Abstract
MicroRNAs are small non-coding RNAs that are able to regulate gene expression and play important roles in some biological and pathological processes, including the myocardial ischemia/reperfusion (I/R) injury. Recent findings demonstrated that miR-1 exacerbated I/R-induced injury. This study was to investigate theanti-apoptotic property of miR-1 inhibition and the potential regulatory mechanism. Results showed miR-1 expression reduced in the heart of rats undergoing myocardial I/R and the cardiomyocytes receiving hypoxia/reoxygenation (H/R) injury, but the serum miR-1 expression increased. The targets of miR-1 were predicted by cDNA microarray, and Bcl-2 and GADD45β were selected as candidate targets. Western blot assay and qPCR showed Bcl-2 and GADD45β protein and mRNA expressions increased after I/R injury and H/R injury. Bcl-2 was a direct target of miR-1 as shown in previous studies. Luciferase assay and Western blot assay revealed GADD45β was a direct target of miR-1, and miR-1 suppressed GADD45β expression via binding to its 3’UTR. Furthermore, miR-1 inhibition increased Bcl-2 expression and reduced IA/AAR (infarct area/area at risk) ratio and cell apoptosis in rats undergoing myocardial I/R as well as in cardiomyocytes receiving H/R injury. Importantly, Bcl-2 knockdown restored these consequences following miR-1 inhibition. However, GADD45β knockdown reduced IA/AAR ratio and cell apoptosis in vivo and in vitro, but failed torestore above consequences after miR-1 inhibition. In conclusion miR-1 inhibition protects against H/R-induced apoptosis of myocytes by directly targeting Bcl-2 but not GADD45β.
Keywords: microRNA-1, hypoxia/reoxygenation, Bcl-2, cardiomyocytes, apoptosis
Introduction
A variety of pathological processes, including myocardial ischemia/reperfusion (I/R) injury may cause hypoxia/reoxygenation (H/R), resulting in cellular injury and death [1,2]. Accumulating evidence shows that myocardial I/R injury may induce myocyte apoptosis [3,4], indicating that apoptosis plays an important role in the development of myocardial diseases. Therefore, the control of apoptosis might be a potential strategy for the treatment of myocardial I/R injury.
MicroRNAs (miRNAs) are a conserved family of small (~22 nt) non-coding RNA molecules that are able to regulate gene expression at the post-transcriptional level [5]. They can bind to the 3’untranslated region (3’UTR) of a target mRNA via the complementarity, and the extent of the base pair between miRNA and its target results in target mRNA cleavage or translation repression [5-7]. A previous study showed that approximately 60% of protein-coding genes were controlled by miRNAs [8], suggesting that miRNAs participate in a large number of biological processes, including cell growth, apoptosis and differentiation [9,10]. Studies have demonstrated that miRNAs play crucial roles in the development of myocardial diseases, including myocardial I/R injury [11]. For example, inhibition of miR-92a suppresses the I/R-induced cardiomyocytes apoptosis by targeting Smad7 [12]; miR-15b promotes H/R-induced apoptosis of cardiomyocytes by down-regulating Bcl-2 through the mitochondrial pathway [13]; miR-1 enhances myocardial I/R injury through increasing myocyte apoptosis in mouse models [14], indicating that miR-1 inhibition hasa therapeutic potential for the myocardial I/R injury. The present study was to investigate the effect of miR-1 inhibition on the I/R (H/R) induced apoptosis of myocytes both in vivo and in vitro, and to elucidate the potential regulatory mechanism. Here, we showed that miR-1 expression decreased in rats undergoing myocardial I/R injuryas well as in cardiomyocytes exposed to H/R, while miR-1 inhibition showed protective effects against I/R (H/R) induced apoptosis of myocytes. Bcl-2 and GADD45β were found to be two targets of miR-1, and Bcl-2, but not GADD45β, could mediate the effects of miR-1 on the I/R or H/R injury.
Materials and methods
Cell culture and transfection
Rat cardiomyocytes H9c2 and HL-1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10% fetal bovine serum (FBS, Gibco), 100 U/ml penicillin and 100 µg/ml streptomycinat 37°C with 5% CO2. miR-1 ASO and miR-1 mimics were introduced into cells in the presence of LipofectamineTM 2000 (Invitrogen) at a final concentration of 100 nM and 40 nM, respectively.
I/R injury model
Adult Sprague-Dawley (SD) rats (280±20 g, n=21) were randomly divided into I/R group, sham group and control group. Rats were anesthetized intraperitoneally with chloral hydrate at 3 µl/g. The I/R injury model was established.
H/R of H9c2 cardiomyocytes
Treated H9c2 cells were maintained in medium containing 1% FBS (low serum medium) followed by exposure to hypoxia (94% N2, 5% CO2 and 1% O2) for 6 h. Then, the medium was refreshed with 10% FBS-containing medium and cells were incubated in an environment with 95% air and 5% CO2 for reoxygenation for 12 h. Cells under normoxic conditions served as a control.
RNA isolation and qPCR
Total RNAs were extracted from cardiac tissues or cardiomyocytes using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Then, 500 ng of RNA was used for reverse transcription using the Reverse transcriptase and oligo (dT) or specific miR-1 primers. β-actin was used as an internal control to normalize Bcl-2 and GADD45β mRNA expressions, while U6 snRNA as an internal control to normalize miR-1 expression. Real time PCR was performed using the RealMasterMixkit (SYBR Green I) on an ABI 7300 real time system. Primers used in reverse transcription reaction and real time PCR are listed as follows: miR-1 reverse transcription primer: 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGATACACAC-3’; U6 reverse transcription primer: 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAAC-3’; miR-1 forward primer: 5’-ACACTCCAGCTGGGGTGTGGAATGTA-3’; miR-1 reverse primer: 5’-TGGTGTCGTGGAGTCG-3’; U6 forward primer: 5’-ACACTCCAGCTGGGGTGCTCGCTTCGGCAGCACA-3’: U6 reverse primer: 5’-AGGGTCCGAGGTATTC-3’; Bcl-2 sense: 5’-CGACTTTGCAGAGATGTCCA-3’; Bcl-2 antisense: 5’-ATGCCGGTTCAGGTACTCAG-3’. GADD45β sense: 5’-GAGGCGGCCAAACTGATGAAT-3’; GADD45β antisense: 5’-CGCAGCAGAACGACTGGAT-3’; β-actin sense: 5’-GTCCACCGCAAATGCTTCTA-3’, β-actin antisense: 5’-TGCTGTCACCTTCACCGTTC-3’.
Northern blot assay
Total RNAs were isolated using miRNeasy Mini Kit (Qiagen) from rat cardiac tissues after I/R. The concentration and quality of RNA were determined using the NanoDrop ND-2000. Then, 10 μg of RNA was separated by 15% denaturing polyacrylamide gel Electrophoresis (Invitrogen), transferred to nylon membrane through a capillary or vacuum blotting system, fixed by UV or heat, and finally hybridized with labeled probes using UltraHyb-Oligo buffer (Ambion). The membrane was washed in 2× SSC buffer (0.1% SDS) and visualized.
cDNA microarray assay
Total RNAs isolated from rat cardiac tissues after I/R as well as rat cardiomyocytes after H/R were used for cDNA microarray assay with the GeneChip® Rat Genome 230 2.0 Array. Each sample was performed in triplicates and the value of gene expression represented a mean of three experiments.
Western blot assay
Western blot assay was performed to determine the protein expressions of Bcl-2 and GADD45β in rat cardiac tissues and cardiomyocytes after I/R and H/R, respectively. Briefly, cardiac tissues or cardiomyocytes were lysed in RIPA buffer (150 mM NaCl, 1.0% NP-40 or 0.1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 50 mM Tris-HCl pH 8.0) containing protease inhibitors. Then, 50 µg of protein was separated on a 12% sodium dodecyl sulphate-polyacrylamide gelelectrophoresis, and transferred onto a PVDF membrane which was blocked in 5% milk and incubated with mouse anti-Bcl-2 or rabbit anti-GADD45β antibodies, followed by incubation with secondary anti-IgG HRP-conjugated antibodies. Finally, the protein bands were visualized using the enhanced chemiluminescence (ECL) kit. GAPDH served as an internal control.
Plasmid construction and luciferase assay
GADD45β 3’UTR containing the binding sites for miR-1 was amplified by PCR and inserted downstream of the luciferase reporter gene. In addition, the mutant GADD45β 3’UTR reporter gene in which several nucleotides within the binding site were mutated was generated using a QuikChange® II Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s instructions. For luciferase assay, the cardiomyocytes were co-transfected with miR-1 ASO or mimic with reporter gene containing wild-type or mutated GADD45β 3’UTR. After transfection for 48 h, cells were harvested and the luciferase intensity was measured using a Dual Luciferase Reporter Gene Assay kit according to the manufacturer’s instructions. Renilla luciferase intensity served as a control to normalize the firefly luciferase intensity.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
Apoptotic cells were measured by TUNEL assay (Roche) according to the manufacturer’s protocols. Briefly, the treated cardiac tissues or cardiomyocytes were fixed in 4% methanol free HCHO for 20 min at room temperature, permeablized in 3-50 μg/ml PK in PBS or the permeabilisation solutionfor 10 min at room temperature, and finally labelled for 60 min at 37°C in a humidified environment indark. After TUNEL staining, sections were incubated with DAPI solution to stain nuclei for 5 min. Apoptotic nuclei were stained red, while normal nucleiblue.
Statistical analysis
All the data are expressed as mean ± standard deviation from three independent experiments. The difference between two groups was analyzed by using two-tailed Student’s t-test. A value of P<0.05 was considered statistically significant.
Results
miR-1 is down-regulated in response to rat myocardial I/R injury
To investigate the roles of miR-1 in the responses to myocardial I/R injury, the miR-1 expression was detected by qPCR in rats of I/R group, sham group and control group. As shown in Figure 1A, results showed miR-1 expression significantly decreased in I/R rats when compared with sham group and control group. Similar findings were observed after Northern blot assay (Figure 1B). However, the serum miR-1 expression increased in I/R rats (Figure 1C). In addition, miR-1 expression was also determined by qPCR in rat myocardiocytes after H/R. In accordance with above results, miR-1 expression was down-regulated in myocardiocytes exposed to H/R (Figure 1D).
Figure 1.
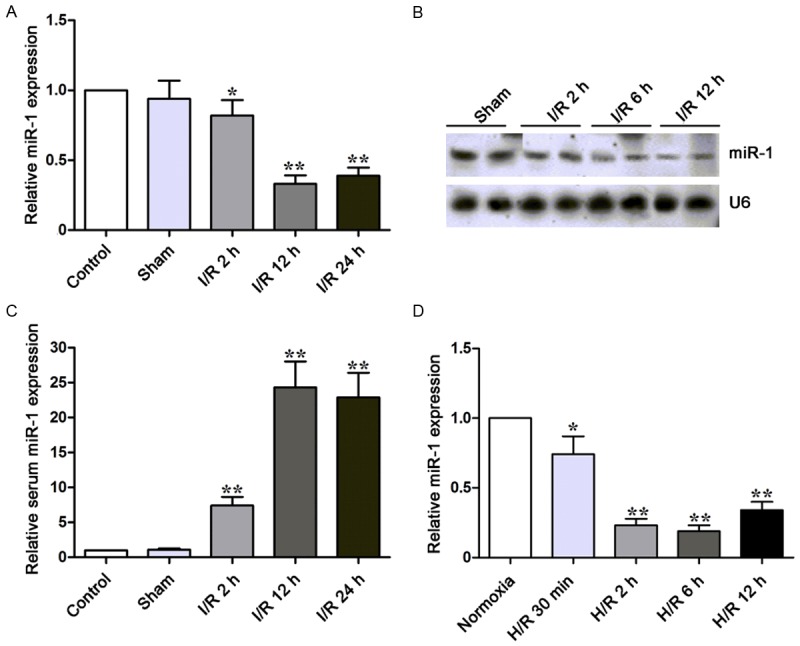
miR-1 expression is down-regulated in I/R rats and H9c2 cells exposed to H/R. (A, B) miR-1 expression was detected by qPCR (A) and Northern blot assay (B) inrats at indicated time points. miR-1 expression decreased in I/R rats as compared to sham group. (C) Serum miR-1 expression was detected by qPCR in rats. Serum miR-1 expression increased in ratsundergoing I/R injury. (D) miR-1 expression was detected in H9c2 cells. U6 was an internal control. *P<0.05, **P<0.01.
Prediction of miR-1 targets in rat I/R injury model
miR-1 targets were predicted by using cDNA microarray assay. Figure 2A showed that the expressions of several genes were up-regulated after I/R or H/R, inverse to the change in miR-1 expression. Therefore, genes with up-regulated expressions were selected as candidate targets. Of these genes, Bcl-2 has been found as a target of miR-1 in a previous study [15]. Western blot assay and qPCR confirmed that Bcl-2 and GADD45β protein and mRNA expressions significantly increased in I/R ratsas compared to sham group (Figure 2B and 2C). Similar findings were observed in cardiomyocytes exposed to H/R (Figure 2D and 2E). Therefore, we further investigated the relationship between GADD45β expression and miR-1 expression.
Figure 2.
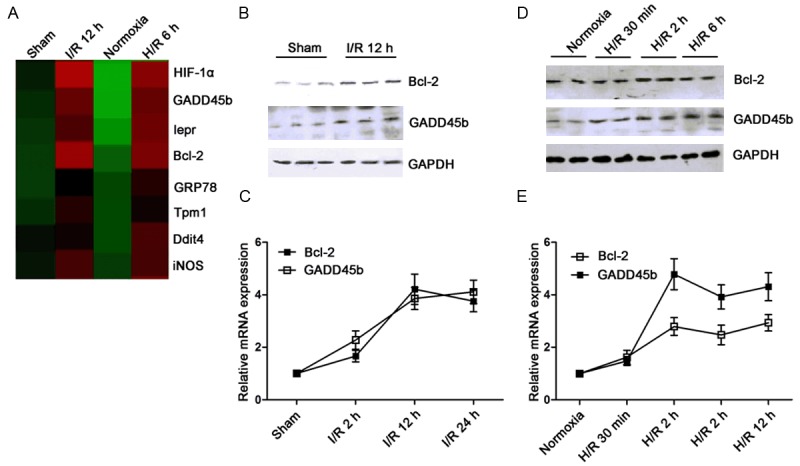
Prediction of candidate targets for miR-1. (A) cDNA array was performed to predict targets for miR-1. The dysregulated genes are shown in the heat map. “Red” represents up-regulated genes, while green represents down-regulated genes. (B, C) Bcl-2 and GADD45β protein and mRNA expressions were detected by Western blot assay (B) and qPCR (C). Bcl-2 and GADD45β expressions were up-regulated in I/R rats as compared to sham group. (D, E) Bcl-2 and GADD45β protein and mRNA expressions were detected by Western blot assay (D) and qPCR (E) in H9c2 cells. GAPDH and U6 served as internal controls.
GADD45β is a direct target of miR-1
Figure 3A showed that there was a binding site for miR-1 on the 3’UTR of GADD45β mRNA, and the seed sequence of miR-1 was conservative among species. A luciferase reporter gene containing GAD45β 3’UTR as well as mutant 3’UTR were constructed and both had mutated binding sites. The luciferase assay was then preformed in H9c2 cells co-transfected with either miR-1 or miR-1 ASO and wild-type or mutant GADD45β 3’UTR construct. As shown in Figure 3B, results showed that miR-1 reduced the luciferase intensity controlled by GADD45β 3’UTR, while miR-1 inhibition increased GADD45β intensity. However, neither miR-1 nor miR-1 inhibition affected the intensity of mutant GADD45β 3’UTR (Figure 3B). These results demonstrate that GADD45β is a direct target of miR-1. To investigate the effect of miR-1 on the GADD45β expression, GADD45β expression was measured by Western blot assay in H9c2 cells and HL-1 cells transfected with an increasing dose of miR-1 mimics or miR-1 ASO. As shown in Figure 3C and 3D, results showed that miR-1 over-expression inhibited GADD45β protein expression in a dose-dependent manner, while miR-1 inhibition increased it. Taken together, these findings indicate that miR-1 directly suppresses GADD45β expression via binding to its 3’UTR.
Figure 3.
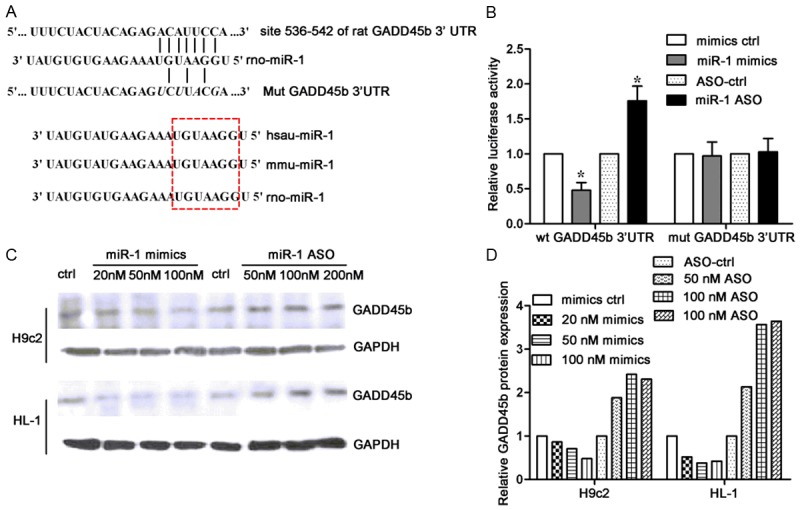
GADD45β is a direct target for miR-1. A. Sequence alignment of rat GADD45β 3’UTR and miR-1. Several bases within the binding site were mutated as shown in italic. The seed sequence in miR-1 was conservative among species. B. H9c2 cells were co-transfected with either miR-1 or miR-1 ASO and luciferase reporter construct controlled by WT or mutant GADD45β 3’UTR. Luciferase assay was performed to detect the effect of miR-1 on the intensity of GADD45β 3’UTR. C. H9c2 cells and HL-1 cells transfected with increasing concentrations of miR-1 mimics or ASO were subjected to Western blot assay to determine GADD45β protein expression. GAPDH was employed as a loading control. D. Relative GADD45β protein expression in different groups.
Silencing of Bcl-2 restores the effects of miR-1 inhibition on I/R-induced apoptosis
Since Bcl-2 is a target of miR-1, whether Bcl-2 is involved in the effects of miR-1 on I/R-induced apoptosis was further investigated. Firstly, Bcl-2 silencing was confirmed in myocardial I/R rats, and found to suppress the increased Bcl-2 expression following miR-1 inhibition (Figure 4A). In addition, miR-1 inhibition reduced the IA/AAR (infarct area/area at risk) ratio, while Bcl-2 knockdown increased it after miR-1 inhibition (Figure 4B). In addition, the apoptotic cells in myocardial tissues decreased after miR-1 ASO was introduced, while in the presence of Bcl-2 knockdown and miR-1 ASO, the apoptotic cells significantly increased. Secondly, the effect of Bcl-2 on the miR-1-induced apoptosis was also examined in H9c2 cells exposed to H/R. As shown in Figure 4D, Bcl-2 knockdown reduced its protein expression following miR-1 inhibition. Moreover, Bcl-2 knockdown increased apoptotic H9c2 cells that were inhibited by miR-1 inhibition. Taken together, these results suggest that miR-1 inhibitionprotects myocardium against I/R and H/R injury, but Bcl-2 knockdown blocks this effect.
Figure 4.
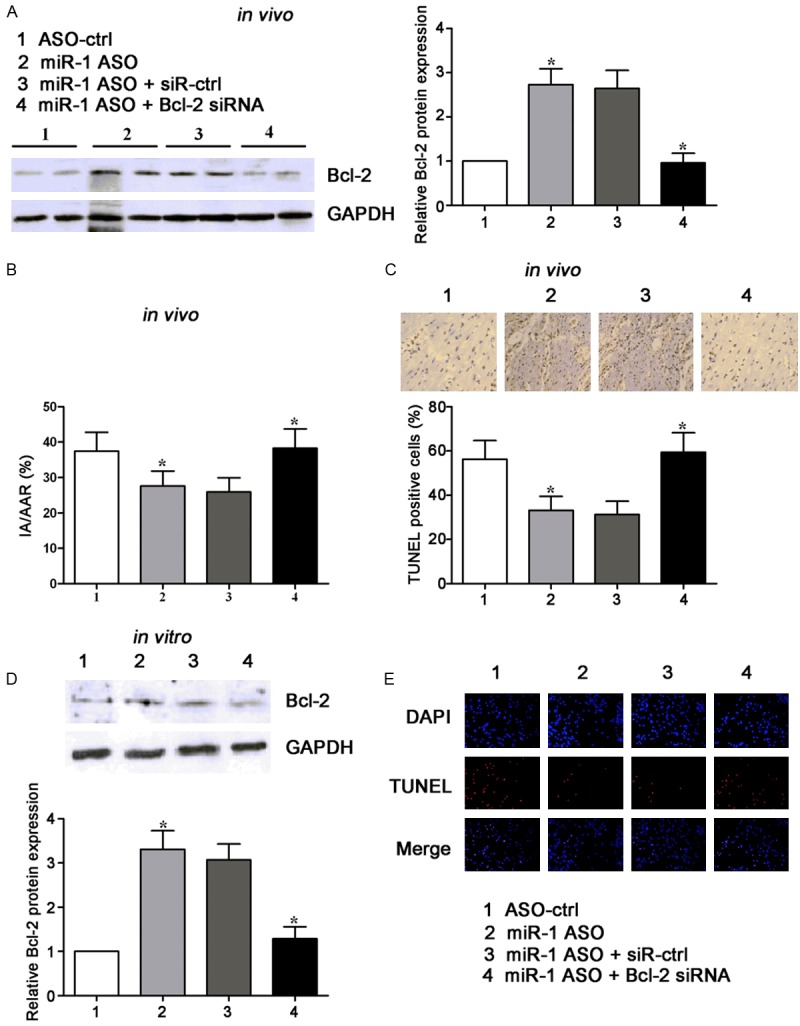
miR-1 inhibition promotes Bcl-2 expression and regulates cardiac infarct area of rats and cardiomyocytes apoptosis after I/R or H/R. A. Western blot assay of Bcl-2 protein expression in rats treated with miR-1 ASO, or co-treated with miR-1 ASO and siRNA against Bcl-2, together with controls. GAPDH wasa loading control. B. Statistical analysis of IA/AAR ratio in rats treated with miR-1 ASO, or co-treated with miR-1 ASO and siRNA against Bcl-2. IA, infarct area; AAR, area at risk. C. Effects of miR-1 inhibition on the apoptotic cells in cardiac tissues subjected to I/R were investigated by TUNEL staining. Graph (below) represents the percentage of apoptotic cells. D. Western blot assay of Bcl-2 protein expression in cardiomyocytes transfected with miR-1 ASO, or co-treated with miR-1 ASO and siRNA against Bcl-2. GAPDH wasa loading control. E. Effects of miR-1 inhibition on the apoptosis of cardiomyocytes subjected to H/R injury were investigated by TUNEL staining. Apoptotic cells were stainedred. *P<0.05.
Silencing of GADD45β fails to restore the effects of miR-1 inhibition on I/R-induced apoptosis
Finally, whether GADD45β mediates the effect of miR-1 on I/R-induced injury was further investigated. GADD45β knockdown was confirmed by Western blot assay in myocardial I/R rats, and it reduced the increased GADD45β protein expression followed miR-1 inhibition (Figure 5A). In addition, GADD45β knockdown reduced IA/AAR ratio (Figure 5B) and apoptotic cells in myocardial tissues exposed to I/R injury (Figure 5C). However, GADD45β failed restore the IA/AAR ratio and apoptotic cells in myocardial tissue that were reduced by miR-1 inhibition. Similar results were observed in myocardiocytes exposed to H/R (Figure 5D and 5E). Overall, these findings indicate that miR-1 exerts its protective effects on I/R injury in a GADD45β independent manner, although GADD45β is a direct target of miR-1.
Figure 5.
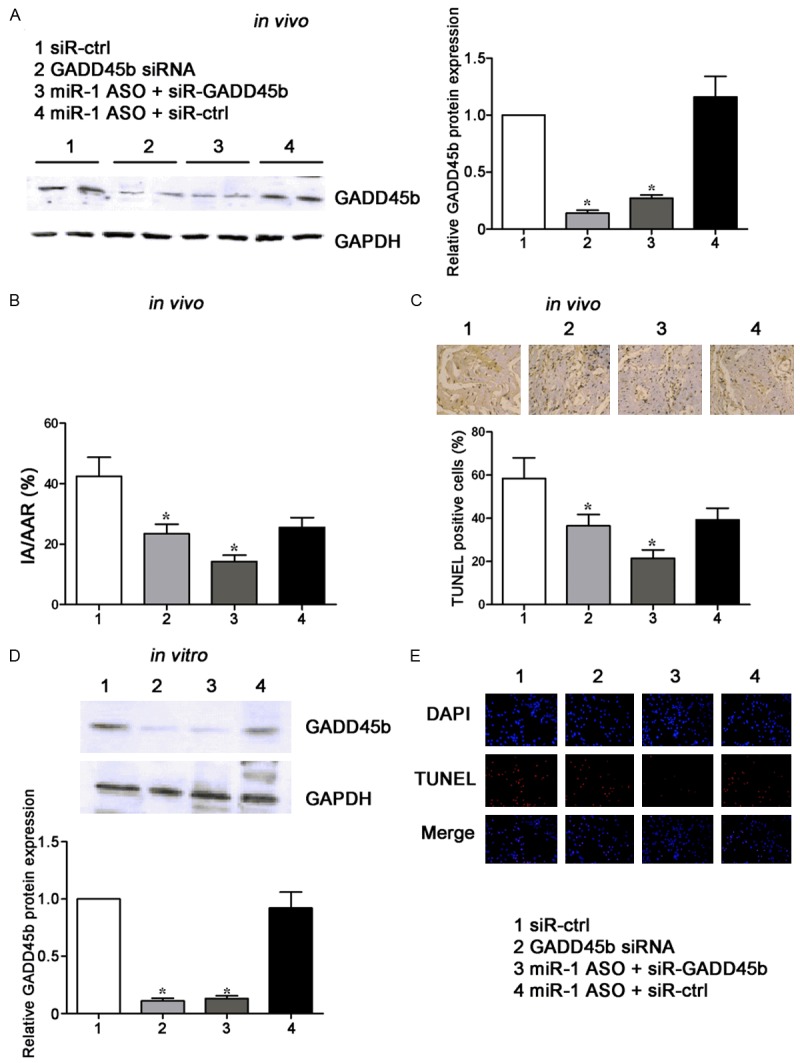
GADD45β knockdown fails to restore the effects of miR-1 inhibition on the cardiac infarct area and cardiomyocytes apoptosis after I/R or H/R injury. A. Western blot assay of GADD45β protein expression in rats treated with GADD45β siRNA, or co-treated with miR-1 ASO and siRNA against GADD45β. GAPDH wasa loading control. B. Statistical analysis of IA/AAR ratio in rats treated with GADD45β siRNA, or co-treated with miR-1 ASO and siRNA against GADD45β. IA, infarct area; AAR, area at risk. C. Effects of miR-1 inhibition on the apoptotic cells in cardiac tissues subjected to I/R injury were investigated by TUNEL staining. Graph (below) represents the percentage of apoptotic cells. D. Western blot assay of GADD45β protein expression in cardiomyocytes transfected with GADD45β siRNA, or co-treated with miR-1 ASO and siRNA against GADD45β. GAPDH wasa loading control. E. Effects of miR-1 inhibition on the apoptosis of cardiomyocytes subjected to H/R injury were investigated by TUNEL staining. Apoptotic cells were stainedred. *P<0.05.
Discussion
Studies have demonstrated that miR-1 plays a crucial role in the development of cardial diseases. β-blocker propranolol exerts protective effect against myocardial ischemic arrhythmogenesis partially viadown-regulating miR-1, indicating the cardioprotection of miR-1 inhibition against myocardial ischemia [16]. Tanshinone IIA suppresses ischemic arrhythmias and cardiac mortality by down-regulating miR-1 in a rat model, suggesting miR-1 may serve as a potential target for the prevention of ischemic arrhythmias [17]. In addition, plasma miR-1 expression is at a low level, while itsignificantly increases in MI patients and rats models [18]. Inrat acute myocardial infarction (AMI) model, serum miR-1 expression significantly increasedand its expression was positively related to the myocardial infarct size and serum creatine kinase-MB, implying that serum miR-1 may become a diagnostic biomarker for AMI [19]. miR-1 expression is down-regulated in MI patients and rats with myocardial I/R injury [20,21]. miR-1 participates in the H2S protection of myocardiocytes against I/R injury-induced apoptosis by regulating Bcl-2, suggesting that miR-1 plays important roles in the myocardial I/R injury [22]. In mouse models, miR-1 over-expression aggravates I/R injury by increasing apoptosis, while miR-1 inhibition attenuates cardiac I/R injury [14]. Our findings also showed that miR-1 expression was down-regulated in myocardial I/R rats and rat cardiomyocytes exposed to H/R, but serum miR-1 increased. Functional studies indicated that miR-1 inhibition reduced apoptotic cells in cardial tissues exposed to I/R as well as apoptotic cardiomyocytes exposed to H/R. In addition, miR-1 inhibition reduced IA/AAR ratio in I/R rats. These results suggest that miR-1 inhibition may serve a potential strategy for the therapy of I/R injury.
miRNAs function by regulating a number of targets via binding to their 3’UTR [5]. In the current study, miR-1 targets were predicted by cDNA microarray assay. Among the dysregulated genes, Bcl-2 and GADD45β were selected as candidate targets and both showed up-regulated expressions following I/R injury and H/R. Findings from cDNA microarray assay were confirmed by Western blot assay and qPCR. Bcl-2 has been found as a target of miR-1 [15]. Our study confirmed that miR-1 inhibition increased Bcl-2 protein expression. Bcl-2 is a member of Bcl-2 familythat either induces or inhibits cell apoptosis [23]. Bcl-2 is known as an important anti-apoptotic protein and involved in a variety of biological processes [24-26]. Increased Bcl-2 expression was found to contribute to the protective effect of high-dose fasudil pre-treatment on the myocardial I/R injury [27]. Bcl-2 expression was also up-regulated during the process that Panax quinquefolium sapnin reduced apoptotic cardiomyocytes exposed to I/R [28]. Studies also confirm that Bcl-2 is protective against myocardial I/R injury. In our study, results indicated that silencing of Bcl-2 increased apoptotic cells in myocardial tissues and apoptotic cardiomyocytes and elevated IA/AAR ratio all of which were inhibited by miR-1 inhibition, suggesting that Bcl-2 knockdown aggravates myocardial I/R injury and miR-1 inhibition is protective against I/R and H/R by up-regulating Bcl-2 expression.
Besides Bcl-2, GADD45β was also found as another target of miR-1. Luciferase assay showed miR-1 inhibited the intensity of GADD45β 3’UTR, while miR-1 inhibition increased it. When the binding site for miR-1 was mutated, the inhibitory effect of miR-1 on GADD45β 3’UTR intensity was abolished. In addition, Western blot assay showedmiR-1 reduced GADD45β protein expression in a dose-dependent manner, while miR-1 inhibition increased it. GADD45β is a member of growth arrest DNA damage-inducible gene (GADD45) family. There is evidence showing that GADD45β promotes sorafenib-induced apoptosis in hepatocellular carcinoma cells [29]. GADD45β contributes to p53 activation and is able to regulate cell cycle and apoptosis [30]. Our findings also revealed that GADD45β knockdown reduced apoptotic cells in cardial tissues and apoptotic cardiomyocytes after I/R (H/R) injury. However, GADD45β knockdown failed to restore the reduction of apoptosis and IA/AAR ratio following miR-1 inhibition. These results indicate that although GADD45β is a direct target of miR-1, miR-1 protects against I/R injury not through regulating GADD45β. There may exist other targets for miR-1 that mediate the effects of miR-1 on I/R injury.
In conclusion, our results show miR-1 inhibition is protective against I/R (H/R) injury by targeting Bcl-2 but not GADD45β. This suggests that anti-miR-1 may become a therapeutic strategy for myocardial I/R injury.
Acknowledgements
The study was supported by 2015 Medical and Health Science and Technology Research Fund of Zhejiang Province (2015KYB387); 2015 Traditional Chinese Medicine Scientific Research Fund of Zhejiang Province (2015ZA203); Jiaxing Cardiovascular Key Discipline Fund (2014-F07); Chronic Diseases Base Fund of Zhejiang Province (2013-JX); JiaxingKey Innovation Team Fund (2015-CX1).
Disclosure of conflict of interest
None.
References
- 1.Wang X, Zhou Y, Kim HP, Song R, Zarnegar R, Ryter SW, Choi AM. Hepatocyte growth factor protects against hypoxia/reoxygenationinduced apoptosis in endothelial cells. J Biol Chem. 2004;279:5237–5243. doi: 10.1074/jbc.M309271200. [DOI] [PubMed] [Google Scholar]
- 2.Kim BM, Chung HW. Hypoxia/reoxygenation induces apoptosis through a ROSmediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem Biophys Res Commun. 2007;363:745–750. doi: 10.1016/j.bbrc.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Abbate A, Bussani R, Amin MS, Vetrovec GW, Baldi A. Acute myocardial infarction and heart failure: role of apoptosis. Int J Biochem Cell Biol. 2006;38:1834–1840. doi: 10.1016/j.biocel.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Freude B, Masters TN, Robicsek F, Fokin A, Kostin S, Zimmermann R, Ullmann C, Lorenz-Meyer S, Schaper J. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol. 2000;32:197–208. doi: 10.1006/jmcc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci U S A. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang LY, Liu M, Li X, Tang H. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3) J Biol Chem. 2013;288:4035–4047. doi: 10.1074/jbc.M112.410506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 10.Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13:790–795. doi: 10.1016/s0960-9822(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 11.Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics. 2011;43:534–542. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Zhou M, Li C, Zhou J, Li H, Zhu D, Wang Z, Chen A, Zhao Q. MicroRNA-92a inhibition attenuates hypoxia/reoxygenationinduced myocardiocyte apoptosis by targeting Smad7. PLoS One. 2014;9:e100298. doi: 10.1371/journal.pone.0100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Zhang G, Liang Z, Liu X, Li T, Fan J, Bai J, Wang Y. MicroRNA-15b enhances hypoxia/reoxygenation-induced apoptosis of cardiomyocytes via a mitochondrial apoptotic pathway. Apoptosis. 2014;19:19–29. doi: 10.1007/s10495-013-0899-2. [DOI] [PubMed] [Google Scholar]
- 14.Pan Z, Sun X, Ren J, Li X, Gao X, Lu C, Zhang Y, Sun H, Wang Y, Wang H, Wang J, Xie L, Lu Y, Yang B. miR-1 exacerbates cardiac ischemiareperfusion injury in mouse models. PLoS One. 2012;7:e50515. doi: 10.1371/journal.pone.0050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50:377–387. doi: 10.1536/ihj.50.377. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, Zhang Y, Shan H, Pan Z, Li X, Li B, Xu C, Zhang B, Zhang F, Dong D, Song W, Qiao G, Yang B. MicroRNA-1 downregulation by propranolol in a rat model of myocardial infarction: a new mechanism for ischaemic cardioprotection. Cardiovasc Res. 2009;84:434–441. doi: 10.1093/cvr/cvp232. [DOI] [PubMed] [Google Scholar]
- 17.Shan H, Li X, Pan Z, Zhang L, Cai B, Zhang Y, Xu C, Chu W, Qiao G, Li B, Lu Y, Yang B. Tanshinone IIA protects against sudden cardiac death induced by lethal arrhythmias via repression of microRNA-1. Br J Pharmacol. 2009;158:1227–1235. doi: 10.1111/j.1476-5381.2009.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M, Micheli B, Biglioli P, Achilli F, Martelli F, Maggiolini S, Marenzi G, Pompilio G, Capogrossi MC. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y, Tan N, Yang J, Liu X, Cao X, He P, Dong X, Qin S, Zhang C. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci (Lond) 2010;119:87–95. doi: 10.1042/CS20090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, Xie B, Gao XG, Wang YW. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci. 2011;18:22. doi: 10.1186/1423-0127-18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostjancic E, Zidar N, Stajer D, Glavac D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115:163–169. doi: 10.1159/000268088. [DOI] [PubMed] [Google Scholar]
- 22.Kang B, Hong J, Xiao J, Zhu X, Ni X, Zhang Y, He B, Wang Z. Involvement of miR-1 in the protective effect of hydrogen sulfide against cardiomyocyte apoptosis induced by ischemia/reperfusion. Mol Biol Rep. 2014;41:6845–6853. doi: 10.1007/s11033-014-3570-2. [DOI] [PubMed] [Google Scholar]
- 23.Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 24.Low IC, Loh T, Huang Y, Virshup DM, Pervaiz S. Ser70 phosphorylation of Bcl-2 by selective tyrosine nitration of PP2A-B56delta stabilizes its antiapoptotic activity. Blood. 2014;124:2223–2234. doi: 10.1182/blood-2014-03-563296. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Yan Q, Liu S, Yang X. Knockdown of EpCAM enhances the chemosensitivity of breast cancer cells to 5-fluorouracil by downregulating the antiapoptotic factor Bcl-2. PLoS One. 2014;9:e102590. doi: 10.1371/journal.pone.0102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leiber A, Graf B, Spring B, Rudner J, Kostlin N, Orlikowsky TW, Poets CF, Gille C. Neonatal monocytes express antiapoptotic pattern of Bcl-2 proteins and show diminished apoptosis upon infection with Escherichia coli. Pediatr Res. 2014;76:142–149. doi: 10.1038/pr.2014.74. [DOI] [PubMed] [Google Scholar]
- 27.Li WN, Wu N, Shu WQ, Guan YE, Jia DL. The protective effect of fasudil pretreatment combined with ischemia postconditioning on myocardial ischemia/reperfusion injury in rats. Eur Rev Med Pharmacol Sci. 2014;18:2748–2758. [PubMed] [Google Scholar]
- 28.Li D, Liu M, Tao TQ, Song DD, Liu XH, Shi DZ. Panax quinquefolium saponin attenuates cardiomyocyte apoptosis and opening of the mitochondrial permeability transition pore in a rat model of ischemia/reperfusion. Cell Physiol Biochem. 2014;34:1413–1426. doi: 10.1159/000366347. [DOI] [PubMed] [Google Scholar]
- 29.Ou DL, Shen YC, Yu SL, Chen KF, Yeh PY, Fan HH, Feng WC, Wang CT, Lin LI, Hsu C, Cheng AL. Induction of DNA damage-inducible gene GADD45beta contributes to sorafenib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res. 2010;70:9309–9318. doi: 10.1158/0008-5472.CAN-10-1033. [DOI] [PubMed] [Google Scholar]
- 30.Salvador JM, Brown-Clay JD, Fornace AJ Jr. Gadd45 in stress signaling, cell cycle control, and apoptosis. Adv Exp Med Biol. 2013;793:1–19. doi: 10.1007/978-1-4614-8289-5_1. [DOI] [PubMed] [Google Scholar]


