Abstract
It should be urgently better understood of the mechanism that contributes cancer aggressiveness. Epithelial-mesenchymal transition (EMT) plays a fundamental role in tumor progression and metastasis formation by invasion, resistance to cell death and senescence, resistance to chemotherapy and immunotherapy, immune surveillance, immunosuppression and inflammation, confers stem cell properties. Tumor-associated macrophages (TAMs) are key orchestrators and a set of macrophages in tumor microenvironment. They are major players in the connection between inflammation and cancer. TAMs could promote proliferation, invasion and metastasis of tumor cells, stimulate tumor angiogenesis, and inhibit anti-tumor immune response mediated by T cell followed by promoting tumor progression. Recently, studies showed that TAMs played critical role in the regulation of EMT in cancer, although the underlying mechanism of TAMs-mediated acquisition of EMT has been largely unclear. This review will discuss recent advances in our understanding of the role of TAMs in the regulation of EMT during tumorigenesis and summarize the recent ongoing experimental and pre-clinical TAMs targeted studies.
Keywords: Cancer, tumor-associated macrophages, epithelial-mesenchymal transition
Introduction
Cancer is a major public health problem in the United States and many other parts of the world. A quarter of the deaths in the United States is due to cancer [1]. It is the leading cause of death in developed countries and the second leading cause of death in developing countries [2]. Currently, one third of women and half of men in the United States developed cancer during their lifetime [3]. In the United States, it was estimated that there was 1,660,290 new cancer cases and 580,350 cancer deaths in 2013. The five-year relative survival rate is approximately 65% for all human cancers due to the lack of understanding of the exact mechanism underlying cancer development and progression [1]. Therefore, a better understanding of the mechanism which contributes cancer aggressiveness is urgently needed.
In 1968, Elizabeth Hey first defined the concept of epithelial-mesenchymal transition (EMT). It is an essential embryonic process during which epithelial cells loose contact with their neighbors and gain mesenchymal properties, this could enable them to break through the basement membrane which separates different tissues from the embryo [4]. Later researchers discovered aberrant reactivation of EMT could promote tumor cell migration and invasion by disruption of apical-basal polarity and loss of E-cadherin expression [5-7]. Hence, it will greatly benefit our understanding of tumor migration and invasion that clarifying the regulation of EMT.
Tumor-associated macrophages (TAMs) are key orchestrators and a set of macrophages of the tumor microenvironment [8,9]. They are major players in the connection between inflammation and cancer [10]. TAMs could promote proliferation, invasion and metastasis of tumor cell, stimulate tumor angiogenesis, and inhibit anti-tumor immune response mediated by T cell, followed by promoting tumor progression [11,12]. Accumulated evidences have demonstrated that TAMs plays critical role in the regulation of EMT in cancer [13-16]. This review will summarize what is known about TAMs in cancer development and progression and the role of TAMs in the regulation of EMT, so as to get a better understanding of the mechanism that contributes to cancer aggressiveness. Lastly, we will describe potential application of TAMs as targets for the prevention and/or treatment of human cancers in the future.
Epithelial-mesenchymal transition
In 1968, Elizabeth Hey first proposed the concept of EMT. It is an essential embryonic process, during which polarized epithelial cells convert to motile mesenchymal cells. The typical characters of this process are lossing of cell-cell adhesions and apical-basal polarity in epithelial cells, and the acquisition of migratory and invasive properties [5]. Later reseachers discovered aberrant reactivation of EMT could promote tumor cell migration and invasion [5-7]. By suppressing the expression of epithelial markers such as E-cadherin, and by increasing the expression of mesenchymal mark-ers, including Vimentin, Slug, Snail, Fibronectin, zinc-finger E-box binding homeobox 1 (ZEB1), ZEB2, and α-smooth muscle action (SMA), the cells acquire the ability to migrate and invade, which could lead to tumor progression and metastasis [5].
EMT plays a fundamental role in tumor progression and metastasis formation by invasion, resistance to cell death and senescence, resistance to chemotherapy and immunotherapy, immune surveillance, immunosuppression, and inflammation, and confers stem cell properties [5]. So far, many growth factors, cytokines and cellular signaling pathways have been found in EMT regulating in cancer, incluing transforming growth factor beta (TGF-β), forkhead box protein M1 (FoxM1), hepatocyte growth factor (HGF), epidermal growth factor factor (EGF), NFκB, Notch, and Wnt [5,17-19]. Recently, the growing body of literature strongly suggested that TAMs played a critical role in regulation of EMT in cancer [13-16]. Therefore, we will highlight the function of TAMs in the regulation of EMT in human cancers.
The promotion role of TAMs in cancer
The polarization of macrophages in cancer
Macrophages can be divided schematically into two main classes in line with the Th1/Th2 dichotomy [10]. They are simplified as either classical ‘M1’ or alternative ‘M2’ [20]. Microbial stimuli such as lipopolysaccharides (LPS) and Th1 cytokines such as interferon gamma (IFN-γ) drive macrophages to the classically activated state. The characters of these cells are increased expression of inflammatory cytokines, chemokines, and reactive nitrogen/oxygen intermediates (RNI/ROI). The functions of these cells including promoting Th1 effector response, anti-microbial ability, protection against various types of bacteria and viruses, and tumoricidal functions. In contrast, Th2 cytokines such as IL-4 and IL-13 drive macrophages to the alternatively activated state [21]. The characters of these cells are increased expression of scavenging receptors and scavenging activity, reduced expression of inflammatory cytokines, and preferentially metabolized arginine to ornithine via arginase. The functions of these cells including anti-inflammatory, the promotion of Th2 response, tissue repairing and remodelling, protection from parasite infection, and tumor promotion. M1- and M2-polarized macrophages have been indicated in tumors [22]. TAMs resemble M2-polarized macrophages [23].
Origin of TAMs
The historic description of tissue macrophages is that they are solely derived from bone marrow (BM). However, evidences from recent studies suggest that most tissue macrophages origin from yolk sac progenitors [24]. Evidence indicate macrophages involved in pathogen responses originate from circulating BM monocytes [25]. Evidences from several recent studies demonstrate that most TAM subpopulations derived from the Ly6C+ population of circulating mouse moncytes in primary mouse mammary tumors, grafted tumors and lung metastases [26-28]. It is reported that TAMs are solely derived from BM as the primary source of monocytes in the Lewis lung carcinoma syngeneic transplant model [29].
Various soluble factors have been reported to regulate the orginal of macrophages at the molecular level. CSF1 is the master regulator and chemotactic factor of most populations of macrophages whether they are derived from the yolk sac or BM [30]. High CSF1 expression in cancer is associated with poor prognosis; while knocking-out CSF-1 receptor (CSF-1R) in cancer shows delayed initation, progression, and metastasis by the depletion of TAMs [12,31]. Many tumors and their stroma could release abundant CSF-1. Tumors could orchestrate the recruitment and differentiation of BM into TAMs by releasing CSF-1 and other monocyte/macrophage chemoattractants [24]. IL-34, the second ligand for CSF-1R, is reported expressing in breast cancer cells following cytotoxic therapy and is associated with the recruitment of TAMs [32,33]. In a xenograft model of skin cancer, VEGFA could recruit macrophage progenitors and then differentiate into TAMs in the presence of IL-4, and the lossing of these TAMs inhibited tumor growth, tumor invasion, proliferation and tumor angiogenesis [34]. In the Polyoma Middle T oncoprotein (PYMT) model, the binding of CCL2 to its receptor CCR2 directly mediates the monocyte recruiment to the primary tumor and metastases in the presence of CSF-1 [26,28,35]. In human breast cancer models, CCL18 binding to its receptor PITPNM3 mediates TAMs recruitment in collaboration with GM-CSF [13]. In colon cancer models, CCL9 bindsto its receptor CCR1 and mediates the immature myeloid cells recruiment [36]. The ablation of these chemokines got in the loss of monocytes and/or TAMs and the inhibition of malignancy.
The origins of TAMs in many cancers is still uncertain. Further study characterizing TAMs in different human cancers and their relationship to the possibly existing different macrophages is now warranted.
Roles of TAMs in cancer initiation and promotion
TAMs are key orchestrators and a set of macrophages of the tumor microenvironment [8,9]. They are major players in the connection between inflammation and cancer [10]. In 2009, Colotta firstly defined cancer-related inflammation (CRI) as the seventh hallmark of cancer [37]. CRI is one of the ten redefined hallmarks of cancer [38]. The reason why CRI associates with increaseing cancer risk is that chronic infection or persistent irritation is often called “smoldering inflammation” [39]. Activated macrophages work in concert with other immune cells in this type of immune response as major players [40]. Evidences show inflammatory microenvironment promote genetic instability within the developing tumor epithelial cells, the infiltrating or resident immune cells in inflammatory microenvironment including macrophages [37]. Studies have demonstrated the essential role for macrophages in promoting tumor progression through NF-κB-induced expression of cytokines such as TNF and IL-6 [41,42]. Recently, evidences showed TAMs-derived proinflammatory cytokine IL-23 and the Th17 pathway were associated with progression of spontaneous colon cancer [43].
Roles of TAMs in cancer angiogenesis
Tumor and its stromal cells produce differernt mediators that promote angiogenesis. Growth factors such as VEGF, TGF-β, PDGF and members of the FGF family released from TAMs possess proangiogenic role in many tumors [44-47]. TAMs secrete angiogenic factor thymidine phosphorylase and produce several angiogenesis modulating enzymes including MMP-2, MMP-7, MMP-9, MMP-12, and cyclooxygenase-2 [48-51]. Tie-2 expressing monocytes/macrophages (TEMs) have been reported to possess proangiogenic role in many human and mice tumors [52,53]. TAMs release many chemokines involved in angiogenic processes such as CXCL12, CCL2, CXCL8, CXCL1, CXCL13, and CCL5 [54,55]. Hypoxia is a major driver of angiogenesis. The hypoxic areas of the tumor can see the accumulation of macrophages, particularly in necrotic tissue [56]. HIF1α, which is expressed in macrophages, modulates the recruitment of macrophages to hypoxic regions of the tumor. This recruitment is through CCL-2, endothelins and other chemokines [56,57]. HIF1α regulates the transcription of a large panel of genes associated with angiogenesis at the hypoxic site including VEGF [56,58]. TAMs are also involved in lymphangiogenesis, and the lymphatic endothelial growth factors secreted by TAMs are associated with peritumoral lymphangiogenesis [59,60].
Roles of TAMs in cancer invasion and metastasis
Evidences from a study in the PyMT mouse model and in breast cancer cell xenografts show that TAMs are required for tumor cell migration and invasion [61]. The mechanism is that CSF-1 synthesized from tumor cells stimulates TAMs to move and produce EGF, afterward activates migration in the tumor cells [62]. Co-culture of tumor cells with TAMs increases invasiveness of cancer cells through TNF-α dependent MMP induction in TAMs [63,64]. Evidences from tissue culture experiments show Wnt5a acting through the noncanonical pathway in organoids and TNF-α via NFκB in co-culture promote tumor cell invasion [65,66]. Reduction of the number of TAMs by genetic methods or inhibition of EGF or CSF-1 signaling in wild-type mice bearing mammary tumors reduces the number of circulating tumor cells [67,68]. TAMs produces the most Urokinase/Plasminogen activator (uPA), and in the PyMT model its loss inhibits metastasis [69]. A CSF1-EGF paracrine loop between TAMs and tumor cells has been implicated in breast cancer cell invasion and intravasation [61]. Evidence show macrophages are essential for malignant progression and metastasis in a spontaneous model of skin carcinoma [70]. An in vivo study indicates high cathepsin activity in TAMs mediates tumor growth, angiogenesis, and invasion in pancreatic cancer [68].
Roles of TAMs in cancer immunoregulation
Macrophages are the major immonoregulatory cells in tumors, and they take great part in immune responses in tumors [12]. In 1988, Fidler first reported that activated macrophages could kill tumor cells and eliminate metastases [71]. Later evidences show activating macrophages by either overexpressing GM-CSF or treating tumors with CpG plus anti-IL-10 could inhibit tumor growth in xenograft models [72,73]. The characters of these immonoregulatory TAMs are downregulation of IL-12, IL-18, and TLR signaling pathway and upregulation of arginase [74,75]. This is in agreement with TAMs are M2-polarized population [47]. GM-CSF controll TAMs differentiate to trophic TAMs and then apart from immunologically activated ones in the presence of high concentrations of CSF-1 [39,76]. TAMs could also inhibit cytotoxic T cell responses. The production of IL-10 from TAMs can induce the expression of costimulatory molecule programmed death ligand (PD)-L1 in monocytes, these monocytes could inhibit cytotoxic T cell responses [77]. In human ovarian cancers the production of CCL22 from TAMs regulates the influx of reulatory T cells that inhibit cytotoxic T cell responses [78]. TAMs in mammary tumor xenografts cytotoxic T cell responses by the synthesis of PGE2 and TGF-β [79]. These facts indicate the immunoregulation roles of TAMs in cancer (Table 1).
Table 1.
Role of TAMs in cancer
| Role of TAMs in cancer | Mechanism | References |
|---|---|---|
| Cancer initiation and promotion | Through NF-κB-induced expression of cytokines such as TNF and IL-6, proinflammatory cytokine IL-23 and the Th17 pathway | [41-43] |
| Cancer angiogenesis | Through secrete VEGF, TGF-β, PDGF, members of the FGF family, MMP-2, MMP-7, MMP-9, MMP-12, cyclooxygenase-2, CXCL12, CCL2, CXCL8, CXCL1, CXCL13, CCL5 and HIF1α | [44-51,54-57] |
| Cancer invasion and metastasis | CSF-1 signal, TNF-α dependent MMP induction, secrete EGF, Urokinase/Plasminogen activator (uPA), high cathepsin activity | [62-64,67-69] |
| Cancer immunoregulation | Downregulation of IL-12, IL-18 and TLR signaling pathway and upregulation of arginase, inhibit cytotoxic T cell responses, the production of CCL22 | [74-75,77,78] |
Roles of TAMs in regulating cancer EMT
EMT plays a fundamental role in tumor progression and metastasis formation, clarifying the regulation of EMT will greatly benefit our understanding of tumor migration and invasion. Increasing evidences have revealed that many growth factors, cytokines and cellular signaling pathways play great roles in initiation and execution of EMT, including TGF-β, FoxM1, HGF, EGF, NFκB, Notch, Snail, ZEB1, ZEB2, Twist1, KLF4, KLF8, Sox9 and Wnt [5,17-19,80]. Accumulated evidences have demonstrated that TAMs played critical role in the regulation of EMT in cancers [13-16]. In the following sections, we will discuss the crosstalk between TAMs and selected signaling pathways, including which play critical roles in EMT.
Crosstalk between TAMs and NFκB
NFκB is a major inflammation mediator and an oncogenic key transcription factor, it is activated in breast cancer and its activation leads to direct suppression of miR488, an anti-metastatic microRNA; and the downregulation of miR488 subsequently increased the expression of SATB1 and Twist1, which are two major EMT initiators leading to the acquisition of mesenchymal phenotype [81]. Resveratrol inhibit LPS-induced EMT in mouse melanoma through the down-regulation of NF-κB activity [82]. In oral squamous cell carcinoma (OSCC), TAMs play a protumor role and promote EMT through activation of Gas6/Axl-NF-κB [83]. Pterostilbene, a natural stilbene isolated from blueberries, possesses anti-cancer effects in a variety of cancer types [84]. In breast cancer, a recent study demonstrates that pterostilbene effectively suppresses the generation of CSCs and metastatic potentialunder the influence of M2 TAMs via modulating EMT associated signaling pathways, specifically NFκB/miR488 circuit [85].
Crosstalk between TAMs and TLR4/IL-10
Toll-like receptors (TLRs) play critical roles in innate immunity and are primarily expressed on macrophages and dentritic cells. The activation of TLRs in these cells induce cytokine secretion and inflammatory responses. TLR4, one of this class of receptors, has been demonstrated closely to the connection between inflammation-mediated carcinogenesis and tumor progression [86]. The inhibition of TLR4 signaling in TAMs reduced cytokines and weakened their tumor-promoting activity in experimental lung metastasis [87]. In human hepatocellular carcinoma, evidence indicated that TLR4/JNK/MAPK signaling was required for LPS-induced EMT [88,89]. IL-10, a type II cytokine, has been demonstrated to play a critical role in regulating macrophages. It is produced by monocytes, a subtype of dendritic cells, and activated macrophages [90]. Besides, additional sources of IL-10 in cancers include the alternatively activated M2 TAMs [91]. The local production of IL-10 results in a tumor microenvironment fa-vors cancer cells survival and metastases [91-94]. Evidence indicates the activation of TLR4 signaling on M2-polarized TAMs stimulates an increased release of IL-10 [95]. Recently, the potential role of TLR4/IL-10 signaling in the EMT of pancreatic cancer is clarified, M2-polarized TAMs promoted EMT in pancreatic cancer cells partially through TLR4/IL-10 signaling [15].
Crosstalk between TAMs and TGF-β1
Transforming growth factor-β (TGF-β) influences cell differentiation, proliferation, motility, and apoptosis as a multifunctional cytokine [96,97]. The expression of TGF-β is abundant in chronic inflammatory diseases and cancer [98]. TGF-β1 is one of the most important members of TGF-β. TGF-β1 plays critical roles in cancer progression and metastasis [99,100], which induces epithelial plasticity leading to EMT in cancer cells [98,101]. TGF-β1/Smad signaling directly activates the expression of EMT transcription factors, including ZEB1, ZEB2, Snail, Slug and Twist. In breast cancer, evidence shows the Ras effector Blimp-1 play an essential role in TGF-β1-induced EMT via repression of BMP-5 [102]. KLF8 involves in TGF-β1-induced EMT and promotes invasion and migration in gastric cancer cells [98]. MiRNA-200b represses TGF-β1-induced EMT and fibronectin expression in kidney proximal tubular cells [103]. TAMs can induce EMT in intratumoral cancer cells through TGF-β signaling and activation of the β-catenin pathway [104]. Two double-negative feedback loops: one between the transcription factor SNAIL1 and miR-34 family and the other between the transcription factor ZEB1 and miR-200 family, have been found involved in TGF-β1-induced EMT of MCF10A cells [105]. NFκB signalling is involed in TGF-β1-induced EMT [106-108].
A recent study showed that TAMs promote hepatocellular carcinoma CSC-like properties via TGF-β1-induced EMT. Depletion of TGF-β1 blocked acquisition of CSC-like properties through inhibition of TGF-β1-induced EMT [14]. In human cholangiocarcinoma (CCA) cell lines, the various cytokines secreted by TAMs including TGF-β1 induced EMT [109]. Evidence showed that TAMs co-culture enhanced invasion of gastric cancer cells via TGF-β and BMP pathways [110]. These findings provided convincing evidence that there was a tight crosstalk between TAMs and TGF-β1 signaling pathways, and TAMs may play a central role in tumor EMT through interaction with TGF-β1 signaling.
Crosstalk between TAMs and FoxQ1
Forkhead box Q1 (FoxQ1) is a member of the forkhead transcription factor family [111]. FoxQ1 plays critical roles in hair follicle morphogenesis and gastric epithelial differentiation [112]. Evidences from studies demonstrated that increased FoxQ1 expressionis correlated with metastasis and poor prognosis for colon cancer, lung cancer, and breast cancer [113-116]. FoxQ1 induces an EMT change, gain of stem cell-like properties, and acquisition of resistance to chemotherapy-induced apoptosis [114]. FoxQ1 represses E-cadherin expression via binding to the E-box in its promoter region, and the knockdown of FoxQ1 blocks TGF-β-induced EMT in breast cancer cells [116]. The suppression of FoxQ1 is implicated in benzyl isothiocyanate-mediated inhibition of EMT in human breast cancer cells [117]. In bladder cancer, it has been demonstrated short hairpin RNA targeting FoxQ1 inhibit invasion and metastasis of cancer cells via the reversal of EMT [118]. In Hela cells, evidence show FoxQ1 promote TGF-β1 expression and induce EMT [119]. In NSCLC, FoxQ1 is implicated in regulating EMT and increasing chemosensitivity, downregulation of FoxQ1 promotes the expression of epithelial markers, decreases several mesenchymal markers in vitro and in vivo, and increases resistance to conventional chemotherapeutic agents [120]. Versican V1 is an aggregating chondroitinsulfate proteoglycan, which is secreted by both tumor cells and TAMs [121]. It is an important proinflammatory mediator in the tumor microenvironment, and the increased expression of Versican V1 correlates with metastasis and poor survival in many human cancers [122-124]. Recently a study reported FoxQ1 expression is an independent and significant risk factor for hepatocellular carcinoma. FoxQ1 induces EMT via the transactivation of ZEB2 expression by directly binding to the ZEB2 promoter; Versican V1 was identified as a direct transcriptional target of FoxQ1, the inhibition of Versican V1 inhibits FoxQ1-mediated TAMs migration; animal studies show the up-regulation of FoxQ1 in HCC cells promote HCC metastasis and intratumor TAMs infiltration; in human HCC tissues, FoxQ1 expression is positively correlated with ZEB2 and Versican V1 expression and intratumoral TAM infiltration [125]. Taken together, there is a reciprocal interaction between TAMs and FoxQ1 signaling, which may contribute to the development of EMT in cancer.
TAMs as a cancer therapeutic target
Since TAMs plays a critical role in the development and progression of human cancer, it also plays a critical role in the regulation of EMT in cancer. Targeting TAMs could be a novel strategy for the treatment of human cancers. TAMs could be either tumor killing (M1 or activated) or tumor promoting (M2 or alternatively activated) [126,127]. Large-scale transcriptome analysis results revealled macrophages have a mixed phenotype expressing both M1 and M2 markers [12]. Several recent studies indicated the approach of block macrophage trophic phenotypes together with their immunosuppressive behaviors and enhance their activation, and antitumoral activities is feasible and therapeutic [128,129]. The major strategy targeting TAMs for cancer therapy based upon genetic experiments is inhibition of CSF-1 signaling by anti-CSF1 receptor-neutralizing antibodies or small-molecule inhibitors [128]. The inhibition of CSF-1R causes TAMs to repolarize to a state regulated by GM-CSF in glibastoma, cervical and breast cancer models have been demonstrated to be antitumoral [8,130]. Small-molecule inhibitors to CSF1R could deplete some populations of TAMs and enhance tumor responses to chemotherapy by the removal of macrophage-mediated immunosuppression during the tumor recovery period [33,131]. Study results showed low-dose irradiation of tumors programs macrophages to an activated state could orchestrate T cell immunotherapy [132]. Macrophages could also enhance monoclonal antibodies therapeutic efficacy of monoclonal antibodies [129]. Trabectedin could directly kill monocytes and/or macrophages and could be a therapeutic agent against tumors in mice models [133]. Recently a study indicated amphotericin B could enhance macrophage-mediatedinhibition of glioma tumor-initiating cells [134]. Striking, using a neutralizing antibody to the CSF1R in a single-molecule approach has been applied in diffuse-type giant cell tumors that overexpress CSF1 in a recent clinical trial [135].
Conclusions and future directions
In conclusion, TAMs play critical roles in the development and progression of human cancer, in which it is mediated through regulation of cancer intiation and promotion, cancer angiogenesis, cancer invasion and metastasis, cancer immunoregulation. EMT plays a fundamental role in tumor progression and metastasis formation by invasion, resistance to cell death and senescence, resistance to chemotherapy and immunotherapy, immune surveillance, immunosuppression and inflammation, and confers stem cell properties [5]. Accumulated evidences have demonstrated that TAMs plays critical role in the regulation of EMT in cancer [13-16]. This review discussed the role of TAMs in regulation EMT with signaling pathways, including NFκB, TLR4/IL-10, TGF-β1 and FoxQ1 (Figure 1). Therefore, targeting TAMs could be a promising strategy for the treatment of cancers. Therecent ongoing experimental and pre-clinical TAMs targeted studies have indeed made some encouraging progress. We believe that TAMs targeted strategy will be applied in clinic for cancer patients in the future.
Figure 1.
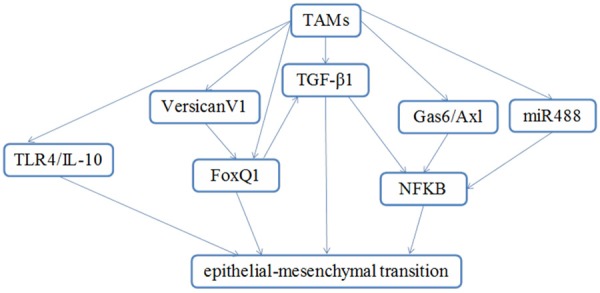
A proposed model for TAMs that controls the processes of EMT. TAMs triggers EMT through regulation of TLR4/IL-10, Versican V1, TGF-β1, Gas6/Ax1, miR488, FoxQ1 and NFκB.
Acknowledgements
This work was supported by the Scientific and Technological Planning project of Shaanxi Province (No. 2014KW23-02) and the Fundamental Research Funds for the Central Universities.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 7.Savagner P, Boyer B, Valles AM, Jouanneau J, Thiery JP. Modulations of the epithelial phenotype during embryogenesis and cancer progression. Cancer Treat Res. 1994;71:229–249. doi: 10.1007/978-1-4615-2592-9_12. [DOI] [PubMed] [Google Scholar]
- 8.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su S, Liu Q, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, Cui X, Zheng F, Li H, Yao H, Su F, Song E. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, Wei LX. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93:844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 16.Helm O, Held-Feindt J, Grage-Griebenow E, Reiling N, Ungefroren H, Vogel I, Kruger U, Becker T, Ebsen M, Rocken C, Kabelitz D, Schafer H, Sebens S. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer. 2014;135:843–861. doi: 10.1002/ijc.28736. [DOI] [PubMed] [Google Scholar]
- 17.Grego-Bessa J, Diez J, Timmerman L, de la Pompa JL. Notch and epithelial-mesenchyme transition in development and tumor progression: another turn of the screw. Cell Cycle. 2004;3:718–721. [PubMed] [Google Scholar]
- 18.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-beta and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–2374. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 20.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 23.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anticancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 25.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C (high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 28.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shand FH, Ueha S, Otsuji M, Koid SS, Shichino S, Tsukui T, Kosugi-Kanaya M, Abe J, Tomura M, Ziogas J, Matsushima K. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc Natl Acad Sci U S A. 2014;111:7771–7776. doi: 10.1073/pnas.1402914111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55:861–867. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34:81–89. doi: 10.1016/j.it.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 33.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012;227:17–28. doi: 10.1002/path.3989. [DOI] [PubMed] [Google Scholar]
- 35.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B, Gorbatov R, Forghani R, Novobrantseva TI, Koteliansky V, Figueiredo JL, Chen JW, Anderson DG, Nahrendorf M, Swirski FK, Weissleder R, Pittet MJ. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, Aoki M, Taketo MM. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci U S A. 2010;107:13063–13068. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 45.Bingle L, Lewis CE, Corke KP, Reed MW, Brown NJ. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br J Cancer. 2006;94:101–107. doi: 10.1038/sj.bjc.6602901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol. 2004;99:1667–1674. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- 47.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumorassociated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 48.Hotchkiss KA, Ashton AW, Klein RS, Lenzi ML, Zhu GH, Schwartz EL. Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Res. 2003;63:527–533. [PubMed] [Google Scholar]
- 49.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 50.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 51.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 52.Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, Di Serio C, Naldini L, De Palma M. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 53.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 55.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 57.Grimshaw MJ, Wilson JL, Balkwill FR. Endothelin-2 is a macrophage chemoattractant: implications for macrophage distribution in tumors. Eur J Immunol. 2002;32:2393–2400. doi: 10.1002/1521-4141(200209)32:9<2393::AID-IMMU2393>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 58.Lewis CE, Hughes R. Inflammation and breast cancer. Microenvironmental factors regulating macrophage function in breast tumours: hypoxia and angiopoietin-2. Breast Cancer Res. 2007;9:209. doi: 10.1186/bcr1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji RC. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006;25:677–694. doi: 10.1007/s10555-006-9026-y. [DOI] [PubMed] [Google Scholar]
- 61.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 63.Mytar B, Woloszyn M, Szatanek R, Baj-Krzyworzeka M, Siedlar M, Ruggiero I, Wieckiewicz J, Zembala M. Tumor cell-induced deactivation of human monocytes. J Leukoc Biol. 2003;74:1094–1101. doi: 10.1189/jlb.0403140. [DOI] [PubMed] [Google Scholar]
- 64.Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon cocultivation with macrophages is due to TNFalpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 65.Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103:5454–5459. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 67.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 69.Almholt K, Lund LR, Rygaard J, Nielsen BS, Dano K, Romer J, Johnsen M. Reduced metastasis of transgenic mammary cancer in urokinase-deficient mice. Int J Cancer. 2005;113:525–532. doi: 10.1002/ijc.20631. [DOI] [PubMed] [Google Scholar]
- 70.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fidler IJ, Schroit AJ. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta. 1988;948:151–173. doi: 10.1016/0304-419x(88)90009-1. [DOI] [PubMed] [Google Scholar]
- 72.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 73.Eubank TD, Roberts RD, Khan M, Curry JM, Nuovo GJ, Kuppusamy P, Marsh CB. Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res. 2009;69:2133–2140. doi: 10.1158/0008-5472.CAN-08-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A. A distinct and unique transcriptional program expressed by tumorassociated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 75.Ojalvo LS, King W, Cox D, Pollard JW. Highdensity gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 77.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 79.Torroella-Kouri M, Silvera R, Rodriguez D, Caso R, Shatry A, Opiela S, Ilkovitch D, Schwendener RA, Iragavarapu-Charyulu V, Cardentey Y, Strbo N, Lopez DM. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 2009;69:4800–4809. doi: 10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- 80.Li HX, Han M, Bernier M, Zheng B, Sun SG, Su M, Zhang R, Fu JR, Wen JK. Kruppel-like factor 4 promotes differentiation by transforming growth factor-beta receptor-mediated Smad and p38 MAPK signaling in vascular smooth muscle cells. J Biol Chem. 2010;285:17846–17856. doi: 10.1074/jbc.M109.076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li QQ, Chen ZQ, Cao XX, Xu JD, Xu JW, Chen YY, Wang WJ, Chen Q, Tang F, Liu XP, Xu ZD. Involvement of NF-kappaB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell Death Differ. 2011;18:16–25. doi: 10.1038/cdd.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC, Lee CH. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012;18:685–693. doi: 10.1177/1753425912436589. [DOI] [PubMed] [Google Scholar]
- 83.Lee CH, Liu SY, Chou KC, Yeh CT, Shiah SG, Huang RY, Cheng JC, Yen CY, Shieh YS. Tumor-associated macrophages promote oral cancer progression through activation of the Axl signaling pathway. Ann Surg Oncol. 2014;21:1031–1037. doi: 10.1245/s10434-013-3400-0. [DOI] [PubMed] [Google Scholar]
- 84.McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res. 2012;173:e53–61. doi: 10.1016/j.jss.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 85.Mak KK, Wu AT, Lee WH, Chang TC, Chiou JF, Wang LS, Wu CH, Huang CY, Shieh YS, Chao TY, Ho CT, Yen GC, Yeh CT. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-kappaB/microRNA 448 circuit. Mol Nutr Food Res. 2013;57:1123–1134. doi: 10.1002/mnfr.201200549. [DOI] [PubMed] [Google Scholar]
- 86.Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7:1271–1285. doi: 10.1016/j.intimp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 87.Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 signaling promotes tumor growth. J Immunother. 2010;33:73–82. doi: 10.1097/CJI.0b013e3181b7a0a4. [DOI] [PubMed] [Google Scholar]
- 88.Jing YY, Han ZP, Sun K, Zhang SS, Hou J, Liu Y, Li R, Gao L, Zhao X, Zhao QD, Wu MC, Wei LX. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 2012;10:98. doi: 10.1186/1741-7015-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li H, Li Y, Liu D, Liu J. LPS promotes epithelial-mesenchymal transition and activation of TLR4/JNK signaling. Tumour Biol. 2014;35:10429–10435. doi: 10.1007/s13277-014-2347-5. [DOI] [PubMed] [Google Scholar]
- 90.Bernert H, Sekikawa K, Radcliffe RA, Iraqi F, You M, Malkinson AM. Tnfa and Il-10 deficiencies have contrasting effects on lung tumor susceptibility: gender-dependent modulation of IL-10 haploinsufficiency. Mol Carcinog. 2003;38:117–123. doi: 10.1002/mc.10151. [DOI] [PubMed] [Google Scholar]
- 91.Zeni E, Mazzetti L, Miotto D, Lo Cascio N, Maestrelli P, Querzoli P, Pedriali M, De Rosa E, Fabbri LM, Mapp CE, Boschetto P. Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J. 2007;30:627–632. doi: 10.1183/09031936.00129306. [DOI] [PubMed] [Google Scholar]
- 92.Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, Selvan SR. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res. 2011;51:170–182. doi: 10.1007/s12026-011-8262-6. [DOI] [PubMed] [Google Scholar]
- 93.Lee JH, Lee GT, Woo SH, Ha YS, Kwon SJ, Kim WJ, Kim IY. BMP-6 in renal cell carcinoma promotes tumor proliferation through IL-10-dependent M2 polarization of tumor-associated macrophages. Cancer Res. 2013;73:3604–3614. doi: 10.1158/0008-5472.CAN-12-4563. [DOI] [PubMed] [Google Scholar]
- 94.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Banerjee S, Halder K, Bose A, Bhattacharya P, Gupta G, Karmahapatra S, Das S, Chaudhuri S, Bhattacharyya Majumdar S, Majumdar S. TLR signaling-mediated differential histone modification at IL-10 and IL-12 promoter region leads to functional impairments in tumor-associated macrophages. Carcinogenesis. 2011;32:1789–1797. doi: 10.1093/carcin/bgr208. [DOI] [PubMed] [Google Scholar]
- 96.Szkaradkiewicz A, Karpinski TM, Drews M, Borejsza-Wysocki M, Majewski P, Andrzejewska E. Natural killer cell cytotoxicity and immunosuppressive cytokines (IL-10, TGFbeta1) in patients with gastric cancer. J Biomed Biotechnol. 2010;2010:901564. doi: 10.1155/2010/901564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grandclement C, Pallandre JR, Valmary Degano S, Viel E, Bouard A, Balland J, Remy-Martin JP, Simon B, Rouleau A, Boireau W, Klagsbrun M, Ferrand C, Borg C. Neuropilin-2 expression promotes TGF-beta1-mediated epithelial to mesenchymal transition in colorectal cancer cells. PLoS One. 2011;6:e20444. doi: 10.1371/journal.pone.0020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H, Liu L, Wang Y, Zhao G, Xie R, Liu C, Xiao X, Wu K, Nie Y, Fan D. KLF8 involves in TGF-beta-induced EMT and promotes invasion and migration in gastric cancer cells. J Cancer Res Clin Oncol. 2013;139:1033–1042. doi: 10.1007/s00432-012-1363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Su HT, Weng CC, Hsiao PJ, Chen LH, Kuo TL, Chen YW, Kuo KK, Cheng KH. Stem cell marker nestin is critical for TGF-beta1-mediated tumor progression in pancreatic cancer. Mol Cancer Res. 2013;11:768–779. doi: 10.1158/1541-7786.MCR-12-0511. [DOI] [PubMed] [Google Scholar]
- 100.Cho KH, Jeong KJ, Shin SC, Kang J, Park CG, Lee HY. STAT3 mediates TGF-beta1-induced TWIST1 expression and prostate cancer invasion. Cancer Lett. 2013;336:167–173. doi: 10.1016/j.canlet.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 101.Katsuno Y, Lamouille S, Derynck R. TGFbeta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 102.Romagnoli M, Belguise K, Yu Z, Wang X, Landesman-Bollag E, Seldin DC, Chalbos D, Barille-Nion S, Jezequel P, Seldin ML, Sonenshein GE. Epithelial-to-mesenchymal transition induced by TGF-beta1 is mediated by Blimp-1-dependent repression of BMP-5. Cancer Res. 2012;72:6268–6278. doi: 10.1158/0008-5472.CAN-12-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang O, Chen XM, Shen S, Hahn M, Pollock CA. MiRNA-200b represses transforming growth factor-beta1-induced EMT and fibronectin expression in kidney proximal tubular cells. Am J Physiol Renal Physiol. 2013;304:F1266–1273. doi: 10.1152/ajprenal.00302.2012. [DOI] [PubMed] [Google Scholar]
- 104.Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang J, Tian XJ, Zhang H, Teng Y, Li R, Bai F, Elankumaran S, Xing J. TGF-beta-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci Signal. 2014;7:ra91. doi: 10.1126/scisignal.2005304. [DOI] [PubMed] [Google Scholar]
- 106.Kawata M, Koinuma D, Ogami T, Umezawa K, Iwata C, Watabe T, Miyazono K. TGF-betainduced epithelial-mesenchymal transition of A549 lung adenocarcinoma cells is enhanced by pro-inflammatory cytokines derived from RAW 264.7 macrophage cells. J Biochem. 2012;151:205–216. doi: 10.1093/jb/mvr136. [DOI] [PubMed] [Google Scholar]
- 107.Tobar N, Villar V, Santibanez JF. ROSNFkappaB mediates TGF-beta1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol Cell Biochem. 2010;340:195–202. doi: 10.1007/s11010-010-0418-5. [DOI] [PubMed] [Google Scholar]
- 108.Boreddy SR, Srivastava SK. Deguelin suppresses pancreatic tumor growth and metastasis by inhibiting epithelial-to-mesenchymal transition in an orthotopic model. Oncogene. 2013;32:3980–3991. doi: 10.1038/onc.2012.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Techasen A, Loilome W, Namwat N, Dokduang H, Jongthawin J, Yongvanit P. Cytokines released from activated human macrophages induce epithelial mesenchymal transition markers of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2012;13(Suppl):115–118. [PubMed] [Google Scholar]
- 110.Shen Z, Kauttu T, Cao J, Seppanen H, Vainionpaa S, Ye Y, Wang S, Mustonen H, Puolakkainen P. Macrophage coculture enhanced invasion of gastric cancer cells via TGF-beta and BMP pathways. Scand J Gastroenterol. 2013;48:466–472. doi: 10.3109/00365521.2013.772226. [DOI] [PubMed] [Google Scholar]
- 111.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 112.Feuerborn A, Srivastava PK, Kuffer S, Grandy WA, Sijmonsma TP, Gretz N, Brors B, Grone HJ. The Forkhead factor FoxQ1 influences epithelial differentiation. J Cell Physiol. 2011;226:710–719. doi: 10.1002/jcp.22385. [DOI] [PubMed] [Google Scholar]
- 113.Kaneda H, Arao T, Tanaka K, Tamura D, Aomatsu K, Kudo K, Sakai K, De Velasco MA, Matsumoto K, Fujita Y, Yamada Y, Tsurutani J, Okamoto I, Nakagawa K, Nishio K. FOXQ1 is overexpressed in colorectal cancer and enhances tumorigenicity and tumor growth. Cancer Res. 2010;70:2053–2063. doi: 10.1158/0008-5472.CAN-09-2161. [DOI] [PubMed] [Google Scholar]
- 114.Qiao Y, Jiang X, Lee ST, Karuturi RK, Hooi SC, Yu Q. FOXQ1 regulates epithelial-mesenchymal transition in human cancers. Cancer Res. 2011;71:3076–3086. doi: 10.1158/0008-5472.CAN-10-2787. [DOI] [PubMed] [Google Scholar]
- 115.Feng J, Zhang X, Zhu H, Wang X, Ni S, Huang J. FoxQ1 overexpression influences poor prognosis in non-small cell lung cancer, associates with the phenomenon of EMT. PLoS One. 2012;7:e39937. doi: 10.1371/journal.pone.0039937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang H, Meng F, Liu G, Zhang B, Zhu J, Wu F, Ethier SP, Miller F, Wu G. Forkhead transcription factor foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res. 2011;71:1292–1301. doi: 10.1158/0008-5472.CAN-10-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sehrawat A, Kim SH, Vogt A, Singh SV. Suppression of FOXQ1 in benzyl isothiocyanate-mediated inhibition of epithelial-mesenchymal transition in human breast cancer cells. Carcinogenesis. 2013;34:864–873. doi: 10.1093/carcin/bgs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Z, Pang Z, Xing Y, Wan F, Lan D, Wang H. Short hairpin RNA targeting FOXQ1 inhibits invasion and metastasis via the reversal of epithelial-mesenchymal transition in bladder cancer. Int J Oncol. 2013;42:1271–1278. doi: 10.3892/ijo.2013.1807. [DOI] [PubMed] [Google Scholar]
- 119.Fan DM, Feng XS, Qi PW, Chen YW. Forkhead factor FOXQ1 promotes TGF-beta1 expression and induces epithelial-mesenchymal transition. Mol Cell Biochem. 2014;397:179–186. doi: 10.1007/s11010-014-2185-1. [DOI] [PubMed] [Google Scholar]
- 120.Feng J, Xu L, Ni S, Gu J, Zhu H, Wang H, Zhang S, Zhang W, Huang J. Involvement of FoxQ1 in NSCLC through regulating EMT and increasing chemosensitivity. Oncotarget. 2014;5:9689–9702. doi: 10.18632/oncotarget.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28:233–245. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 122.Said N, Sanchez-Carbayo M, Smith SC, Theodorescu D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J Clin Invest. 2012;122:1503–1518. doi: 10.1172/JCI61392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kischel P, Waltregny D, Dumont B, Turtoi A, Greffe Y, Kirsch S, De Pauw E, Castronovo V. Versican overexpression in human breast cancer lesions: known and new isoforms for stromal tumor targeting. Int J Cancer. 2010;126:640–650. doi: 10.1002/ijc.24812. [DOI] [PubMed] [Google Scholar]
- 124.Damasceno KA, Bertagnolli AC, Estrela-Lima A, Ribeiro LG, Rabelo BS, Campos CB, Barros AL, Cassali GD. Versican expression in canine carcinomas in benign mixed tumours: is there an association with clinical pathological factors, invasion and overall survival? BMC Vet Res. 2012;8:195. doi: 10.1186/1746-6148-8-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xia L, Huang W, Tian D, Zhang L, Qi X, Chen Z, Shang X, Nie Y, Wu K. Forkhead box Q1 promotes hepatocellular carcinoma metastasis by transactivating ZEB2 and Versican V1 expression. Hepatology. 2014;59:958–973. doi: 10.1002/hep.26735. [DOI] [PubMed] [Google Scholar]
- 126.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 128.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 130.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, Daniel D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology. 2013;2:e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 133.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, Nebuloni M, van Rooijen N, Mortarini R, Beltrame L, Marchini S, Fuso Nerini I, Sanfilippo R, Casali PG, Pilotti S, Galmarini CM, Anichini A, Mantovani A, D’Incalci M, Allavena P. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 134.Sarkar S, Doring A, Zemp FJ, Silva C, Lun X, Wang X, Kelly J, Hader W, Hamilton M, Mercier P, Dunn JF, Kinniburgh D, van Rooijen N, Robbins S, Forsyth P, Cairncross G, Weiss S, Yong VW. Therapeutic activation of macrophages and microglia to suppress brain tumorinitiating cells. Nat Neurosci. 2014;17:46–55. doi: 10.1038/nn.3597. [DOI] [PubMed] [Google Scholar]
- 135.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, de Visser KE, Italiano A, Le Tourneau C, Delord JP, Levitsky H, Blay JY, Ruttinger D. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]


