NSCLC chemotherapy regimens, significantly improved PFS (2.2 months for erlotinib vs. 1.8 months for placebo) in 2nd-line chemotherapy for patients with advanced NSCLC [5]. In another phase III clinic trial, the other two FDA-approved 2nd-line NSCLC chemotherapy regimens, pemetrexed and docetaxel, were also assessed in 2nd-line chemotherapy setting for patients with NSCLC [6]. In that study, investigators found that median PFS was 2.9 months for both pemetrexed and docetaxel. It is very encouraging to notice that nintedanib is equivalently effective in improving patients’ median PFS (3.9 months) as compared to three FDA-approved 2nd-line NSCLC chemotherapy regimens (2.2 months, 2.9 months and 2.9 months for erlotinib, pemetrexed and docetaxel, respectively). Thus, our data of median PFS strongly suggested that nintedanib could be an effective 2nd-line chemotherapy regimen in treating patients with NSCLC.
In our study, the median OS for nintedanib was 6.7 months (95% CI, 4.8-10.1 months), same as the median OS for erlotinib (6.7 months) [5], and slightly shorter than the median OSs for pemetrexed or docetaxel (8.3 months and 7.9 months, respectively) [6]. It’s worth noting that in the placebo-controlled arm of 2nd-line chemotherapy for patients with advanced NSCLC, the median OS was only 4.7 months [5]. Although no statistical analysis was available to determine whether the OS for nintedanib in our study was significantly better than placebo, this data seems to be line with the conclusion drawn from PFS comparison, suggesting that patients with advanced NSCLC may also benefit on overall survival by the treatment of nintedanib in 2nd-line chemotherapy.
In summary, our study showed that 2nd-line chemotherapy with single-regimen of nintedanib might be equivalently efficient and safe, as compared with three FDA-approved 2nd-line chemotherapy regimens, erlotinib, pemetrexed and docetaxel, to treat patients with advanced NSCLC.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Walker S. Updates in non-small cell lung cancer. Clin J Oncol Nurs. 2008;12:587–596. doi: 10.1188/08.CJON.587-596. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D’Amico TA, Demmy TL, Ganti AK, Govindan R, Grannis FW Jr, Horn L, Jahan TM, Jahanzeb M, Kessinger A, Komaki R, Kong FM, Kris MG, Krug LM, Lennes IT, Loo BW Jr, Martins R, O’Malley J, Osarogiagbon RU, Otterson GA, Patel JD, Pinder-Schenck MC, Pisters KM, Reckamp K, Riely GJ, Rohren E, Swanson SJ, Wood DE, Yang SC, Hughes M, Gregory KM NCCN (National Comprehensive Cancer Network. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–1271. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 4.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J. Clin. Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar , Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA Jr. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J. Clin. Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 7.Haghgoo SM, Allameh A, Mortaz E, Garssen J, Folkerts G, Barnes PJ, Adcock IM. Pharmacogenomics and targeted therapy of cancer: Focusing on non-small cell lung cancer. Eur J Pharmacol. 2015;754:82–91. doi: 10.1016/j.ejphar.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, Heckel A, Rettig WJ. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 9.Kutluk Cenik B, Ostapoff KT, Gerber DE, Brekken RA. BIBF 1120 (nintedanib), a triple angiokinase inhibitor, induces hypoxia but not EMT and blocks progression of preclinical models of lung and pancreatic cancer. Mol Cancer Ther. 2013;12:992–1001. doi: 10.1158/1535-7163.MCT-12-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, Novello S LUME-Lung 1 Study Group. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 11.Daga H, Takeda K, Okada H, Miyazaki M, Ueda S, Kaneda H, Okamoto I, Yoh K, Goto K, Konishi K. Safety and efficacy of nintedanib (BIBF 1120) plus pemetrexed in Japanese patients with advanced or recurrent non-small cell lung cancer (NSCLC): a phase I study. J. Clin. Oncol. 2013;31:8056. [Google Scholar]
- 12.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN. CTCAE v3. 0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]


