Abstract
Our previous work has demonstrated that interleukin-22 (IL-22) enhances the invasiveness of endometrial stromal cells (ESCs) of adenomyosis in an autocrine manner. In the present study, we further investigated whether IL-22 mediated crosstalk between vascular endothelial cells (VECs) and ESCs in vitro. Here we found that VECs in ectopic lesion from women with adenomyosis highly expressed IL-22 receptors IL-22R1 and IL-10R2. Both recombinant human IL-22 (rhIL-22) and IL-22 from ESCs increased IL-22R1 and IL-10R2 expression on human umbilical vein endothelial cells (HUVECs). Treatment with rhIL-22 led to an elevation of HUVECs viability, but did not influence HUVECs apoptosis. In contrast, anti-human IL-22 neutralizing antibody (α-IL-22) inhibited HUVECs viability induced by supernatants of ESCs. Stimulation with rhIL-22 or ESCs up-regulated CD105 expression on HUVECs and promoted angiogenesis, and α-IL-22 could reverse these effect induced by ESC. Compared to non-treated HUVECs, HUVECs educated by rh-IL-22 or ESCs could further up-regulate Ki-67 and proliferating cell nuclear antigen (PCNA) expression, and down-regulate Fas ligand (FasL) expression in ESCs. However, these effects induced by ESC-educated HUVECs were inhibited by α-IL-22. These results suggest that IL-22 derived from ESC promotes IL-22 receptors expression and enhances the viability, activation and angiogenesis of HUVEC. In turn, the educated HUVEC may further stimulate proliferation and restricts apoptosis of ESC. The integral effect may contribute to the progress of adenomyosis. Blocking IL-22 can disturb crosstalk between ESC and VEC mediated by IL-22, suggesting that blocking IL-22 may be a potential treatment strategy for adenomyosis.
Keywords: IL-22, adenomyosis, endometrial stromal cells, vascular endothelial cells, angiogenesis
Introduction
Adenomyosis is a common gynecological disease with a mysterious pathogenesis. Unlike endometriosis, adenomyosis is defined by an abnormal displacement of the eutopic endometrium deeply and haphazardly inside the myometrium [1]. However, the pathogenic mechanism responsible for adenomyosis is not well known as yet. Therefore, appropriate treatments for adenomyosis, especially individual control strategies are still difficult to achieve.
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, is essential for the delivery of nutrients and oxygen to cells that are distant from existing blood vessels [2]. Angiogenesis is an essential component in the physiological processes (wound healing and embryonic development etc) as well as pathological processes (diabetic retinopathy, invasive tumor growth and metastatic dissemination metastasis etc) [3,4]. Neovascularization has been considered to be a major pathological feature of adenomyosis [5,6]. Angiogenesis is thought to be required for the implantation of ectopic endometrial tissues and their subsequent proliferation [6,7].
Accumulated evidence supports that the role of cytokines production from ectopic endometrium in the pathophysiology of adenomyosis, such as IL-6, IL-8, CCL2 (also known as monocyte chemoattractant protein-1) and IL-22 [8-11]. IL-22, as a cytokine is described with opposing pro-inflammatory and anti-inflammatory functions. The functional IL-22 receptor complex consists of two submits, IL-22R1 and IL-10R2, which are ubiquitously expressed in various organs and cell types [12-14]. IL-22 activates a signal transduction cascade that results in the rapid activation of several transcription factors including Signal Transducers and Activators of Transcription (STAT) proteins via binding the receptor complex [14,15].
Our previous works had established that IL-22 secreted by ESCs promotes the growth and invasiveness in an autocrine manner [11,16]. In addition, we found that IL-22 stimulates the production of IL-6, IL-8 and VEGF from ESCs [11,16]. These cytokines play an important role in angiogenesis, and contribute to the development of adenomyosis [5]. However, whether IL-22 produced by ESCs regulates the biological behaviors of VECs and promotes the dialogue between ESCs and VECs remain unclear.
Therefore, the present study is undertaken to investigate whether VECs in ectopic lesion from women with adenomyosis express IL-22 receptors, and further analyze the role of ESCs-derived IL-22 in viability, apoptosis and angiogenesis of HUVECs, and the effect of IL-22-educated HUVECs on ESCs in vitro.
Materials and methods
Tissue collection
All tissue samples were collected with informed consent in accordance with the requirements of the Research Ethics Committee in Hospital of Obstetrics and Gynecology, Fudan University. The eutopic endometrium tissues (n=20, for isolation and culture of ESCs) and ectopic lesions from women (n=10, for immunohistochemistry) with adenomyosis were obtained undergoing hysterectomy. All the samples were confirmed histologically according to established criteria [17].
Immunohistochemistry (IHC)
Immunohistological staining was performed as previously described [11,18]. The IL-22, IL-22R1 and IL-10R2 protein levels in the ectopic lesions (n=10) from women with adenomyosis were dehydrated in graded ethanol and incubated with hydrogen peroxide in 1% bovine serum albumin in Tris-buffered saline (TBS) to block endogenous peroxidase. The samples were then incubated with mouse anti-human IL-22R1 antibody (25 ug/ml) (R&D Systems, USA), mouse anti-human IL-10R2 antibody (25 ug/ml) (R&D Systems) or mouse IgG isotype antibody overnight at 4°C in a humid chamber. After washing three times with TBS, the sections were overlaid with peroxidase-conjugated anti-mouse IgG antibody (Golden Bridge International, Inc., Beijing, China), and the reaction was developed with 3,3-diaminobenzidine (DAB), and counterstained with hematoxylin. The experiments were repeated three times.
Cell isolation and culture
The ESCs were isolated according to the previous methods [19]. The eutopic endometrial tissues (n=20) from women with adenomyosis were collected under sterile conditions and transported to the laboratory on ice in DMEM (Dulbecco’s modified Eagle’s medium)/F-12 (Gibco, USA) with 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA). Flow cytometry showed ≥95% vimentin-positive ESCs.
HUVECs were maintained as monolayers in Kaighn Modification of DMEM/F-12 medium supplemented with 10% FCS and 10 ng/ml human EGF (R&D Systems). These cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Treatment with rhIL-22 protein and α-IL-22
HUVECs were treated with recombinant human IL-22 (rhIL-22) (1, 10, 100 ng/ml) (R&D Systems) or the supernatants from ESCs treated with anti-human IL-22 neutralizing antibody (α-IL-22) (0.05, 0.5 or 5 ug/ml) for 48 h, with vehicle as control. Then the cell-counting kit-8 (CCK-8) assay, apoptosis assay and flow cytometry (FCM) assay (The concentration of rhIL-22 was 100 ng/ml for the latter two assays) were performed to detect the effect of rhIL-22 on the proliferation, apoptosis, and the expression IL-22R1 and IL-10R2 in HUVECs, respectively.
Co-culture of HUVECs with ESCs
HUVECs (2×105 cells/well) were plated in the lower chamber and then ESCs were plated in the upper chamber using transwell system (0.4 mm pore size, 6.5 mm diameter, Corning, USA), and formed a untouched co-culture unit. In co-culture system, these cells were incubated with or without α-IL-22 (5 ug/ml). After 48 h, HUVECs in the lower chamber were collected, and the expression of CD105, CD62E, VE-cadherin, CXCR1 and CXCR2 was analyzed by flow cytometry.
The cell-counting kit-8 (CCK-8) assay and apoptosis assay
HUVECs were seeded in 96-well plates (5×103 cells/well) and treated as described above. Then these cells were collected and detected the viability and apoptosis by the CCK-8 (Dojindo, Japan). According to the manufacturer’s protocol, the CCK8 reagent was added to each well and cells were incubated at 37°C for 1-4 h. The absorbance (optical density) at 450 nm was measured and used to represent the viability of cells. Each experiment was performed in six parallel wells, and repeated three times.
For apoptosis assay, HUVECs were seeded in 24-well plates (2×105 cells/well) and treated as described above. Then the apoptosis level HUVECs was analyzed by annexin V-FITC apoptosis assay (Invitrogen, USA) according to the manufacturer’s protocol.
FCM
HUVECs were digested with 0.25% trypsin only for 30-50 s, and blown off gently and washed with phosphate-buffered saline (PBS). After blocking with 10% FBS, the recovered cells were mixed with anti-human IL-10R2 (R&D Systems), CXCR1 (R&D Systems), CXCR2 (R&D Systems), CD105 (Biolegend, San Diego, CA, USA), CD62E (Biolegend) or VE-cadherin (Biolegend) antibody in darkness for 30 min at room temperature. As a negative control, an isotope control was used. After incubation, the cells were washed and analyzed immediately by FCM analysis was performed on a Beckman-Coulter CyAN ADP Analyzer (Beckman Coulter, Inc. Kraemer Boulevard Brea, CA, USA). Data were analyzed with FlowJo Version 6.1 software (TreeStar, Ashland, OR, USA). The statistical analysis was conducted using isotype-matched controls as references. The experiments were repeated three times.
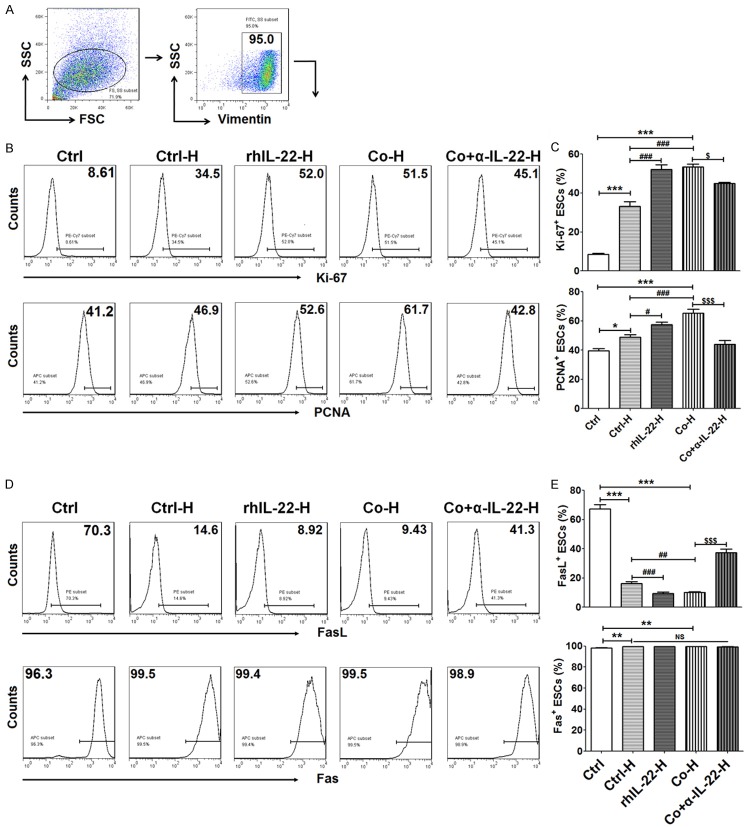
After stimulation with or without rhIL-22, or co-culture with ESCs (plus α-IL-22 or not) for 48 h, HUVECs were collected and further co-cultured with fresh ESCs for another 48 h. Subsequently, the expression of Ki-67, PCNA, Fas and FasL (all antibodies were from Biolegend) in ESCs was analyzed by FCM.
Tube formation assay (Matrigel assay)
The tube formation was performed as previously described [20]. HUVECs were seeded on growth factor-reduced Matrigel in normal growth medium with VEGF (10 ng/ml, as a positive control), rhIL-22, and the supernatants from ESCs (these ESCs were treated with or without α-IL-22 for 48 h). Capillary-like tube formation was quantified by counting numbers of junctions/enclosed circles in 5 randomly chosen optical fields with light microscopy after 16 hours.
Statistics
All values are shown as the mean ± SEM. The data were analyzed with GraphPad Prism version 5 by t-test or one-way ANOVA. Differences were considered statistically significant at P<0.05.
Results
VECs in ectopic lesion from women with adenomyosis highly express IL-22R1 and IL-10R2
Our previous work showed that ESCs form women with adenomyosis secreted IL-22. To further the regulation of IL-22 produced by ESCs on VECs, IHC was performed to analyze the expression of IL-22 receptors (IL-22R1 and IL-10R2) in VECs of ectopic lesion. The results in Figure 1 showed that VECs from women with adenomyosis (n=10) were strong positive stain of IL-22R1 and IL-10R2, which echoed our previous work [11]. These data suggested that IL-22 derived from ESCs may regulate the biological behaviors of VECs in ectopic lesion of adenomyosis in a paracrine manner.
Figure 1.
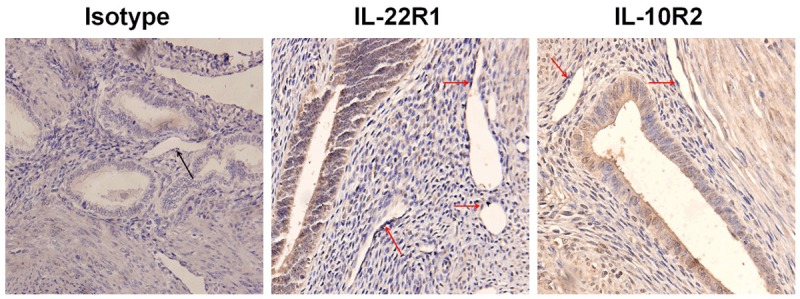
VECs in ectopic lesion from women with adenomyosis highly express IL-22R1 and IL-10R2. Immunohistochemistry analysis for IL-22R1 and IL-10R2 expression in ectopic lesion from women with adenomyosis (n=10). Original magnification: ×400. Red arrows: VEC (vascular endothelial cells).
IL-22 secreted by ESCs up-regulates IL-22R1 and IL-10R2 expression on VECs
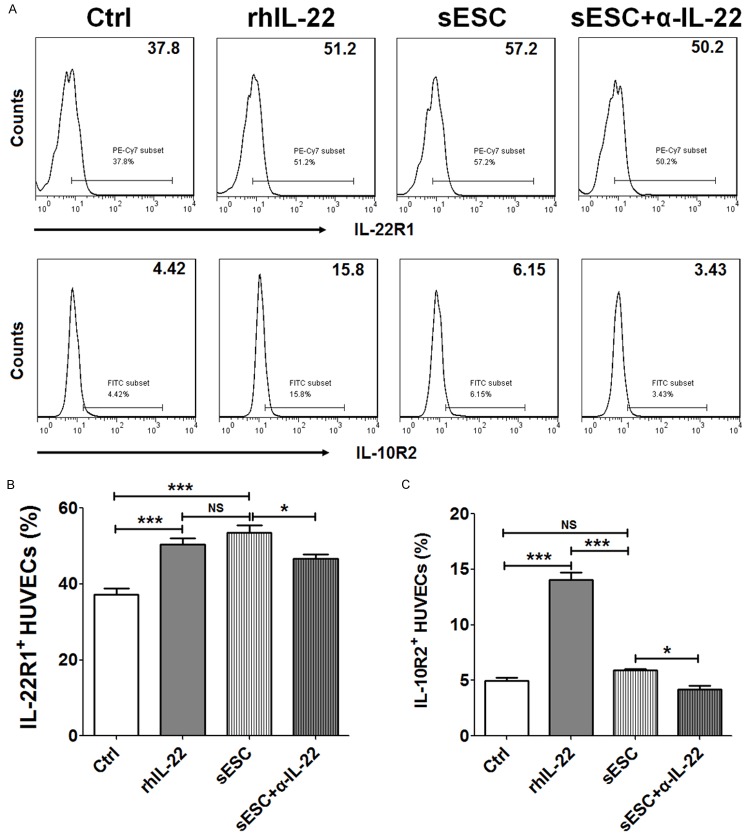
It has previously been reported rhIL-22 protein can directly increase the expression of IL-22R1 and IL-10R2 on ESCs [11]. For adenomyosis, the endometrial implants that grow into the wall of the uterus bleed during menstruation (the same as endometrial tissue bleeds), so there has a close contact between ESC and VEC in the local ectopic foci. In order to evaluate the role of IL-22 from ESC on regulation of its receptors on VEC, we treated HUVECs with rhIL-22, or co-cultured with ESCs plus α-IL-22 or not. As shown in Figure 2, rhIL-22 significantly up-regulated IL-22R1 and IL-10R2 expression on HUVECs (P<0.001) (Figure 2A-C). Further analysis showed that co-culture with ESCs led to an increase of IL-22R1 not IL-10R2 level in HUVECs (P<0.001 or P>0.05) (Figure 2A-C). However, compared to co-culture group, combination of co-culture and α-IL-22 resulted in the decrease of both IL-22R1 and IL-10R2 (P<0.05) (Figure 2A-C), suggesting that ESCs-secreted IL-22 could up-regulate IL-22R1 and IL-10R2 on VECs.
Figure 2.
IL-22 secreted by ESCs up-regulates IL-22R1 and IL-10R2 expression on VECs. We treated HUVECs with rhIL-22 (100 ng/ml), or the supernatants from ESCs (n=6) pre-treated with or without α-IL-22 (5 ug/ml). After 48 h, the expression of IL-22R1 (A, B) and IL-10R2 (A, C) on HUVECs was analyzed by FCM. rhIL-22: recombinant human IL-22 protein; α-IL-22: anti-human IL-22 neutralizing antibody; sESCs: the supernatants from ESCs; sESCs+α-IL-22: the supernatants from ESCs, which were pre-treated with α-IL-22. The data are expressed as the mean ± SEM. *P<0.05 and ***P<0.001 (one-way ANOVA). NS: no statistically difference.
ESC-derived IL-22 stimulates the viability of VECs
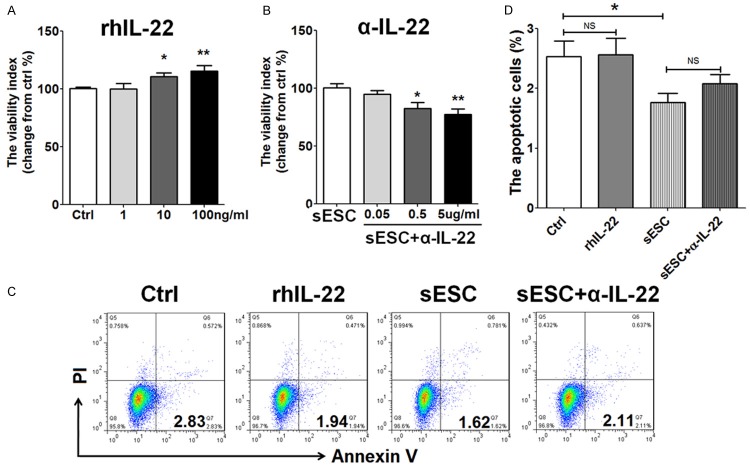
To investigate the potential effect of IL-22 on VEC in vitro, HUVECs were incubated with rhIL-22 (1, 10 or 100 ng/ml) or the supernatant from ESCs plus α-IL-22 (0.05, 0.5 or 5 ug/ml) for 48 h. The results showed that treatment with rhIL-22 led to a significant increase of HUVECs viability in a dosage-dependent manner (P<0.05 or P<0.01) (Figure 3A). In contrast, blocking IL-22 with α-IL-22 inhibited HUVECs viability when incubation with the supernatants from ESCs (P<0.05 or P<0.01) (Figure 3A). The optimum concentration of rhIL-22 and α-IL-22 was 100 ng/ml and 5 ug/ml, respectively. However, rhIL-22 had no effect on HUVECs apoptosis (P>0.05) (Figure 3C and 3D). Although there was a decrease of HUVECs apoptosis after co-culture with ESCs (P<0.05) (Figure 3C and 3D), blocking IL-22 did not influence this effect induced by ESCs (P>0.05) (Figure 3C and 3D). Collectively, these results indicated that ESCs-derived IL-22 could promote the viability not apoptosis of HUVECs. ESCs inhibited HUVECs apoptosis, but this effect was not dependent on IL-22.
Figure 3.
ESC-derived IL-22 stimulates the viability of VECs. HUVECs were treated with rhIL-22 (1, 10 or 100 ng/ml for CCK8 assay; 100 ng/ml for apoptosis assay), or the supernatants from ESCs (n=6) pre-treated with or without α-IL-22 (0.05, 0.5 or 5 ug/ml for CCK8 assay; 5 ug/ml for apoptosis assay). After 48 h, the viability (A, B) and apoptosis (C, D) of HUVECs were detected by CCK8 assay and apoptosis assay. The data are expressed as the mean ± SEM. *P<0.05 and **P<0.01 (one-way ANOVA). NS: no statistically difference.
The up-regulation of CD105 and VE-cadherin on HUVECs is dependent and independent on IL-22
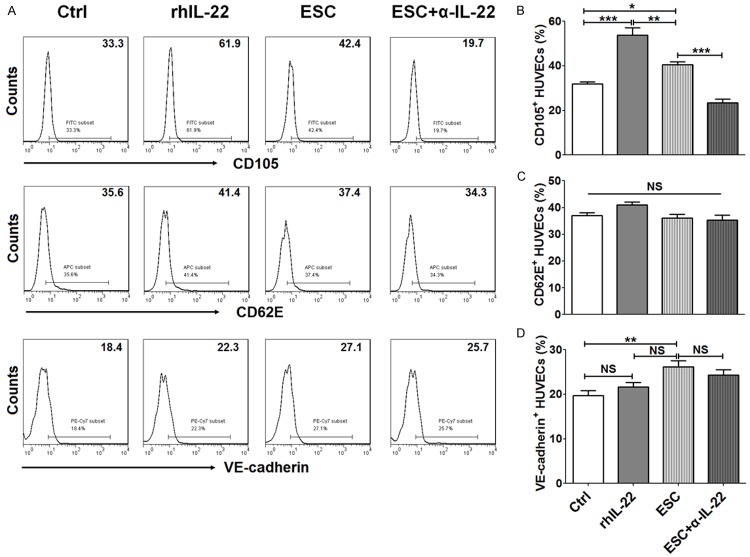
We next detected the effect of IL-22 on CD105, CD62E and VE-cadherin expression on HUVECs, and found that both rhIL-22 and co-culture with ESCs could enhance CD105 expression on HUVECs (P<0.05 or P<0.001) (Figure 4A and 4B). Similarly, the up-regulation of CD105 on HUVECs could be reversed by α-IL-22. It implied that ESCs increased CD105 expression on HUVECs by secreting IL-22. FCM results showed that CD62E expression on HUVECs did not change after IL-22 stimulation or co-culturing with ESCs (P>0.05) (Figure 4A and 4C). Of note, culture with ESC promoted VE-cadherin expression (P<0.01) (Figure 4A and 4D). But neither rhIL-22 nor blocking IL-22 modulated VE-cadherin expression on HUVECs (P>0.05) (Figure 4A and 4D), suggesting that both endogenous and exogenous IL-22 were not involved in VE-cadherin regulation of HUVECs.
Figure 4.
The up-regulation of CD105 and VE-cadherin on HUVECs is dependent and independent on IL-22. After treatment with rhIL-22 (100 ng/ml), or indirectly co-culture with ESCs (In co-culture unit, 5 ug/ml α-IL-22 was added or not) (n=6) for 48 h, HUVECs were collected and used to evaluate CD105 (A, B), CD62E (A, C) and VE-cadherin (A, D) expression by FCM. ESCs: co-culture with ESCs; ESCs+α-IL-22: co-culture with ESCs plus α-IL-22. The data are expressed as the mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001 (one-way ANOVA). NS: no statistically difference.
IL-22 secreted by ESCs promotes angiogenesis of VECs
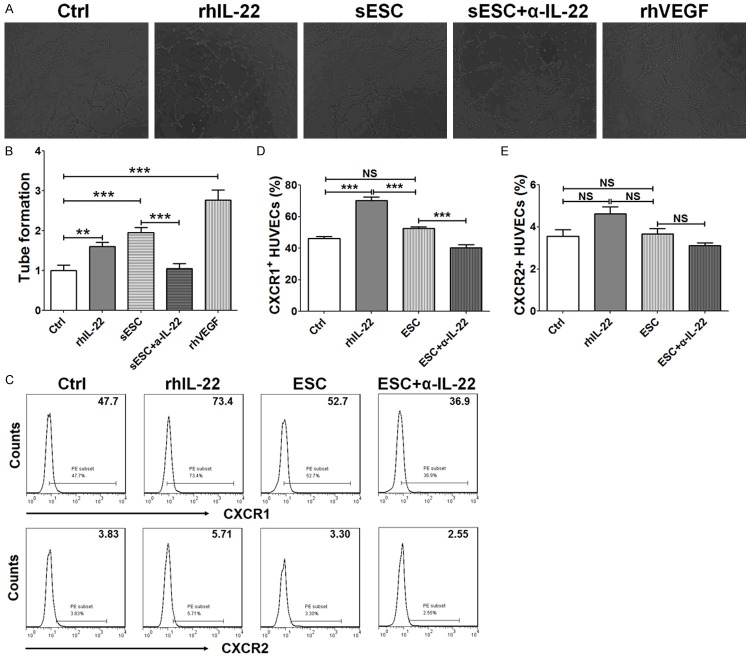
Taking into account the important role of CD105 and VE-cadherin in the regulation of proliferation, activation and angiogenesis in VEC [21,22], we speculated that ESCs and IL-22 might promote angiogenesis. So we used tube formation assay to evaluate the role of rhIL-22, the supernatants from ESCs or the supernatants from ESC treated with α-IL-22 on angiogenesis regulation of HUVECs. As shown in Figure 5, rhVEGF protein obviously stimulated angiogenesis of HUVECs as a positive control (P<0.001) (Figures 5A and 4B). RhIL-22 stimulation or the supernatants from ESCs promotes angiogenesis of HUVECs (P<0.01 or P<0.001) (Figure 5A and 5B). In addition, the supernatants from ESCs treated with α-IL-22 led to a decrease of tube formation of HUVECs compared to the supernatants from ESCs (P<0.001) (Figure 5A and 5B). These findings suggested that ESCs promoted angiogenesis of HUVECs by producing IL-22 in vitro, this effect may be associated with the regulation of CD105 mediated by IL-22.
Figure 5.
IL-22 secreted by ESCs promotes angiogenesis of VECs. (A, B) HUVECs were treated with rhIL-22 (100 ng/ml) or the supernatant from ESCs (n=6) pre-treated with or without α-IL-22 (5 ug/ml), with recombinant human VEGF (rhVEGF, 10 ng/ml) as positive control. Then tube formation assay was performed to analyze the angiogenesis of HUVECs. In addition, HUVECs were treated as described in Figure 4, and then the expression of CXCR1 and CXCR2 was detected by FCM (C-E). The data are expressed as the mean ± SEM. **P<0.01 and ***P<0.001 (one-way ANOVA). NS: no statistically difference.
It had been reported that IL-22 could stimulated the secretion of IL-6, IL-8 and VEGF, especially IL-8 [11]. Thus, we then analyzed the role of IL-22 in IL-8 receptors regulation on HUVECs. As shown, both endogenous and exogenous IL-22 up-regulated CXCR1 (P<0.001) (Figure 5C and 5D) not CXCR2 (P>0.05) (Figure 5C and 5E) expression on HUVECs. Taken together, these data suggested that the stimulatory effect on angiogenesis of VECs induced by IL-22 from ESCs might be associated with CD105, IL-6, VEGF, and interaction of IL-8/CXCR1.
VECs educated by IL-22 from ESCs enhanced ESC proliferation
In order to know the role of cross-talk between ESCs and VECs in ESCs biological behaviors, we collected HUVECs after treatment with rhIL-22 protein, co-culture with ESCs or plus α-IL-22 or not, then further co-cultured with fresh vimentin+ ESCs (Figure 6A). As shown, compared to ESC alone, co-culture with HUVECs significantly increased proliferation-related molecules Ki-67 and PCNA expression in ESCs (P<0.01, P<0.001) (Figure 6B and 6C). RhIL-22-treated or ESC-co-cultured HUVECs could further elevated Ki-67 and PCNA expression in ESCs (P<0.01 or P<0.001) (Figure 6B and 6C), and this effect induced by ESC-co-cultured HUVECs could be inhibited by blocking IL-22 (P<0.01 or P<0.001) (Figure 6B and 6C). Therefore, HUVECs educated by IL-22 from ESCs enhanced the proliferation of ESCs.
Figure 6.
VECs educated by IL-22 from ESCs enhanced ESC proliferation. After stimulation with rhIL-22 or co-culture with ESCs plus α-IL-22 or not for 48 h, HUVECs were collected and further co-cultured with fresh ESCs (n=6) for another 48 h. Then, the expression of Ki-67, PCNA (B, C), FasL and Fas (D, E) in these vimentin+ ESCs (A) was analyzed by FCM. Ctrl H: co-culture with control HUVECs; rhIL-22-H: co-culture with HUVECs, which were pre-treated with rhIL-22; Co-H: co-culture with HUVECs, which were pre-cocultured with ESCs; Co+α-IL-22-H: co-culture with HUVECs, which were pre-cocultured with ESCs plus α-IL-22. The data are expressed as the mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001 compared to ctrl group; #P<0.05, ##P<0.01 and ###P<0.001 compared to Ctrl H group; $P<0.05 and $$$P<0.001 compared to Co-H group (one-way ANOVA). NS: no statistically difference.
Subsequently, we found that co-culture with HUVECs also led to a decrease of FasL expression on ESCs (P<0.001) (Figure 6D and 6E), and rhIL-22 or co-culture with ESC could amply this effect (P<0.01 or P<0.001) (Figure 6D and 6E). On the contrary, Blocking IL-22 completely reversed the effect induced by HUVECs co-culture with ESCs (P<0.001) (Figure 6D and 6E). Of note, both co-culture with HUVECs and ESC-co-cultured HUVECs gave rise to the up-regulation of Fas, but IL-22 had no similar effect (P>0.05) (Figure 6D and 6E). Therefore, these findings provided evidence that IL-22 could strengthen dialogue between ESCs and HUVECs, and further amply the stimulatory effect on ESCs growth by HUVECs.
Discussion
The major characteristics of adenomyotic cells include reactivity to steroid hormones, increased angiogenesis and increased migration and invasion [23,24]. The vascularity density of the myometrium by Doppler sonography in women with adenomyosis was significantly higher than that of control group from women without adenomyosis, especially in the ectopic lesions and surrounding sites (data not shown). This observation indicated that there should be a close relationship between VECs and ESCs in ectopic lesions. However, the molecular of crosstalk between VECs and ESCs, and its roles in regulation of angiogenesis, biological behavior of ESCs and progress of adenomyosis were not understood.
IL-22 was first identified as an IL-10-related T cell-derived inducible factor (IL-TIF) from a lymphoma cell line and mainly secreted by active T helper cells and NK cells [25]. We had reported that ESCs from women with adenomyosis expressed IL-22. Here, the results of IHC showed that VECs in ectopic lesion of adenomyosis highly expressed the receptors of IL-22, IL-22R1 and IL-10R2, which partly echoed previous reports [26]. Further analysis found that rhIL-22 and IL-22 secreted by ESCs would up-regulate IL-22R1 and IL-10R2 expression. Other unknown molecules might be also involved in the regulation of IL-22R1 mediated by ESCs. Thus, it can be concluded that high level of IL-22 from ESCs leads to an increase of IL-22 receptors of VECs in a positive feedback dependent manner. This effect will further strengthen the interaction between VECs and ESCs.
IL-22 has an important role in host defense at mucosal surfaces as well as in tissue repair [27]. As we know, adenomyosis is also caused by trauma, known as tissue injury and repair [28]. The response to any implants is wound healing comprised of inflammation and tissue remodeling. The wound healing process involves extensive tissue remodeling through production of extracellular matrix (ECM) components, remodeling enzymes, cellular adhesion molecules, growth factors and cytokines genes. In this process, angiogenesis is a critical event in tissue remodeling. Therefore, we next investigated the role of IL-22 on the biological function of VECs, and found that both rhIL-22 protein and IL-22 from ESCs promoted the viability but not regulated the apoptosis in HUVECs. Of note, the supernatants from ESCs restricted the apoptosis in HUVECs, the exact molecular mechanism need to further research.
Endoglin is a type I membrane glycoprotein located on cell surfaces and is part of the transforming growth factor-β1 (TGF-β1) receptor complex [29]. Up-regulation of CD105 expression is found in tumor vasculature and proliferating cells, suggesting that CD105 is a biomarker for endothelial proliferation or angiogenesis [30-33]. In current study, ESCs up-regulated CD105 expression on HUVECs in co-culture unit by secreting IL-22, suggesting IL-22 derived from ESC may be involved in the proliferation or angiogenesis of HUVECs. Angiogenesis-relative molecule CD62E is also known as E-selectin. It is found to mediate the adhesion of tumor cells to endothelial cells, by binding to CD62E ligands, and play a role in cancer metastasis [34]. However, we observed that both ESCs and IL-22 did not influence CD62E expression. VE-cadherin, also known as CD144, is a calcium-dependent transmembrane cell-cell adhesion molecule localized at the intercellular boundaries of endothelial cells and hematopoietic stem cells. It mediates calcium-dependent homophilic cell adhesion, plays fundamental roles in microvascular permeability and in the morphogenic and proliferative events associated with angiogenesis [35]. Interestingly, indirectly co-culture with ESCs led to the increase of VE-cadherin on HUVECs, but this effect was not dependent on IL-22.
Our previous work had showed that IL-22 stimulated the production of IL-6, IL-8 and VEGF [11]. In the current study, the results by tube formation assay showed that rhVEGF protein was a positive control. It significantly stimulated angiogenesis. Either rhIL-22 or the supernatants from ESCs promoted angiogenesis of HUVECs, and blocking IL-22 could abolish this effect, suggesting that ESCs promoted angiogenesis of HUVECs through producing IL-22. In addition, we found that IL-22 elevated CXCR1 not CXCR2 expression on HUVECs. These data above suggest that IL-22 from ESCs promotes angiogenesis possibly by secreting IL-6 and VEGF of ESCs, up-regulation CD105 expression of VECs and strengthening interaction of IL-8/CXCR1 on VECs.
In order to analyze the increase of angiogenesis on the biological behaviors on ESCs, we collected HUVECs educated by IL-22 or ESCs, and further used these cells to stimulate fresh ESCs, and found that co-culture with HUVECs resulted in the up-regulation of Ki-67 and PCNA, and the down-regulation of FasL in ESCs. Moreover, rhIL-22 stimulation or pre-culture with ESCs could enlarge this effect mediated by HUVECs. But blocking IL-22 could significantly inhibit this effect. Taken together, it can be concluded that IL-22-educated HUVECs enhanced the proliferation and probably repressed apoptosis in ESCs by regulating the proliferation and apoptosis-related molecules. Of note, HUVECs also increased Fas expression on ESCs, suggesting that there also had a restricting effect on ESCs growth along with the stimulatory effect. This complex interaction mechanism of ESCs and VECs still need to research.
Collectively, as shown in Figure 7, accompanied by implantation of endometrium into myometrium, IL-22 was produced by ESC. IL-22, on the one hand, strengthens the dialogue between ESC and VEC by up-regulating IL-22 receptors expression on VEC; on the other hand, promotes the activation and angiogenesis of VEC possibly by up-regulation CD105 and CXCR1 expression on VECs, and simulating IL-6, IL-8 and VEGF secretion. In addition, the unknown molecules of ESCs may also be involved in the regulation angiogenesis by increasing VE-cadherin expression on VEC. In turns, VEC enhances proliferation and probably inhibited apoptosis of ESC by regulating Ki-67, PCNA and FasL expression. These integral effects will further promote angiogenesis and ESC growth, and accelerate the progress of adenomyosis by this vicious circle (Figure 7).
Figure 7.
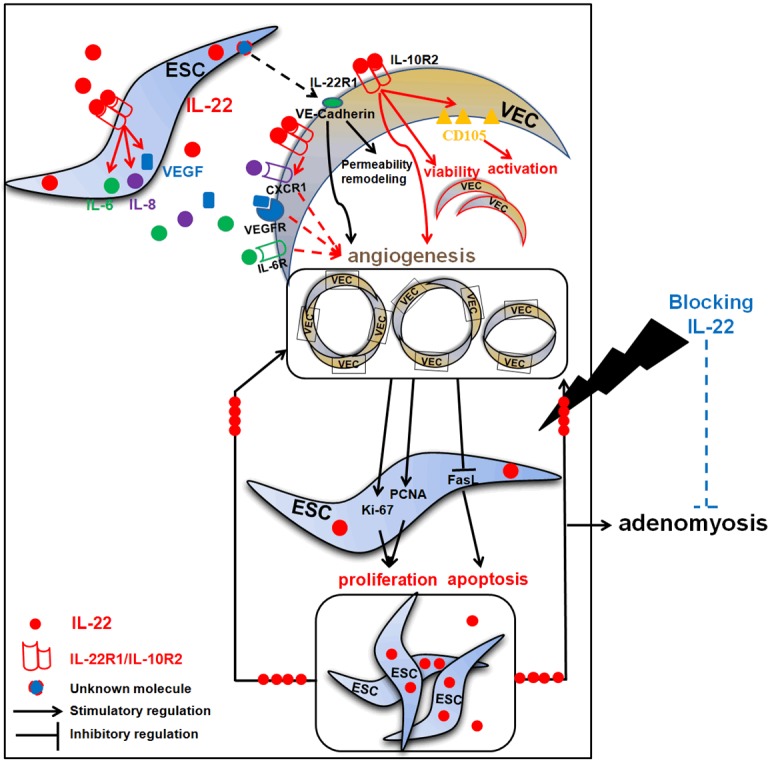
The role of IL-22 in the crosstalk between ESC and VEC, and the progression of adenomyosis. Accompanied by implantation of endometrium into myometrium, IL-22 was produced by ESC. IL-22 not only strengthens the dialogue between ESC and VEC by up-regulating IL-22 receptors expression on VEC, but also promotes the activation and angiogenesis of VEC possibly by up-regulation CD105 and CXCR1 expression on VECs, and simulating IL-6, IL-8 and VEGF secretion. In addition, the unknown molecules of ESCs may also be involved in the regulation angiogenesis by increasing VE-cadherin expression on VEC. In turns, VEC enhances proliferation and probably inhibits apoptosis of ESC by regulating Ki-67, PCNA and FasL expression. These integral effects will further promote angiogenesis and ESC growth, and accelerate the progress of adenomyosis by this vicious circle. Blocking IL-22 will suppress angiogenesis and ESC growth by inhibiting dialogue between ESC and VEC. These findings indicate blocking IL-22 may be a potential treatment strategy for adenomyosis.
Hysterectomy is the major strategy for adenomyosis. But fertility and uterus preservation is compromised. Moreover, the effect of removing adenomyotic lesions is temporary, and most women quickly develop adenomyosis. As traditional pharmacological therapies for adenomyosis, the GnRH agonists and low-dose oral contraceptives are applied primarily for the suppression of endogenous estrogen production. But the results are also unsatisfying [36]. Therefore, there is an urgent need to look for novel treatment strategies for adenomyosis. Our data bring new insight into the role of blocking IL-22 in the angiogenesis and ESC growth by strengthening dialogue between ESC and VEC. These findings indicate blocking IL-22 may be a potential treatment strategy for adenomyosis. Further research is warranted to verify it relying on an animal model of adenomyosis in vivo.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) 81471513, the Training Program for Young Talents of Shanghai Health System XYQ2013104, and the Program for Zhuoxue of Fudan University (all to M-Q Li), and the Research Program of Shanghai Health Bureau (20134185) to Qing Wang.
Disclosure of conflict of interest
None.
References
- 1.Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312–322. doi: 10.1093/humupd/4.4.312. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 3.Breier G. Angiogenesis in embryonic development - A review. Placenta. 2000;21:S11–15. doi: 10.1053/plac.1999.0525. [DOI] [PubMed] [Google Scholar]
- 4.Iruela-Arispe ML, Dvorak HF. Angiogenesis: A dynamic balance of stimulators and inhibitors. Thromb Haemost. 1997;78:672–677. [PubMed] [Google Scholar]
- 5.Goteri G, Lucarini G, Montik N, Zizzi A, Stramazzotti D, Fabris G, Tranquilli AL, Ciavattini A. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1alpha (HIF-1alpha), and microvessel density in endometrial tissue in women with adenomyosis. Int J Gynecol Pathol. 2009;28:157–163. doi: 10.1097/PGP.0b013e318182c2be. [DOI] [PubMed] [Google Scholar]
- 6.Kang S, Zhao J, Liu Q, Zhou R, Wang N, Li Y. Vascular endothelial growth factor gene polymorphisms are associated with the risk of developing adenomyosis. Environ Mol Mutagen. 2009;50:361–366. doi: 10.1002/em.20455. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht ED, Babischkin JS, Lidor Y, Anderson LD, Udoff LC, Pepe GJ. Effect of estrogen on angiogenesis in co-cultures of human endometrial cells and microvascular endothelial cells. Hum Reprod. 2003;18:2039–2047. doi: 10.1093/humrep/deg415. [DOI] [PubMed] [Google Scholar]
- 8.Yang JH, Chen MJ, Wu MY, Chen YC, Yang YS, Ho HN. Decreased suppression of interleukin-6 after treatment with medroxyprogesterone acetate and danazol in endometrial stromal cells of women with adenomyosis. Fertil Steril. 2006;86:1459–1465. doi: 10.1016/j.fertnstert.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 9.Yang JH, Wu MY, Chang DY, Chang CH, Yang YS, Ho HN. Increased interleukin-6 messenger RNA expression in macrophage-cocultured endometrial stromal cells in adenomyosis. Am J Reprod Immunol. 2006;55:181–187. doi: 10.1111/j.1600-0897.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Ulukus EC, Ulukus M, Seval Y, Zheng W, Arici A. Expression of interleukin-8 and monocyte chemotactic protein-1 in adenomyosis. Hum Reprod. 2005;20:2958–2963. doi: 10.1093/humrep/dei154. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Wang L, Shao J, Wang Y, Jin LP, Li DJ, Li MQ. L-22 enhances the invasiveness of endometrial stromal cells of adenomyosis in an autocrine manner. Int J Clin Exp Pathol. 2014;7:5762–5771. [PMC free article] [PubMed] [Google Scholar]
- 12.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10R beta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cellderived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 14.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Chen Y, Liu LB, Chang KK, Li H, Li MQ, Shao J. IL-22 in the endometriotic milieu promotes the proliferation of endometrial stromal cells via stimulating the secretion of CCL2 and IL-8. Int J Clin Exp Pathol. 2013;6:2011–2020. [PMC free article] [PubMed] [Google Scholar]
- 17.Uduwela AS, Perera MA, Aiqing L, Fraser IS. Endometrial-myometrial interface: relationship to adenomyosis and changes in pregnancy. Obstet Gynecol Surv. 2000;55:390–400. doi: 10.1097/00006254-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Li MQ, Shao J, Meng YH, Mei J, Wang Y, Li H, Zhang L, Chang KK, Wang XQ, Zhu XY, Li DJ. NME1 suppression promotes growth, adhesion and implantation of endometrial stromal cells via Akt and MAPK/Erk1/2 signal pathways in the endometriotic milieu. Hum Reprod. 2013;28:2822–2831. doi: 10.1093/humrep/det248. [DOI] [PubMed] [Google Scholar]
- 19.Li MQ, Wang Y, Chang KK, Meng YH, Liu LB, Mei J, Wang Y, Wang XQ, Jin LP, Li DJ. CD4+Foxp3+ regulatory T cell differentiation mediated by endometrial stromal cell-derived TECK promotes the growth and invasion of endometriotic lesions. Cell Death Dis. 2014;5:e1436. doi: 10.1038/cddis.2014.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie F, Meng YH, Liu LB, Chang KK, Li H, Li MQ, Li DJ. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am J Reprod Immunol. 2013;70:69–79. doi: 10.1111/aji.12104. [DOI] [PubMed] [Google Scholar]
- 21.Wang JM, Kumar S, Pye D, van Agthoven AJ, Krupinski J, Hunter RD. A monoclonal antibody detects heterogeneity in vascular endothelium of tumours and normal tissues. Int J Cancer. 1993;54:363–370. doi: 10.1002/ijc.2910540303. [DOI] [PubMed] [Google Scholar]
- 22.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 23.Benagiano G, Brosens I. The endometrium in adenomyosis. Womens Health (Lond Engl) 2012;8:301–312. doi: 10.2217/whe.12.8. [DOI] [PubMed] [Google Scholar]
- 24.Chen YJ, Li HY, Huang CH, Twu NF, Yen MS, Wang PH, Chou TY, Liu YN, Chao KC, Yang MH. Oestrogeninduced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol. 2010;222:261–270. doi: 10.1002/path.2761. [DOI] [PubMed] [Google Scholar]
- 25.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 26.Qin X, Zhang H, Wang F, Xue J, Wen Z. Expression and possible role of interleukin-10 receptors in patients with adenomyosis. Eur J Obstet Gynecol Reprod Biol. 2012;161:194–198. doi: 10.1016/j.ejogrb.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 28.Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet. 2009;280:529–538. doi: 10.1007/s00404-009-1191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interact with the signaling receptor complex of multiple members of the transforming growth factor-h superfamily. J Biol Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 30.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K, Lloyd RV. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–2290. [PubMed] [Google Scholar]
- 31.Wang JM, Kumar S, Pye D, van Agthoven AJ, Krupinski J, Hunter RD. A monoclonal antibody detects heterogeneity in vascular endothelium of tumours and normal tissues. Int J Cancer. 1993;54:363–370. doi: 10.1002/ijc.2910540303. [DOI] [PubMed] [Google Scholar]
- 32.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 33.Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984–992. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- 34.Tabatabai G, Herrmann C, von Kürthy G, Mittelbronn M, Grau S, Frank B, Möhle R, Weller M, Wick W. VEGF-dependent induction of CD62E on endothelial cells mediates glioma tropism of adult haematopoietic progenitor cells. Brain. 2008;131:2579–2595. doi: 10.1093/brain/awn182. [DOI] [PubMed] [Google Scholar]
- 35.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelialsurvival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 36.Wood C. Surgical and medical treatment of adenomyosis. Hum Reprod Update. 1998;4:323–336. doi: 10.1093/humupd/4.4.323. [DOI] [PubMed] [Google Scholar]