Abstract
Ligustrazine, a compound extracted from roots of Ligusticum chuanxiong, is widely used in Chinese traditional medicine to treat cardiac and cerebrovascular diseases and pain, including angina. The mechanism(s) of ligustrazine’s effect to reduce angina is not clear. Angina is mediated by cardiac afferent sensory neurons. These neurons display a large acid-evoked depolarizing sodium current that can initiate action potentials in response to acidification that accompanies myocardial ischemia. Acid-sensing ion channels (ASICs) mediate this current. Here we tested the hypothesis that ligustrazine reduces ischemia-induced cardiac dysfunction and acid-evoked pain by an action to inhibit ASIC-mediated current. The effects of ligustrazine to attenuate ischemia-induced ST-segment depression, T wave changes, and myocardial infarct size in hearts of anesthetized rats were determined. Effects of ligustrazine on currents mediated by ASICs expressed in cultured Chinese hamster ovary cells, and effects of the drug on acid-induced nociceptive behavior and acid-induced currents in isolated dorsal root ganglions cells were measured. Ligustrazine significantly attenuated acid-induced ASIC currents, reduced cardiac ischemia-induced electrical dysfunction and infarct size, and decreased the nociceptive response to injection of acid into the paw of the rat hindlimb. The ASIC channel inhibitor A-317567 similarly reduced electrical dysfunction, infarct size, and nociceptive behavior in the rat. Inhibition of ASICs by ligustrazine may explain at least in part the beneficial effects of the drug that are observed in patients with ischemic heart disease and angina.
Keywords: Angina, ligustrazine, ASIC3 channels
Introduction
Chuanxiong is a dry root of the plant Ligusticum chuanxiong that is used to treat cerebro- and cardiovascular diseases in Chinese traditional medicine [1-3]. Ligustrazine (2, 3, 5, 6-tetramethylpyrazine; Figure 1) is a major active component of Chuanxiong, and it has been used since the 1970s to treat ischemia-induced cerebro- and cardiovascular diseases in China [3]. Ligustrazine has been shown to have vasodilator and anti-hypertensive effects, to relieve pain and suppress inflammation, and to reduce tissue damage in a variety of diseases [2-9].
Figure 1.
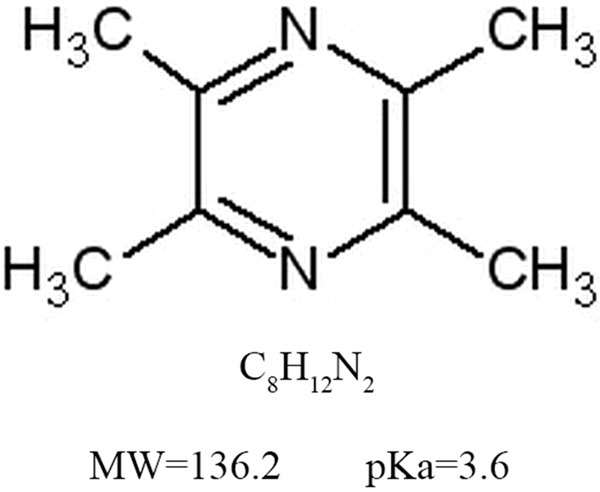
The chemical structure of ligustrazine. The molecular formula of ligustrazine is C8H12N2. The molecular weight of ligustrazine is 136.2, and the value of pKa is 3.6.
Angina is a radiating chest pain that occurs during myocardial ischemia when the blood supply to heart muscle is insufficient to meet the metabolic needs of the tissue [10]. Ischemia leads to increased activity of afferent sympathetic “nociceptive” nerves in the heart. Endogenous agents that are reported to activate sympathetic afferents and mediate angina include bradykinin, prostaglandin, ATP, and adenosine [11]. Lactic acid has been reported to stimulate the activity of cardiac sensory neurons and is also a potential mediator of angina [12]. The occlusion of a coronary artery leads to an increase in formation of lactic acid [13,14] and a drop of pH in the heart to 6.7-7.0 [11].
Acid-sensing ion channels (ASICs) belong to the degenerin family of two transmembrane domains, amiloride-sensitive Na+ channels. ASICs are highly expressed in sensory neurons and their opening leads to an increase of inward Na+ current, depolarization, and firing of action potentials. Evidence indicates that ASICs in sensory neurons mediate acidosis-associated pain [15-19]. Functional ASICs are thought to be composed of three subunits (homo- and heteromultimeric). Subunit composition of a given functional ASIC channel determines its pH sensitivity and response to amiloride. Amiloride and A-317567 block ASIC channel currents at high micromolar concentrations. Highly potent and selective small molecule blockers of ASICs are currently unavailable.
The present study tested the hypothesis that the cardioprotective and anti-anginal effects of ligustrazine are at least in part due to an effect of the compound to block ASIC ion channel currents. The results of the study indicated that ligustrazine blocks acid-induced inward current in cultured cells expressing ASIC channels, and in dorsal root ganglion cells expressing native ASIC channels. Ligustrazine also reduced the detrimental effects of myocardial ischemia on cardiac electrical parameters and viability of myocardium in hearts of anesthetized rats. We conclude that the effect of ligustrazine to reduce ASIC channel current induced by ischemia in the heart may contribute to its reported therapeutic benefits in treatment of patients with ischemic cardiac disease and angina.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (4 and 8-10 weeks old) were purchased from Fujian Experimental Animal Center (Fujian, China).Rats were housed and maintained on a 12 hours light/dark cycle with food and water ad libitum. Test drugs were given orally to conscious rats, 30 min or 1 hour before the vasoconstrictor vasopressin injection. All animal-based experiments were proceeded according to requirements of the Ethics Committee of Fuzhou General Hospital of Nanjing Command, Fuzhou, Fujian, China. All tests were performed based on a double-blind pharmaceutical study.
Vasopressin-induced and Isoproterenol-induced ST-segment depression
Experiments were carried out as described before [20]. In brief, rats were injected i.v with [Arginine8]-vasopressin (0.25 IU/kg). Lead II electrocardiogram was used to record ST-segment depression The data were then fed into Axoscope (Axon Instruments, USA) for real-time analysis via an electrocardiographic amplifier (A-M Systems, USA). The maximal ST-segment depression was obtained5 min after vasopressin injection based on our former study. For the isoproterenol (beta-adrenergic agonist) induced ST-segment depression record, the heart rate, R-wave QQ line (baseline) was obtained simultaneously by Axoscope.
Coronary artery occlusion-induced T-wave elevation
Experiments were based on the method in the previous research [21]. Briefly, anesthetized rats were thoracotomized and placed with a braided silk suture with a 10-mm tapered needle around the coronary artery. The epicardial electrocardiogram was obtained through a unipolar electrode attached to the left ventricular ischemic region and the changes in the T-wave after coronary artery occlusion were analyzed with Axoscope.
Myocardial infarction
Rats were anesthetized and their coronary artery was occluded. Then the coronary artery was reperfused by removal of the polyethylene tube for 24 hours, followed by the ligation of coronary artery and injection with 2 mL of 1% Evans blue solution through the tail vein. After rat heart was cut off, the left ventricle was isolated and transferred into a 1 mm-thick section to judge the area at risk. To detect the necrotic area, the section was incubated with 1% triphenyltetrazolium chloride (TTC) at 37°C for 10 min. The recorded data was input and analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, USA).
Cell culture and expression plasmids
Chinese hamster ovary (CHO) cells were cultured in F12 medium, supplemented with 2 mM glutamine, 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin. All plasmids used in the study were described before [22]. For the cell transfection, shortly, the CHO cells were cotransfected with different ASIC subunits expression vectors and pcDNA3-GFP (Invitrogen, San Diego, CA) using Lipo 2000 (Invitrogen). Tracing data for patch-clamp recording was collected 24 to 48 hours after seeding.
Preparation and recording of acute dorsal root ganglions (DRG) neuron
Base on previous study [23], rats were anesthetized and finished with sevoflurane. The DRGs were stripped off the vertebral column and desheathed at 37°C for 30 min in Hank’s balanced salt solution (HBSS), followed by 1.25 mg/mL trypsin treatment. Ganglia was dissociated by Pasteur pipette and then plated on glass coverslips (Corning, Corning, NY) pretreated with poly (D-lysine) on culture dishes. Neurons were incubated in a humidified atmosphere for 2 to 8 hours in a CO2 water-jacketed incubator (NuAire, Plymouth, MN). The plating medium contained DMEM (Gibco, Long Island, NY), supplemented with 2 mM glutamine, 10% fetal bovine serum (Invitrogen) and B27 (2%, Gibco). Patch-clamp recording was performed on individual DRG neurons 3-8 hours after isolation.
Electrophysiology
Neuron-cell electrophysiological data, filtered at 2 kHz and digitized at 5 kHz using a Digidata 1332A or/and MiniDigi 1A, was traced by the Axopatch-1D amplifier (Molecular Devices, Sunnyvale, CA) at room temperature. In addition, the parameter of cell membrane potential was set at -60 mV or otherwise indicated.
Patch pipettes (3-5 MΩ) contained (in mM): 140 NaCl, 2.5 KCl, 1.8 CaCl2, 1 MgCl2 and 10 N-2-Hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES), pH 7.4 adjusted with NaOH and osmolarity ranging from 320 to 330 mOsm. The intracellular solution consisted of (in mM) 140 CsF, 11 Ethylene glycol-bis (2-aminoethyl ether)-N,N,N,N’-tetraacetic acid (EGTA) as intracellular calcium chelating buffer, 10 HEPES, 2 MgCl2, 1 CaCl2, and 4 K2ATP with pH adjusted by CsOH. For current-clamp recording, the pipette solution contained (in mM): 140 KCl, 5 NaCl, 10 HEPES, 5 EGTA, 2 MgCl2, 2 K2ATP, pH was adjusted by KOH. A piezoelectric-driven rapid application system (Burleigh Instruments, Fishers, NY) was used to apply different pH buffers and used drugs in each cell. ASIC currents were induced by 15 s and pH 5.0 evoke. Dose-dependent response and pH-dependent relationship were evaluated by using buffers of decremental pH or incremental concentrations of tested subjects every 3 min each time with pH 5.0.
Electrophysiology using two-electrode voltage clamp
Experimental procedures for whole Xenopus laevis oocyte electrophysiology have been introduced before [17]. To be short, ovaries follicular membrane was first removed by collagenase type II digestion and cRNA sencoding ENaC were injected into stage V-VI oocytes at 19°C for 3 days. Amiloride sensitive currents were measured by the two-electrode voltage clamp. Ligustrazine (300 μM) was applied in the absence of amiloride to determine the effect of ligustrazine on ENaC activities. Whole oocyte amiloride-sensitive currents were recorded at 1 kHz with a TurboTec 03X amplifier (npi electronic).
Nociceptive behavior evaluation by acetic acid
Rats were placed in a 30 cm × 30 cm × 30 cm Plexiglas chamber and allowed to be observed for more than half an hour before nociceptive behavior test. After capsazepine pretreatment, 20 μL 0.6 % acetic acid solution, 20 μL vehicle (0.9% saline) and ligustrazine were coded and put to the dorsal face of the hind paw based on a double-blinded experiment. The number of flinches was recorded immediately for 5 min long after the injection.
Drugs
Ligustrazine was provided by the National Institute for the Control of Pharmaceutical and Biological Products (Shanghai, China). Other drugs were purchased from Sigma unless otherwise mentioned. Drugs were mixed by 0.5% methyl cellulose and taken in half an hour (ligustrazine) or 1 hour (the calcium-channel blocker and vasodilator nifedipine and the beta-adrenergic antagonist propranolol) to get the maximal blood concentration.
Data analysis
Electrophysiology observed data was analyzed using the Clampfit 9.2 (Molecular Devices) software. The cells were incubated in the test solution for 15 s to record the cell currents. We use the following calculation to examine the test subject’s effect Y:
Y=min+(max-min)/(1+(x/EC50)H)
In this calculation, x is referred to the drug concentration, max and min are the maximum and minimum effects, EC50 is the drug concentration when the effect obtained 50% efficiency, and H is the Hill slope constant. To study the current desensitization, its time course was fitted with a single exponential function and a desensitization time constant (τdes) was defined. In addition, the peak of the current (I peak), and the mean of the current in the last 250 ms of the 15-s acid pulse defined as the steady-state amplitude (I ss) were measured. For some situations such as the records did not fit a single exponential model, T 50 was selected as the time at which the current decreased to 50% of its maximum amplitude. To quantify the total charge transfer during current activation we also calculated the integral of the current (I int). The peak (Peak), the mean in the last 250 ms of the 15-s acid pulse (SS) and the voltage decay time constant (τ) of the voltage depolarization induced by 15-s, pH 5.0 acid pulses were measured.
Statistical analysis
To calculate the statistical significance, paired Student’s t test was used and P<0.05 was considered significant. Experimental data are presented as the mean ± SEM unless a large set of cells were included, in which case, mean ± S.D was used instead. One- or two-way ANOVA was used to compare the difference among different groups. Statistical analysis was performed using Origin 7 (Originlab Corporation, Northhampton MA, USA).
Results
Ligustrazine dose-dependently inhibited vasopressin and isoproterenol induced ST-segment in rat jugular vein
Vasopressin and isoproterenol are two well-known reagents used in rats angina models. We initially test the effect of ligustrazine on these two angina models. Injection of vasopressin could provoke a transient ST-segment depression with the maximal depression at 3 min and return to the quiescent state in less than 10 min (Figure 2). Two already characterized antianginal drugsnifedipine and nicorandil treated groups were set as the positive control. Compared to vehicle solution (0.5% methylcellulose), ligustrazine, similar to nifedipine and nicorandil, inhibited ST-segment changes dose-dependently. The minimum doses which completely inhibited ST-segment depression 1 hour after oral administration were as follows: ligustrazine 1-10 mg/kg (Figure 2A); nifedipine 3 mg/kg (Figure 2B) and nicorandil 10 mg/kg (Figure 2C). Infusion of isoproterenol (10 μg/kg/min, i.v.) induced a rapid increase in heart rate (>100 beats/min) followed by an ST-segment gradual depression. We evaluated the effect of ligustrazine on ST-segment depression 6 min after isoproterenol infusion as described before [20]. Similarly, ligustrazine could significantly depressed the isoproterenol-induced ST-segment suppression dose-dependently (Figure 2D). It appears that ligustrazine (3 mg/kg and 10 mg/kg) repressed ST-segment in two rat angina models with statistical significance.
Figure 2.
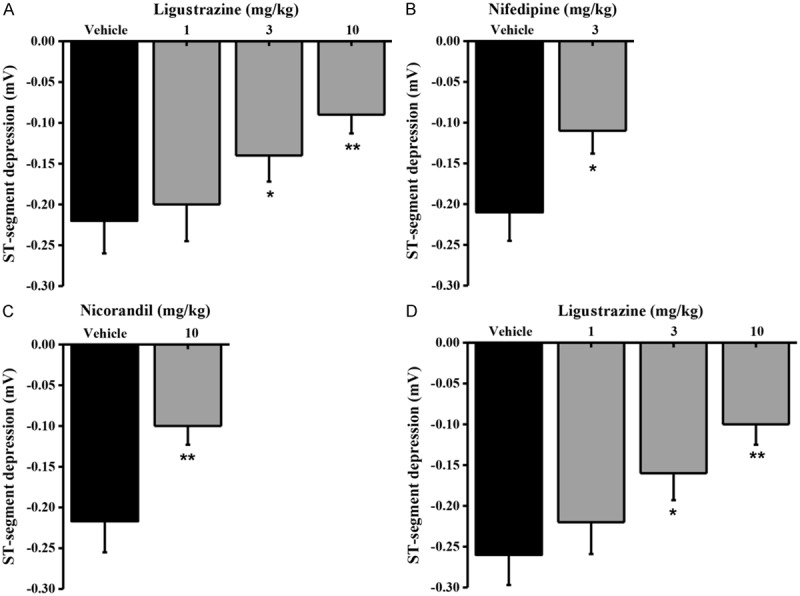
Effects of ligustrazine on the maximum changes in drug-induced ST-segment depression in the electrocardiogram (lead II) in anesthetized rats. A. Means ± SEM ligustrazine and vehicle on the maximum changes in vasopressin-induced ST-segment depression. Ligustrazine was administered orally 30 min prior to vasopressin infusion (0.25 IU/kg, i.v.). Columns indicate the maximal ST-segment depression induced by vasopressin. n=8-9. *p=0.0208 and **p=0.0073, different from this in the vehicle. B. Means ± SEM nifedipine and vehicle on the maximum changes in vasopressin-induced ST-segment depression. Nifedipine was administered orally 1 hourprior to vasopressin infusion (0.25 IU/kg, i.v.). Columns indicate the maximal ST-segment depression induced by vasopressin. n=8-10. *p=0.0437, different from this in the vehicle. C. Means ± SEM nicorandil and vehicle on the maximum changes in vasopressin-induced ST-segment depression. Nifedipine was administered orally 1 hour prior to vasopressin infusion (0.25 IU/kg, i.v.). Columns indicate the maximal ST-segment depression induced by vasopressin. n=8-9. **p=0.0029, different from this in the vehicle. D. Means ± SEM various concentrations ligustrazine and vehicle on the maximum changes in isoproterenol-induced ST-segment depression. Ligustrazine was administered orally 30 min prior to isoproterenol infusion (10 μg/kg/min, i.v.). Columns indicate the ST-segment depression at 5 min after isoproterenol infusion. n=8-10. *p=0.0362 and **p=0.0054<0.01, different from those in the vehicle.
Effect of ligustrazine on coronary artery occlusion induced T-wave
Coronary artery occlusion plays a vital role during angina heart disease development. We have detected that T-wave elevation could be stimulated by coronary artery occlusion in 20 to 40 seconds and the maximal peak occurred within 5 min. Ligustrazine administration greatly blunted coronary artery occlusion-related T-wave compared with vehicle control dose-dependently (Figure 3A). In comparison with the other two antianginal drugs Propranolol (100 mg/kg, p.o.) and nifedipine (3 mg/kg, p.o.), ligustrazine also showed similar inhibition effect on T-wave elevation (Figure 3B).
Figure 3.
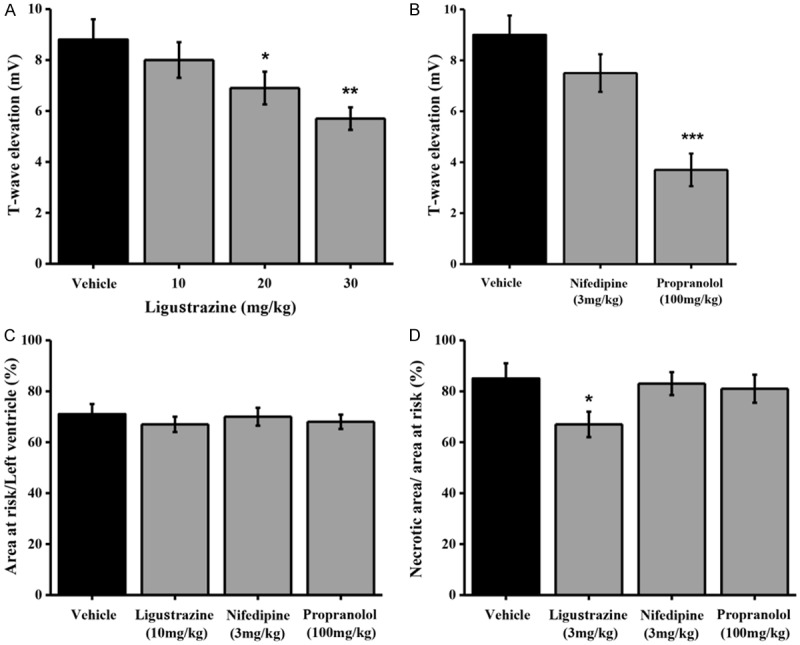
Effects of ligustrazine on the T-wave elevation and myocardial infarct size induced by coronary artery occlusion in anesthetized rats. A. Means ± SEM ligustrazine and vehicle on the T-wave elevation induced by coronary artery occlusion in anesthetized rats. Ligustrazine was administered orally 30 min prior to coronary artery occlusion. Columns indicate the maximal T-wave elevation induced by coronary artery occlusion. n=8-10. *p=0.0258 and **p=0.0017, different from this in the vehicle. B. Means ± SEM nifedipine, propranolol and vehicle on on the T-wave elevation induced by coronary artery occlusion in anesthetized rats. Nifedipine (3 mg/kg) and propranolol (100 mg/kg) were administered orally 1 hour prior to coronary artery occlusion. Columns indicate the maximal T-wave elevation induced by coronary artery occlusion. n=8-9. ***p=0.0002, different from this in the vehicle. C. Means ± SEM ligustrazine, nifedipine, and propranolol on myocardial infarct size after 40 min of coronary artery occlusion and in rats. Drugs were administered orally 30 min (ligustrazine) or 1 hour (nifedipine and propranolol) prior to coronary artery occlusion. Columns indicate the myocardial infarct size induced by coronary artery occlusion. n=8-10. *p=0.0193, different from this in the vehicle. D. Means ± SEM ligustrazine, nifedipine, and propranolol on myocardial infarct size after 24 h of coronary artery occlusion and reperfusion in rats. Drugs were administered orally 30 min (ligustrazine) or 1 hour (nifedipine and propranolol) prior to coronary artery occlusion. Columns indicate the myocardial infarct size induced by coronary artery occlusion. n=8-11. *p=0.0324, different from this in the vehicle.
Effect of ligustrazine on myocardial infarction model in rats
To further test ligustrazine’s effect on myocardial infarction, or acute myocardial infarction, the infarct sizes among different treatment groups were calculated and analyzed. Ligustrazine treatment obviously reduced the infarct size compared with vehicle control 24 hours after coronary artery occlusion (Figure 3D). However, there was no significant difference in the percentage of the total infarcted area among control, nifedipine (3 mg/kg, p.o) or propranolol (100 mg/kg, p.o.) groups (Figure 3C). And the above result indicated that liugstrazine has superior therapeutic effect to other anti-ischemia drugs nifedipine (3 mg/kg, p.o) and propranolol (100 mg/kg, p.o.) in inhibition of myocardial infarction.
Effect of ligustrazine on acid-activated currents in DRG neurons
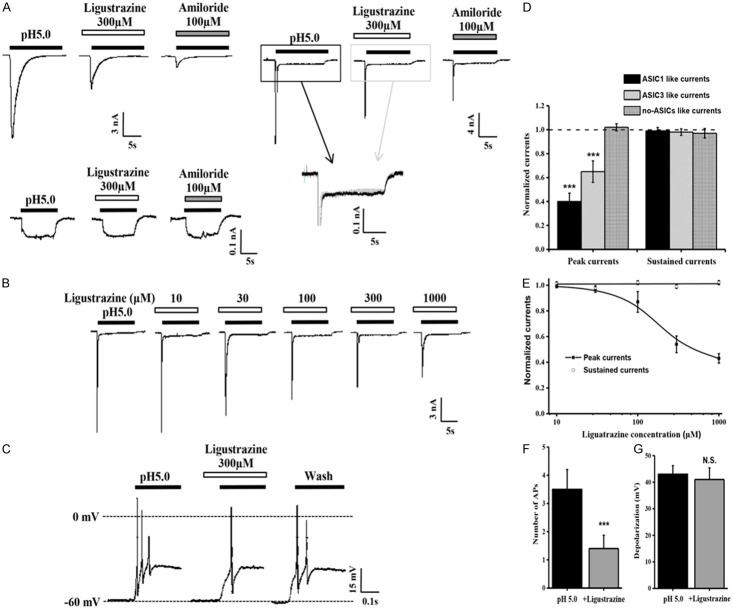
We have demonstrated that ligustrazine was effective in relieving angina in several rat models. As ASIC channels activation in response to acidification is thought to induce ischemic pain, ASICs activity was recorded after ligustrazine administration to further elucidate the possible mechanism of ligustrazine’s protective effect. In acutely dissociated rat DRG neurons in response to acidic-solution perfusion, the DRG neuron cell currents were characterized by three groups, slow-inactivating current (ASIC1-like current), fast-inactivating current (ASIC3-like current), and non-inactivating current (no-ASIC current), suggesting the involvement of diverse ASICs subunits (Figure 4A). 300 mM ligustrazine oral administration generated a significant reduction of the peak amplitude of all ASIC currents at both pH 7.4 and 5.0 (Figure 4D). But ligustrazine was not enough to suppress the continued part of ASIC3-like current (Figure 4A) and neither was amiloride [24]. Figure 4B and 4E showed that ligustrazine dose-dependently decreased the amplitudes and had a maximum effect (43.51±5.26%) at the concentration of 1 mM. The EC50 value of the dose-response curve for ligustrazine was 274.32±10.19 μM.
Figure 4.
Inhibition by ligustrazine of proton-gated currents and membrane excitability in rat DRG neurons. (A) Representative traces of pH 5.0-induced currents in the absence and presence of ligustrazine in isolated rat DRG neurons. slow-inactivating current (left), fast-inactivating current (right), and non-inactivating current (down) induced by pH 5.0, only slow-inactivating current and fast-inactivating current can blocked by amiloride (100 μM). Ligustrazine didn’t depression the sustained currents in ASIC3-like DRG neuron (merge). (B) Representative traces showing the effects of pH 5.0-induced ASIC-like currents in the absence and presence of various concentrations of ligustrazine in rat DRG neurons. The extracellular bath solution contained AMG9810 (10 μM) to block TRPV1 channels. (C) Representative traces of pH 5.0-induced membrane potential depolarization and spike activity in the absence and presence of various concentrations of ligustrazine in rat DRG neurons under current clamp recording model. (D) Means ± SEM peak currents normalized to that induced by pH 5.0-induced in the presence of ligustrazine in rat DRG neurons. n=6-9. ***p=0.0004, different from this in the pH5.0 alone (dashed line). (E) Means ± SEM peak and sustained currents normalized to that induced by pH 5.0 alone in rat DRG neurons. n=7. (F, G) Means ± SEM for number of APs (F) and level of membrane depolarization at the plateau (G) evoked by pH 5.0 in rat DRG neurons under conditions shown in C. n=6. ***p=0.0004.
Effect of ligustrazine on acid-activated membrane excitability in DRG neuron
Activation of ASICs by protons induces sodium influx, resulting in membrane depolarization and neuronal excitation. Further experiments were performed to record DRG neuron excitability in the current-clamp model in the presence of AMG9810 (10 μM) to block proton-induced TRPV1 activation. As shown in Figure 4C, a steep pH drop from 7.4 to 5.0 could trigger bursts of action potentials under current-clamp conditions in the tested neuron. Notably, ligustrazine decreased the number of action potentials evoked by acidosis from 3.57±0.30 of control condition to 1.39±0.28 (Figure 4F). But ligustrazine didn’t decrease the depolarization potentials evoked by acidosis (Figure 4G).
Subunit-specific inhibition of ASIC currents by ligustrazine in CHO cells
Almost all ASIC isoforms, ASIC1a, 1b, 2a, and ASIC3, are present in primary sensory neurons of the trigeminal, vagal, and dorsal root ganglia, which are able to detect noxious chemical, thermal, and high threshold mechanical stimuli. To further investigate different ASIC subunits sensitivity to ligustrazine inhibition, we then studied the effect of ligustrazine on various ASIC subunits expressed CHO cells. It was consistent to find ligustrazine (300 µM) dramatically decreased the current mediated by homomeric ASIC1a (Figure 5A). Therefore, through using the same concentration of ligustrazine as test concentration on other ASIC subunits, we identified ligustrazine dramatically reduced the amplitude of the other ASIC subunits currents (Figure 5B-D). Following a 40 s perfusion of ligustrazine, ASIC1a current was inhibited by 41±3.1%; ASIC1b was inhibited by 86±2.3%; ASIC2a was inhibited by 45±3.4%; and ASIC3 was inhibited by 37±2.9% (Figure 5F). However, Figure 5E showed 300 µM ligustrazine did not inhibit ENaC activities in oocytes.
Figure 5.
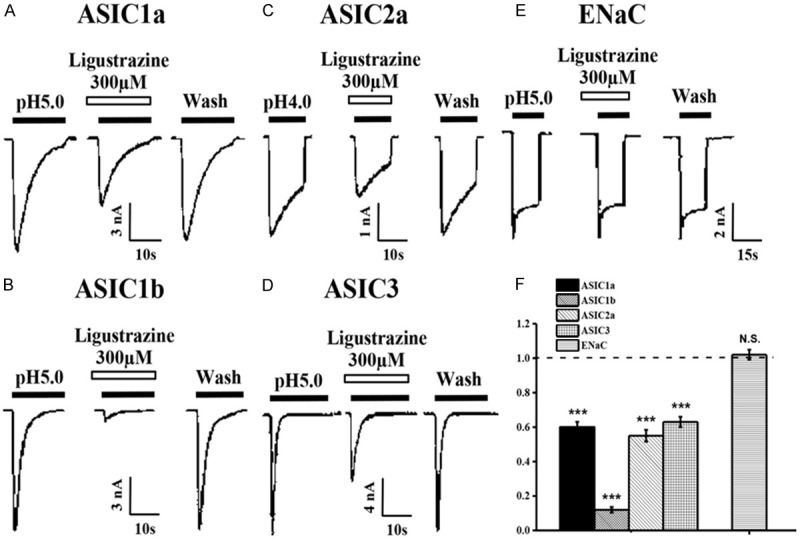
Subunit selectivity of ligustrazine. (A-D) Representative current traces induced by pH 5.0 in the absence or presence of ligustrazine from CHO cells expressing homomeric ASIC1a (A), 1b (B), 2a (C), and ASIC3 (D) channels. (E) Ligustrazine failed to block oocyte-expressing ENaC channels. (F) Means ± SEM peak current of pH 5.0 and ENaC activities-induced currents in the presence of ligustrazine (300 mM) normalized to that induced by pH 5.0 alone (dashed line). n=6-9, ***p=0.0005, compared with pH 5.0 or ENaC activities alone.
As the activation curves of homomeric ASIC1a, 1b, 2a and ASIC3 were very steep for pH activation, we selected pH 5.5 (ASIC1a, 1b), 4.0 (ASIC2a) and 5.0 (ASIC3) as standard stimulus pH values, respectively. A preliminary concentration-response analysis revealed an IC50 of 96.8 μM for the inhibition of the ASIC1a current by ligustrazine. IC50 of ligustrazine for inhibition of the ASIC1b current was 62.0 μM (Figure 6). IC50 of ligustrazine for inhibition of the ASIC2a current was 129.4 μM (Figure 6E). ASIC3 channel showed biphasic proton responses, which were characterized by rapidly desensitizing currents followed by slow and sustained currents that returned to baseline on return to pH 7.4 (Figure 6B). The peak of biphasic currents was remarkably decreased in the presence of ligustrazine and the IC50 was 239.5 mΜ, which was much higher than those of the ASIC1a, 1b and ASIC2a channels (Figure 6E).
Figure 6.
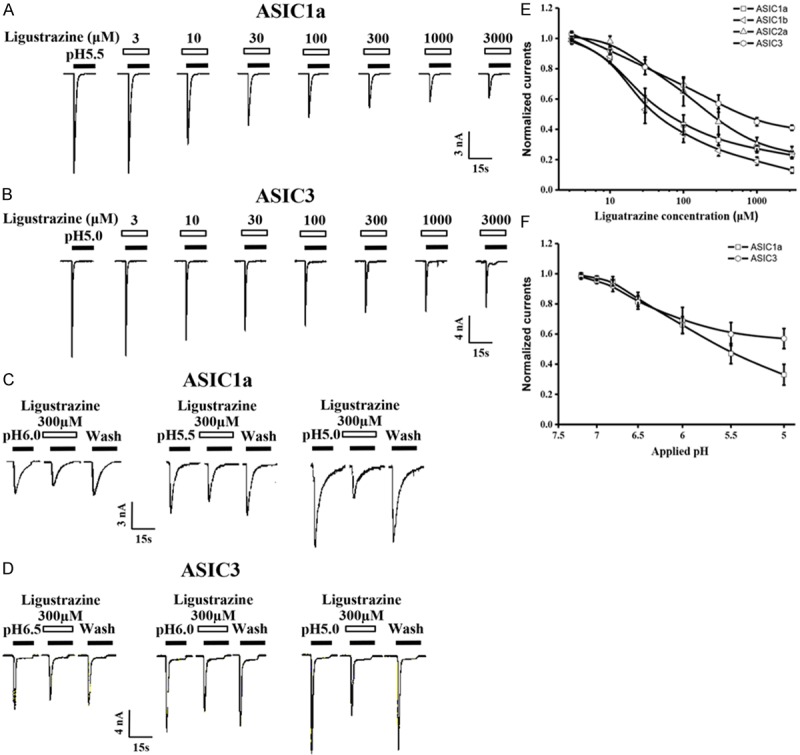
Ligustrazine dose and pH-dependently inhibite the peak currents of ASICs. A. Representative current traces showing the effects of various concentrations of ligustrazine on pH 5.0-induced currents in CHO cells expressing ASIC1a channels. B. Representative current traces showing the effects of various concentrations of ligustrazine on pH 5.0-induced currents in CHO cells expressing ASIC3 channels. C. Representative current traces elicited by application of different pH in the absence or presence of ligustrazine (100 mM) to CHO cells expressing ASIC1a channels. D. Representative current traces elicited by application of different pH in the absence or presence of ligustrazine (100 mM) to CHO cells expressing ASIC3 channels. E. Means ± SEM pH 5.0-induced peak current in the presence of ligustrazine, normalized to that in the absence of ligustrazine under conditions shown in A and B. n=6-9. F. Means ± SEM acid-induced peak current in the presence of ligustrazine, normalized to pH 5.0-induced peak current that in the absence of ligustrazine. n=6-8.
pH dependence of ligustrazine-mediated inhibition of the ASICs currents
To examine whether ligustrazine inhibition was dependent on pH, Figure 6C, 6D showed ligustrazine’s magnitude of inhibition on proton-gated currents was dependent on pH. Figure 6F demonstrated in the presence of ligustrazine (300 μM), the pH 0.5 value of ASIC1a currents changed from 5.82±0.12 to 5.33±0.09 and pH 0.5 value of ASIC3 currents changed from 6.32±0.11 to 5.84±0.08 (data not show) based concentration-response curve. These results proved that ligustrazine also performed a well pH-dependent inhibition on ASICs currents.
Suppression of A-317567 on ASICs and angina in different rat models
In order to confirm that ligustrazine could relieve angina through inhibition of ASICs activation, we next investigated whether ASIC inhibitors exhibited similar effects to ligustrazine in different rat angina models. Amiloride (and amiloride-related compounds), A-317567, PcTx1, and APETx2 are only ASIC blockers so far. A-317567, a novel ASIC blocker, is superior to other drugs for its structure, high specificity and concentration-dependent inhibition of all proton-evoked ASICs currents. Our study found that A-317567 inhibited ST-segment suppression (Figure 7A) and the coronary artery occlusion-induced T-wave elevation in a dose-dependent manner with statistical significance versus vehicle control at 0.3 and 1 mg/kg (Figure 7B). Besides, we also found the administration of A-317567 (1 mg/kg, p.o.) significantly reduced the infarct size (Figure 7C). Taken together, ligustrazine, similar to ASIC inihibitor A-317567, could repress angina through blunting ASIC activation.
Figure 7.
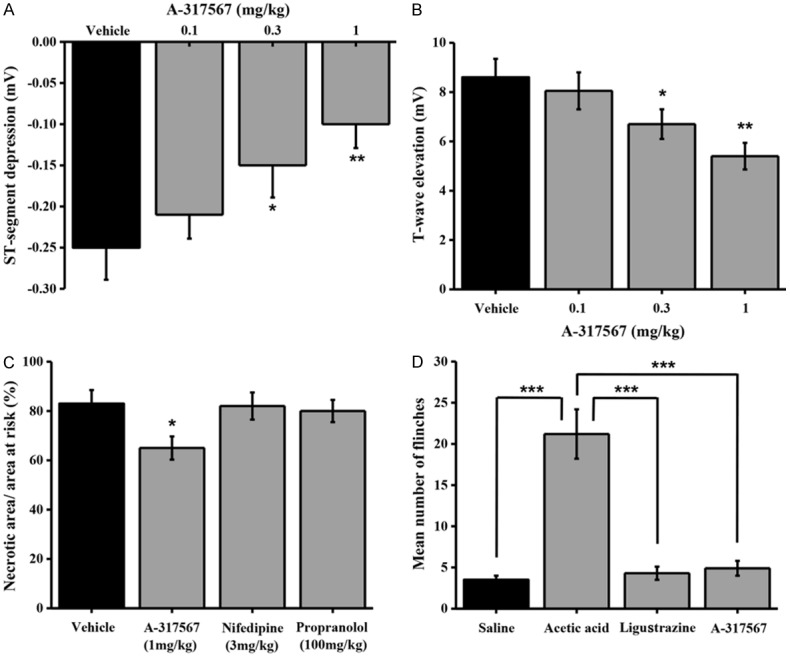
Function of A-317567 on angina model in Rat. A. Means ± SEM A-317567 and vehicle on the maximum changes in vasopressin-induced ST-segment depression in the electrocardiogram (lead II) in anesthetized rats. Ligustrazine was administered orally 30 min prior to vasopressin infusion (0.25 IU/kg, i.v.). Columns indicate the maximal ST-segment depression induced by vasopressin. n=8-10. *p=0.0316 and **p=0.0029, different from this in the vehicle. B. Means ± SEM A-317567 and vehicle on the T-wave elevation induced by coronary artery occlusion in anesthetized rats. A-317567 was administered orally 30 min prior to coronary artery occlusion. Columns indicate the maximal T-wave elevation induced by coronary artery occlusion. n=8-9. *p=0.0463 and **p=0.0058, different from this in the vehicle. C. Means ± SEM A-317567 on myocardial infarct size after 40 min of coronary artery occlusion and in rats. A-317567 was administered orally 30 min prior to coronary artery occlusion. Columns indicate the myocardial infarct size induced by coronary artery occlusion. n=8-10. *p=0.0362, different from this in the vehicle. D. Means ± SEM A-317567 on intraplantar injection acetic acid evoked a flinch/shaking response in rats. Flinching shaking of paw was recorded as the number of flinches per observation period (5 min). n=8-10, ***p=0.0007. Acetic acid, 0.6%; ligustrazine, 0.07 mM; A-317567, 200 μM.
Effect of ligustrazine on nociceptive responses to injection of acetic acid in rats
Usually, the flinch reaction occurs within 5 mins after acetic acid injection in rats. In the present study, this pain induced flinch frequency was significantly blocked by both ligustrazine and A-317567 treatment. (Figure 7D). These results indicated that ligustrazine contributed to acidosis evoked pain extenuation in rats.
Discussion
Ligustrazine, the main bioactive component of a traditional herbal medicine chuanxiong, is widely used in angina treatment because of its low toxicity and therapeutic efficacy [25]. Pain generating processes including inflammation, stroke, and tumor, are always associated with extracellular acidosis [26]. ASIC family, but not TRPV1 receptor, is believed to play a role in acidification related pain [27] because ASIC inhibitor amiloride could extenuate acidosis-induced pain efficiently, whereas TRPV1 antagonist capsazepine could not. Our research characterized the possible explanations of ligustrazine’ santi-ischemic effect through blocking ASIC activation.
Injection of vasopressin and isoproterenol provoked a marked ST-segment repression in rats. The ST-segment elevation was thought to be caused by K+ efflux and is a promising indicative marker for ischemic region [28]. These two models are very useful to evaluate the effect of potential antianginal drugs in vivo. According to present study, invasopressin and isoproterenol-induced angina rat models, we have observed that ligustrazine suppressed the ST-segment elevation dose-dependently (Figure 2A and 2D). The protective effect of ligustrazine on the ischemic myocardium was also fully evaluated on coronary artery occlusion-induced T-wave. Ligustrazine significantly reduced the T-wave elevation dose dependently (Figure 3A). In myocardial infarctions induced by ischemia and reperfusion test, compared to vehicle control, ligustrazine significantly reduced the infarct size but not the other two well-known antianginal drugs nifedipine and propranolol. Taken together, the above results demonstrated that oral administration of ligustrazine could relieve angina severity and myocardial infarction dose-dependently.
The ASICs belong to a distinct member of degenerin super family. The ASIC proteins have been found to be highly expressed in many neurons and sensory cells, such as dorsal root ganglia (DRG) [17]. Through forming various homomeric and heteromeric homoclade channels, ASICs are able to respond to a wide range of pH. Specifically, ASIC3 channelis distinct from the other ASIC subunits because it could generate a biphasic current in response to acidosis [29]. In the heart, ASICs play a significant role in mediating potential pain of angina and ischemic damage.
Several different types of acidosis-evoked currents were detected in immediately dissociated rat DRG cells. Among them, two distinct types of ASIC currents were observed with apparent characteristics such as desensitization rate and low pH related steady cell currents. These two different currents are called ASIC1 and ASIC3-like currents. Interestingly, ASIC1-like currents are more predominant in relatively small neurons but the ASIC3-like currents are more predominant in large neural cells [23]. In rat DRG neurons, transient depolarization induced by small pH changes (from pH 7.4 to pH 5.0) was able to trigger action potentials, followed by a sustained depolarization due to the plateau phase of ASIC3-like currents. The current-clamp experiments showed that ligustrazine decreased action potentials number induced by acidosis (Figure 4C). The ligustrazine altered acid-evoked membrane excitability of DRG neurons and the number of spikes induced by acid stimuli, partially explaining the cellular mechanisms of ligustrazine’s analgesic action on angina in vitro. Furthermore, in consistent with the former observations, ligustrazine inhibited ASIC1a, 1b, 2a and ASIC3 currents based on a dose-dependent and pH-dependent manner (Figure 5). Ligustrazine shifted the proton concentration-response curve downwards, with a decrease in the maximum current response but with no significant change in the pH 0.5 and threshold values. Thus, inhibition of ligustrazine was due to the reduction of the efficacy, but not the affinity of ASICs to proton.
There are few ASIC blockers, such as Amiloride and A-317567. Amiloride has been used to block ASICs in most studies. But A-317567 is a more effective blocker of ASICs which could block ASIC1a, ASIC1b, ASIC2a, and ASIC3 subtypes. In this study, application of A-317567 blocked all ASIC subtypes activation and provided protection from angina in chemical and coronary artery occlusion-induced angina models (Figure 7). A vicious cycle between poststenotic coronary vasoconstriction and sympathetic activation results in severe myocardial ischemia [30]. Similar to ASICs inhibitor A-317567, ligustrazine, could efficiently block all ASICs response (Figure 5). Respectively, ligustrazine is even better than A-317567 because it does not interact in ENaCs as a lack of natriuretic and diuretic activity (Figure 5E).
Activation of these ASICs by acidic pH results in depolarization of nociceptive primary sensory neurons and contributes to nociception [31]. The present study revealed that ligustrazine exerted an analgesic effect by modulating the function of ASICs, since its effect on acidosis-evoked flinch frequency was consistent with that on acid-evoked neuronal excitability of DRG neurons in current clamp experiments (Figure 7D).
In summary, ligustrazine was found to suppress ASIC ion channel currents and acid-evoked pain in the rat hind paw. Ligustrazine also reduced ischemia-induced electrical dysfunction and infarct size in the heart of the anesthetized rat. These results provide a potential biological basis for findings that ligustrazine reduces angina and is beneficial in treatment of ischemic cardiovascular diseases.
Disclosure of conflict of interest
None.
References
- 1.Cai YN, Barer GR. Effect of ligustrazine on pulmonary vascular changes induced by chronic hypoxia in rats. Clin Sci (Lond) 1989;77:515–520. doi: 10.1042/cs0770515. [DOI] [PubMed] [Google Scholar]
- 2.Liu SF, Cai YN, Evans TW, McCormack DG, Barer GR, Barnes PJ. Ligustrazine is a vasodilator of human pulmonary and bronchial arteries. Eur J Pharmacol. 1990;191:345–350. doi: 10.1016/0014-2999(90)94167-v. [DOI] [PubMed] [Google Scholar]
- 3.Zeng SP. Effect of ligustrazine and activating blood circulation injection on experimental glomerulonephritis in rabbits. Zhong xi yi jie he za zhi. 1983;3:357–359. [PubMed] [Google Scholar]
- 4.Cai YN, Bee D, Barer G. Pulmonary vasodilator action of ligustrazine, active principle of a traditional Chinese remedy, in rats and ferrets. Proc Chin Acad Med Sci Peking Union Med Coll. 1989;4:147–152. [PubMed] [Google Scholar]
- 5.Kwan CY, Gaspar V, Shi AG, Wang ZL, Chen MC, Ohta A, Cragoe EJ Jr, Daniel EE. Vascular effects of tetramethylpyrazine: direct interaction with smooth muscle alpha-adrenoceptors. Eur J Pharmacol. 1991;198:15–21. doi: 10.1016/0014-2999(91)90556-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen YJ, Huang CS, Wang F, Gong JY, Pan ZH. [Effect of ligustrazine hydrochloride on coagulation reaction and inflammation reaction in single valve replacement patients with rheumatic heart disease undergoing cardiopulmonary bypass] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:531–535. [PubMed] [Google Scholar]
- 7.Hu JZ, Luo CY, Kang M, Lu HB, Lei GH, Dai Z. [Therapeutic effects of intraarticular injection of ligustrazine on knee osteoarthritis] . Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:591–594. [PubMed] [Google Scholar]
- 8.Xiao S, Li KH, Lu HB. [Effect of ligustrazine on the expression of Bcl-2 protein and apoptosis in rabbit articular chondrocytes in monolayer culture] . Hunan Yi Ke Da Xue Xue Bao. 2003;28:224–226. [PubMed] [Google Scholar]
- 9.Zhou Y, Hu CP, Deng PY, Deng HW, Li YJ. The protective effects of ligustrazine on ischemia-reperfusion and DPPH free radical-induced myocardial injury in isolated rat hearts. Planta Med. 2004;70:818–822. doi: 10.1055/s-2004-827229. [DOI] [PubMed] [Google Scholar]
- 10.Attanasio S, Schaer G. Therapeutic angiogenesis for the management of refractory angina: current concepts. Cardiovasc Ther. 2011;29:e1–e11. doi: 10.1111/j.1755-5922.2010.00153.x. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan JW, Hamm JR, Rothman DL, Shulman RG. Intracellular pH in human skeletal muscle by 1H NMR. Proc Natl Acad Sci U S A. 1988;85:7836–7839. doi: 10.1073/pnas.85.21.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida Y, Murao S. Acid-induced excitation of afferent cardiac sympathetic nerve fibers. Am J Physiol. 1975;228:27–33. doi: 10.1152/ajplegacy.1975.228.1.27. [DOI] [PubMed] [Google Scholar]
- 14.Pal P, Koley J, Bhattacharyya S, Gupta JS, Koley B. Cardiac nociceptors and ischemia: role of sympathetic afferents in cat. Jpn J Physiol. 1989;39:131–144. doi: 10.2170/jjphysiol.39.131. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Li WG, Yu Y, Xiao X, Cheng J, Zeng WZ, Peng Z, Xi Zhu M, Xu TL. Serotonin facilitates peripheral pain sensitivity in a manner that depends on the nonproton ligand sensing domain of ASIC3 channel. J Neurosci. 2013;33:4265–4279. doi: 10.1523/JNEUROSCI.3376-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208:191–194. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- 17.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 18.Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24:10974–10979. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acidsensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto S, Matsui K, Sasabe M, Kitano M, Ohashi N. Effect of SMP-300, a new Na+/H+ exchange inhibitor, on myocardial ischemia and experimental angina models in rats. Jpn J Pharmacol. 2000;84:196–205. doi: 10.1254/jjp.84.196. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Nakatsuji K, Hosoki K, Karasawa T. Preventive effect of a new calcium antagonist, monatepil, on drug-induced ischaemic electrocardiographic changes in rats. Clin Exp Pharmacol Physiol. 1993;20:673–678. doi: 10.1111/j.1440-1681.1993.tb01651.x. [DOI] [PubMed] [Google Scholar]
- 22.Catarsi S, Babinski K, Seguela P. Selective modulation of heteromeric ASIC proton-gated channels by neuropeptide FF. Neuropharmacology. 2001;41:592–600. doi: 10.1016/s0028-3908(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 23.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117:88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H(+)-gated cation channels. Ann N Y Acad Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang P, She G, Yang Y, Li Q, Zhang H, Liu J, Cao Y, Xu X, Lei H. Synthesis and biological evaluation of new ligustrazine derivatives as antitumor agents. Molecules. 2012;17:4972–4985. doi: 10.3390/molecules17054972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zha XM. Acid-sensing ion channels: trafficking and synaptic function. Mol Brain. 2013;6:1. doi: 10.1186/1756-6606-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wemmie JA, Price MP, Welsh MJ. Acidsensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Zuchi C, Tritto I, Ambrosio G. Angina pectoris in women: focus on microvascular disease. Int J Cardiol. 2013;163:132–140. doi: 10.1016/j.ijcard.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Li WG, Yu Y, Xiao X, Cheng J, Zeng WZ, Peng Z, Zhu MX, Xu TL. Serotonin facilitates peripheral pain sensitivity in a manner that depends on the nonproton ligand sensing domain of ASIC3 channel. J Neurosci. 2013;33:4265–4279. doi: 10.1523/JNEUROSCI.3376-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heusch G, Deussen A, Thamer V. Cardiac sympathetic nerve activity and progressive vasoconstriction distal to coronary stenoses: feed-back aggravation of myocardial ischemia. J Auton Nerv Syst. 1985;13:311–326. doi: 10.1016/0165-1838(85)90020-7. [DOI] [PubMed] [Google Scholar]
- 31.Kang S, Jang JH, Price MP, Gautam M, Benson CJ, Gong H, Welsh MJ, Brennan TJ. Simultaneous disruption of mouse ASIC1a, ASIC2 and ASIC3 genes enhances cutaneous mechanosensitivity. PLoS One. 2012;7:e35225. doi: 10.1371/journal.pone.0035225. [DOI] [PMC free article] [PubMed] [Google Scholar]