Abstract
Osteoarthritis (OA) is the most common joint degenerative disease affecting the joint structure, leading to loss of joint function and tissue destruction. Recent studies have demonstrated that miRNAs are involved in many pathological conditions, including OA. The study was to investigate the role of miR-411 in the pathogenesis of OA. The expression of miR-411 was downregulated in OA cartilage compared with in normal cartilage. Conversely, the expression of MMP-13 was upregulated in OA cartilage compared with in normal cartilage. IL-1β treatment repressed miR-411 expression in chondrocytes. Moreover, we identified MMP-13 as a direct target gene of miR-411 in chondrocytes and overexpression of miR-411 inhibited the MMP-13 expression. Furthermore, overexpression of miR-411 increased the expression of type II collagen and type IV collagen expression in chondrocytes. MiR-411 is a crucial regulator of MMP-13 in chondrocytes and may response to the development of OA.
Keywords: Osteoarthritis, chondrocytes, miRNA, miR-411
Introduction
Osteoarthritis (OA) is a degenerative disease that causes physical disability among older persons worldwide [1,2]. Available treatments are limited to pain care and joint replacement surgery in endstage OA patients [3,4]. The etiology of osteoarthritis (OA) is complex, including genetic predisposition, failure of nutrient supply, abnormal mechanical loading and trauma [5,6]. OA is characterized by imbalance between extracellular matrix (ECM) synthesis and degradation and resulted loss of movement, joint pain, and progressive dysfunction [1,7]. Among these, MMP-13 has gained the most interest, due to its capacity to degrade collagens along with a wide range of matrix molecules [8-10]. Further investigation and understanding of OA pathology are needed and important to develop effective therapeutic targets to control OA.
MicroRNAs (miRNAs) are a class of 17-25 nucleotide small endogenous noncoding RNAs which regulate gene expression primarily through base paring to the 3’untranslated region (UTR) of target mRNAs (mRNAs) at posttranscriptional levels. MiRNAs leads to translation repression or mRNA cleavage [11-15]. Recent evidence has demonstrated that miRNAs play crucial role in cell development, proliferation, migration, invasion and differentiation [16-19]. The physiologic and pathogenic role of miRNAs in the maintenance of joint homeostasis and the development of arthritis is currently being elucidated [20-23]. Recent reports have also described a correlation of MMP-13 with specific miRNAs, such as miR-140, miR-126-5p and miR-27a [24-26].
In this study, miR-411 was significantly downregulated in OA cartilage compared with in normal cartilage. Conversely, MMP-13 was upregulated in OA cartilage compared with in normal cartilage. IL-1β treatment repressed miR-411 expression in chondrocytes. Moreover, we identified MMP-13 as a direct target gene of miR-411 in chondrocytes and overexpression of miR-411 inhibited the MMP-13 expression. Furthermore, overexpression of miR-411 increased the type II collagen and type IV collagen expression in chondrocytes.
Materials and methods
Specimen selection and cell culture
OA cartilage samples were isolated from 10 patients with OA (ages 56-64 years) who underwent total knee replacement surgery. The diagnosed of patients were made according to the American College of Rheumatology criteria. Normal knee cartilage was obtained from 10 patients who underwent amputation due to trauma with no history of rheumatoid arthritis or OA. All patients have given written informed consent and agreed to involve in this study. The consent and study was approved by the ethical board of the institute of Affiliated Hospital of Jining Medical College and complied with Declaration of Helsinki.
Oligonucleotides, cell culture and transfections and cell proliferation
The human immortalized juvenile costal chondrocytes cell line C28/I2 was cultured in DMEM/F12 according previously protocol [27,28]. miR-411 mimics and scramble were synthesized by GenePharma (Shanghai, China) and were transfected into the cells with DharmaFECT1 reagent (Dharmacon, TX, USA) with a final oligonucleotide concentration of the 20 nmol/L. Cell proliferation was performed using the CCK-8 (Dojin Laboratories, Japan) at different time after transfection. Absorbance was measured at 450 nm.
RNA extraction and quantitative RT-PCR
Total RNA was extracted using the mirVana miRNA isolation kit (Ambion). miR-411 expression was detected by TaqMan qRT-PCR with TaqMan microRNA assay kits (Ambion) according to manufacturer’s instruction. The expression of miR-411 was normalized to U6 expression. The expression levels of MMP13, Ki-67, type II collagen and type IV collagen were measured by SYBR Green qRT-PCR. Primer sequences are shown in Table S1.
Dual luciferase assays
Cells were transfected with reporter construct, pGL-3 control vector, and either miR-411 or a scramble. The luciferase values were measured by the Dual Luciferase Assay following to manufacturer’s suggestion. Firefly luciferase value was normalized to the Renilla signal and the ratio of the Firefly/Renilla values was detected.
Western blot
Western blot was done according to standard methods. Proteins were resolved by 12% SDS-PAGE and transferred to PVDF membrane (Millipore). Membranes were blocked with 5% milk and probed with primary antibody (MMP-1, type II collagen and type IV collagen, Abcam) at a 1:2000 dilution. After washing, the membranes were measured using HRP-conjugated secondary antibodies and visualized by an enhanced chemiluminescence kit.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 software (IBM). Data are expressed as the mean±SD and were analyzed using one-way ANOVA or Student’s t test. P<0.05 was considered statistically significant.
Results
miR-411 was downregulated in OA cartilage and upregulated in OA cartilage
As shown in Figure 1A, the expression of miR-411 was downregulated in OA cartilage compared with in normal cartilage. Conversely, the expression of MMP-13 was upregulated in OA cartilage compared with in normal cartilage (Figure 1B).
Figure 1.
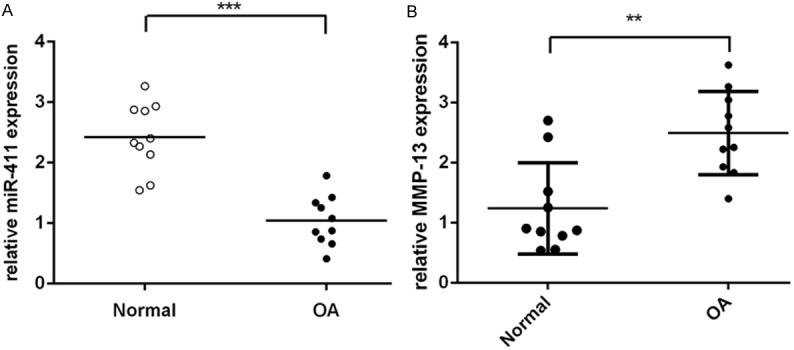
The expression of miR-411 was downregulated in OA cartilage and the expression of MMP-13 was upregulated in OA cartilage. A. The expression of miR-411 was measured in OA cartilage and normal cartilage using qRT-PCR. B. The expression of MMP-13 was measured in OA cartilage and normal cartilage used qRT-PCR.
IL-1β treatment decreased expression of miR-411 and increases MMP-13 expression in chondrocytes
IL-1β (10 ng/ml) treatment repressed miR-411 expression in chondrocytes (Figure 2A). In parallel with the decrease of miR-411 expression, treatment of IL-1β stimulated MMP-13 expression (Figure 2B).
Figure 2.
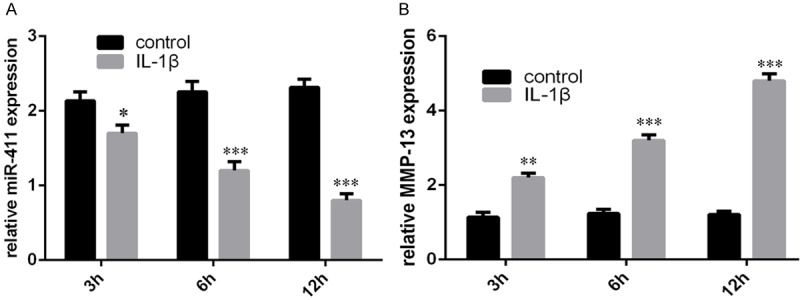
IL-1β treatment decreased expression of miR-411 and increases MMP-13 expression in chondrocytes. A. The expression of miR-411 was measured after treated by IL-1β (10 ng/ml) using qRT-PCR. B. The expression of MMP-13 was measured after treated by IL-1β (10 ng/ml) using qRT-PCR.
MMP-13 is a direct target of miR-411
We found a potential miR-411 binding sequence in the 3’UTR of MMP-13 by using miRNA target prediction software (Figure 3A). Overexpression of miR-411 inhibited the protein expression of MMP-13. Luciferase reporter assay showed that treatment with miR-411 mimic repressed reporter activity (Figure 3C).
Figure 3.
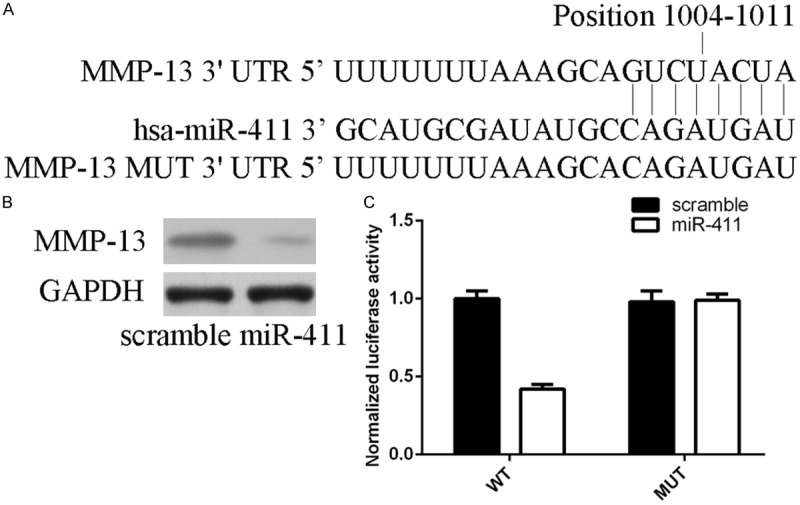
MMP-13 is a direct target of miR-411. A. The 3’UTR of MMP-13 mRNA contains a putative site partially complementary to miR-411. B. Overexpression of miR-411 inhibited the protein expression of MMP-13. C. Overexpression of miR-411 inhibited the luciferase activity of the WT reporter plasmid, but not the MUT reporter plasmid.
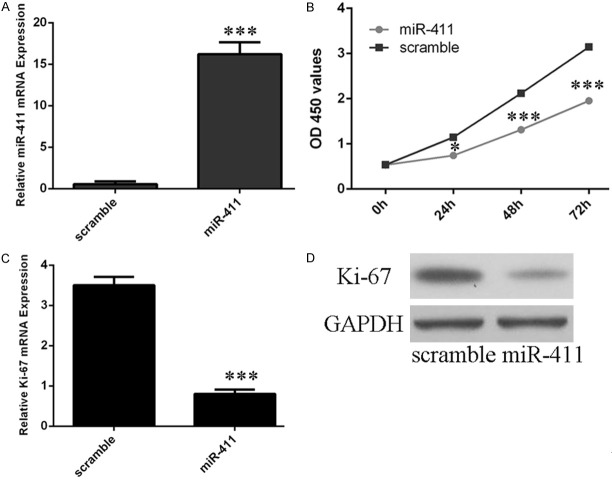
Overexpression of miR-411 inhibited chondrocytes cell proliferation
qRT-PCR analysis demonstrated that miR-411 mimic can enhance the miR-411 expression in chondrocytes (Figure 4A). CCK8 analysis showed that overexpression of miR-411 inhibited chondrocytes cell proliferation (Figure 4B). qRT-PCR and western blot analysis demonstrated overexpression of miR-411 inhibited mRNA and protein expression of Ki-67 in chondrocytes (Figure 4C and 4D).
Figure 4.
Overexpression of miR-411 inhibited chondrocytes cell proliferation. A. The expression of miR-411 was measured using qRT-PCR after transfection miR-411 mimic or scramble. B. CCK8 analysis showed that overexpression of miR-411 inhibited chondrocytes cell proliferation. C. Overexpression of miR-411 inhibited Ki-67 mRNA expression. D. Overexpression of miR-411 inhibited Ki-67 protein expression.
Overexpression of miR-411 increased the expression of the type II collagen and type IV collagen
RT-PCR and western blot analysis demonstrated overexpression of miR-411 increased mRNA and protein expression of type II collagen in chondrocytes (Figure 5A and 5B). Overexpression of miR-411 also enhanced mRNA and protein expression of type IV collagen in chondrocytes (Figure 5C and 5D).
Figure 5.
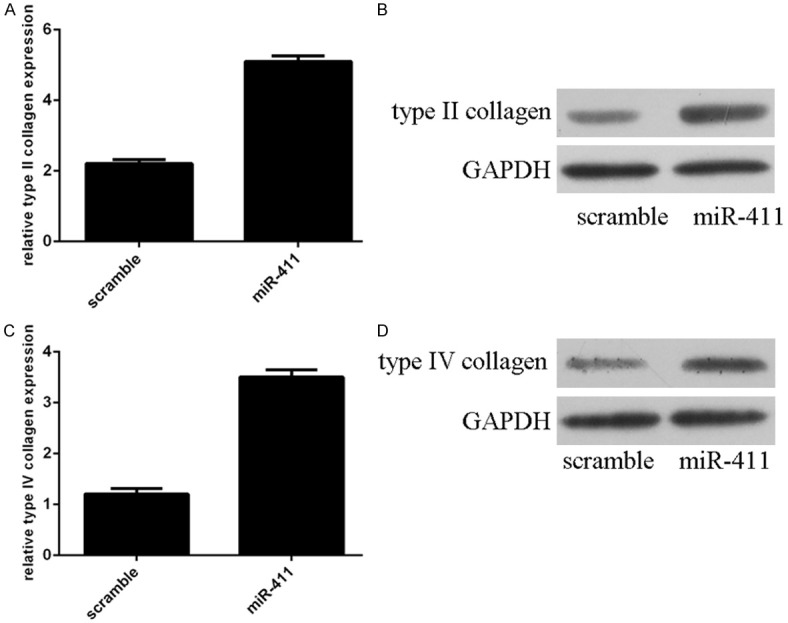
Overexpression of miR-411 increased the type II collagen and type IV collagen expression. A. Overexpression of miR-411 promoted mRNA expression of type II collagen in chondrocytes. B. Overexpression of miR-411 promoted protein expression of type II collagen in chondrocytes. C. The mRNA expression of type IV collagen was detected by qRT-PCR. D. The protein expression of type IV collagen was detected by Western blot.
Discussion
In this study, miR-411 was downregulated in OA cartilage compared with in normal cartilage. Conversely, MMP-13 was upregulated in OA cartilage compared with in normal cartilage. IL-1β treatment repressed miR-411 expression in chondrocytes. Moreover, we identified MMP-13 as a direct target gene of miR-411 in chondrocytes and overexpression of miR-411 inhibited the MMP-13 expression. Furthermore, overexpression of miR-411 increased the expression of the type II collagen and type IV collagen in chondrocytes. Taken together, miR-411 acts as a crucial regulator of the MMP-13 and ECM synthesis and degradation in human chondrocytes.
Harafuji et al [29] reported that the expression of miR-411 was increased in primary immortalized and facioscapulohumeral muscular dystrophy (FSHD) myoblasts in comparison with in control myoblasts. They also identified YAF2 as a direct target of miR-411 and overexpression of miR-411 inhibited Myod, Myh1, and myogenin in C2C12 cells. Xia et al [30] demonstrated that miR-411 was downregulated in hepatocellular carcinoma cells and tissues. Overexpression of miR-411 increased hepatocellular carcinoma cells anchorage-independent growth and proliferation by regulating ITCH expression. However, the role of miR-411 in OA remains unknown. In our study, miR-411 was downregulated in OA cartilage compared with normal cartilage. Moreover, IL-1β treatment repressed miR-411 expression in chondrocytes. There results suggest that miR-411 play important roles in pathogenesis and development of OA.
Proteolytic degradation of cartilage by matrix-degrading enzymes is a hallmark of OA [2,31]. MMp-13 is a critical MMP collagenase and belongs to a family of extracellular matrix-degrading endopeptidases [8,32,33]. Previous studies demonstrated that MMP-13 was at low levels in articular cartilage during physiologic ECM turnover and is overexpression in human OA [34-36]. MMP-13 could degrade type 2 collagen and aggrecan [37]. Several cytokines are capable to stimulate MMP-13 expression in human OA [38,39]. In line with previous data, we demonstrated that MMP-13 was upregulated in human OA compared with normal cartilage. Moreover, treatment of IL-1β stimulated MMP-13 expression and repressed miR-411 expression in chondrocytes. Furthermore, we identified MMP-13 as a direct target gene of miR-411 and overexpression of miR-411 inhibited the MMP-13 expression. We also showed that ectopic expression of miR-411 inhibited chondrocytes cell proliferation and increased the type II collagen and type IV collagen expression.
In conclusion, miR-411 was downregulated in human OA, acting as a crucial regulator of the MMP-13 and catabolic signaling pathways in chondrocytes. Our data provide an insight into the roles of miRNA in OA pathogenesis and provide the possibility of miR-411 as a therapeutic target for the treatment of OA.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (Grant Numbers: ZR2010HQ036).
Supporting Information
References
- 1.Huang K, Wu LD. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36:1149–1160. doi: 10.1177/147323000803600601. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Wu M, Jiang S, Ding W, Luo Q, Shi J. Expression of ADAMTs-5 and TIMP-3 in the condylar cartilage of rats induced by experimentally created osteoarthritis. Arch Oral Biol. 2014;59:524–529. doi: 10.1016/j.archoralbio.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Cutolo M, Berenbaum F, Hochberg M, Punzi L, Reginster JY. Commentary on recent therapeutic guidelines for osteoarthritis. Semin Arthritis Rheum. 2015;44:611–617. doi: 10.1016/j.semarthrit.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Campos GC, Rezende MU, Pasqualin T, Frucchi R, Bolliger Neto R. Lateral wedge insole for knee osteoarthritis: randomized clinical trial. Sao Paulo Med J. 2015;133:13–19. doi: 10.1590/1516-3180.2013.6750002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brody LT. Knee osteoarthritis: Clinical connections to articular cartilage structure and function. Phys Ther Sport. 2015;16:301–16. doi: 10.1016/j.ptsp.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Pathak NN, Balaganur V, Lingaraju MC, Kant V, Kumar D, Sharma AK, Tandan SK. Effect of atorvastatin, a HMG-CoA reductase inhibitor in monosodium iodoacetate-induced osteoarthritic pain: Implication for osteoarthritis therapy. Pharmacol Rep. 2015;67:513–519. doi: 10.1016/j.pharep.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Yan D, Chen D, Hawse JR, van Wijnen AJ, Im HJ. Bovine lactoferricin induces TIMP-3 via the ERK1/2-Sp1 axis in human articular chondrocytes. Gene. 2013;517:12–18. doi: 10.1016/j.gene.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piecha D, Weik J, Kheil H, Becher G, Timmermann A, Jaworski A, Burger M, Hofmann MW. Novel selective MMP-13 inhibitors reduce collagen degradation in bovine articular and human osteoarthritis cartilage explants. Inflamm Res. 2010;59:379–389. doi: 10.1007/s00011-009-0112-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee YJ, Lee EB, Kwon YE, Lee JJ, Cho WS, Kim HA, Song YW. Effect of estrogen on the expression of matrix metalloproteinase (MMP)-1, MMP-3, and MMP-13 and tissue inhibitor of metalloproternase-1 in osteoarthritis chondrocytes. Rheumatol Int. 2003;23:282–288. doi: 10.1007/s00296-003-0312-5. [DOI] [PubMed] [Google Scholar]
- 10.Kalva S, Saranyah K, Suganya PR, Nisha M, Saleena LM. Potent inhibitors precise to S1’ loop of MMP-13, a crucial target for osteoarthritis. J Mol Graph Model. 2013;44:297–310. doi: 10.1016/j.jmgm.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Yu M, Liu C, Zhu H, He X, Peng S, Hua J. miR-34c works downstream of p53 leading to dairy goat male germline stem-cell (mGSCs) apoptosis. Cell Prolif. 2013;46:223–231. doi: 10.1111/cpr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Yu X, Shen J, Wu WK, Chan MT. MicroRNA expression and its clinical implications in Ewing’s sarcoma. Cell Prolif. 2015;48:1–6. doi: 10.1111/cpr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo X, Dong Z, Chen Y, Yang L, Lai D. Enrichment of ovarian cancer stem-like cells is associated with epithelial to mesenchymal transition through an miRNA-activated AKT pathway. Cell Prolif. 2013;46:436–446. doi: 10.1111/cpr.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohdaira H, Sekiguchi M, Miyata K, Yoshida K. MicroRNA-494 suppresses cell proliferation and induces senescence in A549 lung cancer cells. Cell Prolif. 2012;45:32–38. doi: 10.1111/j.1365-2184.2011.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu LL, Zhao X, Xu HL, Wen X, Wang SY, Liu B, Bao JK, Wei YQ. Identification of microRNAregulated autophagic pathways in plant lectininduced cancer cell death. Cell Prolif. 2012;45:477–485. doi: 10.1111/j.1365-2184.2012.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu X, Li Z, Liu J. MiRNAs in primary cutaneous lymphomas. Cell Prolif. 2015;48:271–277. doi: 10.1111/cpr.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J, Feng F. By downregulating TIAM1 expression, microRNA-329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–17569. doi: 10.18632/oncotarget.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X, Qiu G. MicroRNA-10b Promotes Nucleus Pulposus Cell Proliferation through RhoC-Akt Pathway by Targeting HOXD10 in Intervetebral Disc Degeneration. PLoS One. 2013;8:e83080. doi: 10.1371/journal.pone.0083080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Yu C, Chen WP, Wang XH. MicroRNA in osteoarthritis. J Int Med Res. 2011;39:1–9. doi: 10.1177/147323001103900101. [DOI] [PubMed] [Google Scholar]
- 21.Steck E, Boeuf S, Gabler J, Werth N, Schnatzer P, Diederichs S, Richter W. Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med (Berl) 2012;90:1185–1195. doi: 10.1007/s00109-012-0895-y. [DOI] [PubMed] [Google Scholar]
- 22.Swingler TE, Wheeler G, Carmont V, Elliott HR, Barter MJ, Abu-Elmagd M, Donell ST, Boot-Handford RP, Hajihosseini MK, Munsterberg A, Dalmay T, Young DA, Clark IM. The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum. 2012;64:1909–1919. doi: 10.1002/art.34314. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Gibson G, Kim JS, Kroin J, Xu S, van Wijnen AJ, Im HJ. MicroRNA-146a is linked to painrelated pathophysiology of osteoarthritis. Gene. 2011;480:34–41. doi: 10.1016/j.gene.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, Yin H, Liu T, Yan W, Li Z, Chen J, Chen H, Wang T, Jiang Z, Zhou W, Xiao J. MiR-126-5p regulates osteoclast differentiation and bone resorption in giant cell tumor through inhibition of MMP-13. Biochem Biophys Res Commun. 2014;443:944–949. doi: 10.1016/j.bbrc.2013.12.075. [DOI] [PubMed] [Google Scholar]
- 25.Liang ZJ, Zhuang H, Wang GX, Li Z, Zhang HT, Yu TQ, Zhang BD. MiRNA-140 is a negative feedback regulator of MMP-13 in IL-1betastimulated human articular chondrocyte C28/I2 cells. Inflamm Res. 2012;61:503–509. doi: 10.1007/s00011-012-0438-6. [DOI] [PubMed] [Google Scholar]
- 26.Tardif G, Hum D, Pelletier JP, Duval N, Martel-Pelletier J. Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes. BMC Musculoskelet Disord. 2009;10:148. doi: 10.1186/1471-2474-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldring MB. Immortalization of human articular chondrocytes for generation of stable, differentiated cell lines. Methods Mol Med. 2004;100:23–36. doi: 10.1385/1-59259-810-2:023. [DOI] [PubMed] [Google Scholar]
- 28.Lago R, Gomez R, Otero M, Lago F, Gallego R, Dieguez C, Gomez-Reino JJ, Gualillo O. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthritis Cartilage. 2008;16:1101–1109. doi: 10.1016/j.joca.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Harafuji N, Schneiderat P, Walter MC, Chen YW. miR-411 is up-regulated in FSHD myoblasts and suppresses myogenic factors. Orphanet J Rare Dis. 2013;8:55. doi: 10.1186/1750-1172-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia K, Zhang Y, Cao S, Wu Y, Guo W, Yuan W, Zhang S. miR-411 regulated ITCH expression and promoted cell proliferation in human hepatocellular carcinoma cells. Biomed Pharmacother. 2015;70:158–163. doi: 10.1016/j.biopha.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Morris KJ, Cs-Szabo G, Cole AA. Characterization of TIMP-3 in human articular talar cartilage. Connect Tissue Res. 2010;51:478–490. doi: 10.3109/03008201003686958. [DOI] [PubMed] [Google Scholar]
- 32.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higashiyama R, Miyaki S, Yamashita S, Yoshitaka T, Lindman G, Ito Y, Sasho T, Takahashi K, Lotz M, Asahara H. Correlation between MMP-13 and HDAC7 expression in human knee osteoarthritis. Mod Rheumatol. 2010;20:11–17. doi: 10.1007/s10165-009-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niebler S, Schubert T, Hunziker EB, Bosserhoff AK. Activating enhancer binding protein 2 epsilon (AP-2epsilon)-deficient mice exhibit increased matrix metalloproteinase 13 expression and progressive osteoarthritis development. Arthritis Res Ther. 2015;17:119. doi: 10.1186/s13075-015-0648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim H, Min DS, Kang Y, Kim HW, Son KH, Kim HP. Inhibition of matrix metalloproteinase-13 expression in IL-1beta-treated articular chondrocytes by a steroidal saponin, spicatoside A, and its cellular mechanisms of action. Arch Pharm Res. 2015;38:1108–1116. doi: 10.1007/s12272-015-0581-z. [DOI] [PubMed] [Google Scholar]
- 36.Yammani RR, Loeser RF. Brief report: stress-inducible nuclear protein 1 regulates matrix metalloproteinase 13 expression in human articular chondrocytes. Arthritis Rheumatol. 2014;66:1266–1271. doi: 10.1002/art.38391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nummenmaa E, Hamalainen M, Moilanen T, Vuolteenaho K, Moilanen E. Effects of FGF-2 and FGF receptor antagonists on MMP enzymes, aggrecan, and type II collagen in primary human OA chondrocytes. Scand J Rheumatol. 2015;44:321–30. doi: 10.3109/03009742.2014.1000372. [DOI] [PubMed] [Google Scholar]
- 38.Long DL, Ulici V, Chubinskaya S, Loeser RF. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is increased in osteoarthritis and regulates chondrocyte catabolic and anabolic activities. Osteoarthritis Cartilage. 2015;23:1523–1531. doi: 10.1016/j.joca.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong Y, Huang Y, Santoso MB, Wu LD. Sclareol exerts anti-osteoarthritic activities in interleukin-1beta-induced rabbit chondrocytes and a rabbit osteoarthritis model. Int J Clin Exp Pathol. 2015;8:2365–2374. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.