Abstract
Cisplatin has been a key chemotherapy drug for treatment of non-small cell lung cancer (NSCLC) for decades. However, the efficacy of Cisplatin is usually reduced by the occurrence of drug-resistance of cancer cells. Fisetin is a flavonol naturally found in many fruits and vegetables, which has been reported to suppress cell proliferation and induce apoptosis in various cancers. In this study, we aimed to investigate whether Fisetin was capable of enhancing cytotoxicity of Cisplatin in Cisplatin-resistant NSCLC cells, and explore the possible signaling pathways involved. Cisplatin-resistant NSCLC cells, A549-CR, was established by repeated subculturing of A549 cells with increasing Cisplatin. Proliferation ability was assessed by MTT analysis and apoptosis was detected by flow cytometry. The results showed that Fisetin effectively increased sensitivity of A549-CR cells to Cisplatin, possibly mediated by inhibiting aberrant activation of MAPK signaling pathways. This increases the possibility of Fisetin as a promising agent for lung cancer therapy.
Keywords: Cisplatin, fisetin, resistance, NSCLC A549 cells, MAPK signaling pathway
Introduction
Lung cancer is the first most common and lethal human malignant tumor worldwide, of which NSCLC constitutes about 80% of cancer cases [1]. The clinical strategy for treatment of early-stage NSCLC has been surgery or surgery with chemotherapy, with 30%-60% of five-year survival after intervention. At the time of diagnosis, a proportion of NSCLCs are often in an advanced stage and the tumor is unresectable, for which systematic chemotherapy has been the main treatment method [2,3].
In systematic chemotherapy, Cisplatin, a DNA damaging agent, is the most widely used chemotherapy agent for treatment of a variety of cancers, including NSCLC [4]. However, the therapeutic effect of Cisplatin is often weakened by acquired resistance of tumor cells during the process of treatment [5]. Owing to the resistance, relapse and metastasis are common, resulting in high mortality. It is believed that Cisplatin resistance is an important obstacle to NSCLC chemotherapy [6]. For this reason, it is necessary to explore the mechanisms underlying the chemoresistance and to find new methods for enhancing the sensitivity of NSCLC cells to Cisplatin.
Recently, much attention has been focused on natural sources of drugs, especially dietary sources of drugs. Fisetin (3,7,3’,4’-tetrahydroxyflavone) is a natural flavonoid found in various fruits and vegetables, such as strawberry, apple, persimmon, grape, onion, and cucumber, which exhibits a wide variety of functions including neurotrophic, anti-carcinogenic, anti-inflammatory, anti-oxidant, antiangiogenic and antiproliferative effects [7]. In recent years, as a diet-derived botanical flavonoid, Fisetin has been reported to inhibit cell proliferation, migration and invasion, and induce apoptosis in several cancer types, such as glioma cancer [8], colon cancer [9], lung cancer [10], melanoma [11], prostate cancer [12], nasopharyngeal carcinoma [13] and bladder cancer [14]. Importantly, even in some drug-resistant cancer cells, fisetin could provide biological inhibitory effects. For instance, reports showed that Fisetin could reverse multi-drug resistance by inhibiting P-gp function in breast cancer [15], and induce apoptosis as well as repress invasion in chemoresistant pancreatic cancer cells [16]. Besides, several studies have demonstrated that Fisetin mediated a series of aberrant signal pathway molecules, including Nitric oxide [17], NF-kappa B [14], mTORC1 [18], PI3-K/AKT/mTOR [19], Wnt/EGFR [20] and MAPK [21]. Moreover, Fisetin was capable of enhancing the effects of antitumor drugs cooperatively or synergistically on some cancer cells. For example, Tripathi et al. [22] found that addition of Fisetin to Cisplatin enhanced its cytotoxic effect on embryonal carcinoma by activating both mitochondrial and cell death receptor pathway. Touil et al. [23] reported that combination of Fisetin and cyclophosphamide presented improved antiangiogenic and antitumour activity in Lewis lung carcinoma. In addition, Fisetin was reported to alleviate Cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence [24]. These studies indicated that Fisetin might have a potential to treat cancer and simultaneously decrease the toxicity of common chemotherapy drugs through various mechanisms, which led us to hypothesize that Fisetin might reverse the Cisplatin-resistance of lung cancer cells.
To date, the effect of combined Fisetin and Cisplatin treatment on Cisplatin-resistant lung cancer cells has not been reported in the literature. Thus, the aim of this study was to elucidate whether addition of Fisetin could increase cytotoxicity of Cisplatin on Cisplatin-resistant NSCLC cells, and further investigate the possible involvement of signaling transduction pathways during this process, thereby helping us understand the possible benefits and mechanisms of Fisetin in treatment of NSCLC.
Materials and methods
Establishment of cisplatin-resistant lung adenocarcinoma cells
The human lung adenocarcinoma cell lines, A549, was obtained from the American Type Culture Collection (Manassas, VA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Hyclone, Logan) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT) in a humidified atmosphere with 5% CO2. The acquired Cisplatin-resistant subline of A549, A549-CR, was established by repeated subculturing with gradual increase of Cisplatin (1, 2, 4, 6, 8 and 10 μM) over 6 months, which consulted the procedure provided by Selvi, et al. [25].
Cell viability analysis
The cell viability was evaluated by MTT assay. In brief, the cells (1×104) were plated in 96-well cell culture plates in DMEM containing 10% FBS in a final volume of 0.2 ml. When the cells reached 50% confluence, agents were added to appropriate concentrations and incubation continued for an additional 72-96 h. Then, MTT reagent was added to 400 μg/ml and incorporated for 4 hours. Afterwards, the MTT medium mixture was removed and 200 µl of dimethyl sulfoxide was added to each well. Absorbance was measured at 490 nm by a multi-well spectrophotometer (Thermo Electron, Andover, USA).
Cell apoptosis analysis
Apoptotic cells were evaluated using an annexin V-FITC kit (Beyotime, China). The cells were scraped and stained with annexin V-FITC and propidium iodide according to the manufacturer’s protocol. In brief, the cells were washed with PBS. After 195 µl of the binding buffer was added, 5 µl of FITC-labeled annexin V was added and incubated for 10 min at 25°C. The cells were then incubated with 10 µl propidium iodide for 10 min in an ice bath in the dark and the apoptotic cells were determined by flow cytometry (FACS) analysis.
Western blot assay
The cells were harvested, pelleted by centrifugation, washed with ice-cold PBS, and lysed with RIPA buffer [150 mM NaCl, 50 mM Tris base (pH 8.0), 1 mM EDTA, 0.5% sodium deoxycholate, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 1 mM DTT, 1 mM PMSF, and 1 mM Na3VO4] that was supplemented with a protease inhibitor. The proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Life Technologies, Gaithersburg, MD). The blots were then incubated in a fresh blocking solution with an appropriate dilution of the primary antibody at 4°C for 24 h.
The sources of antibodies were as follows: GAPDH mouse polyclonal antibody (Santa Cruz); p-MAPK (Thr202/Tyr204), MAPK rabbit monoclonal antibody (Cell Signaling); Survivin and Cytochrome C mouse monoclonal antibody (Santa Cruz), Caspase-3 and Caspase-8 rabbit polyclonal antibody (Santa Cruz).
After the blots were extensively washed, the membranes were incubated with horseradish peroxidase-coupled secondary antibody (1:2000, Zhongshan Biotech Company, China) at 25°C for 1 h. The bands were visualized and quantified using the Image-Pro Plus 5.0 software (Media Cybernetics). p-MAPK band intensities were normalized to MAPK intensities and other factors were adjusted by the GAPDH band intensities.
Statistical analysis
Data were expressed as mean value ± SD. Differences between groups were analyzed using ANOVA or a t-test. These analyses were performed on SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL). A P-value of <0.05 was considered statistically significant.
Results
Establishment of acquired Cisplatin-resistant A549-CR cells
Cisplatin-resistant A549-CR was obtained by gradual increase of Cisplatin in cell culture. To evaluate the chemotherapy sensitivity of A549 and A549-CR cells, we treated these cells with Cisplatin at different concentration (0, 5, 10, 20, 40, 80, 160, 320 and 640 µM) for 48 h incubation, respectively, and then calculated the IC50 of Cisplatin. As shown in Figure 1A, the IC50 of Cisplatin for A549 and A549-CR was 24.27 μM and 176.64 μM, respectively, which showed the IC50 of A549-CR was about 7.25 times higher than that of A549 cells, implying the establishment of acquired Cisplatin-resistant lung cancer cell line. Notably, when the concentration of Cisplatin was increased to 10 μM, compared with that of the control, the survival rate of A549 cells (77.41±5.34%) was significantly decreased (P<0.05), whereas the survival rate of A549-CR cells (98.41±4.63%) still remained stable (P>0.05). The data indicated that the Cisplatin-resistant A549 cell line, A549-CR was well established and cytotoxicity of 10 μM Cisplatin was markedly different between A549 and A549-CR cells. Therefore, we used the concentration of 10 μM for further experiments.
Figure 1.

A549 and A549-CR cells growth inhibition by Fisetin or Cisplatin. Cell viability was determined by the MTT assay. The data is presented as mean ± SD of three separate experiments. *P<0.05, vs control group (0 μM). A: Cisplatin. IC50 values of 24.27 and 176.64, as well as IC10 values of 5.46 and 34.75, for A549 and A549-CR cells respectively (μM). B: Fisetin. IC50 values of 214.47 and 320.42, as well as IC10 values of 31.91 and 39.39, for A549 and A549-CR cells respectively (μM).
The cytotoxicity of Fisetin in A549 and Cisplatin-resistant A549-CR cells
To determine the effects of Fisetin on the viability of A549 and A549-CR cells, respectively, the cells were incubated with various concentrations (0, 5, 10, 20, 40, 80, 160, 320 and 640 μM) of Fisetin (Sigma, St Louis, MO) for 48 h. Then, the cell viability was tested by MTT assay. As illustrated in Figure 1B, the cell viability was inhibited by Fisetin, and the inhibitory effect was strengthened with an increase in Fisetin concentration, suggesting that Fisetin could inhibit cell viability of both A549 and A549-CR cells, severally, in a dose-dependent manner. IC50 of Fisetin was 214.47 μM for A549 and 320.42 for A549-CR cells, respectively. Likewise, IC10 of Fisetin was 31.91 μM for A549 and 39.39 μM for A549-CR cells, respectively. Importantly, according to the measured data, when treated with 40 μM Fisetin, the survival rates of A549-CR cells (89.81±3.21%) decreased significantly (P<0.05), compared to the controls. Hence, we used the concentration of 40 μM for the next experiments.
Fisetin enhances chemotherapeutic effect of Cisplatin on A549-CR cells
To determine whether Fisetin could overcome Cisplatin-resistance of A549-CR to any extent, we conducted further experiments. A549-CR cells were divided into four groups and treated with 40 μM Fisetin, 10 μM Cisplatin, 40 μM Fisetin+10 μM Cisplatin, and DMEM as a control for 48 h, respectively. Then, the cell viability and apoptosis were tested.
As shown in Figure 2, Single use of 10 μM Cisplatin had little influence on the cell viability and apoptosis of A549-CR cells, confirming that the A549-CR cells were markedly resistant to 10 μM Cisplatin. Then, 40 μM Fisetin alone slightly suppressed cell viability and induced apoptosis of A549-CR. However, the combinational treatment of Fisetin (40 μM) with Cisplatin (10 μM) resulted in an intense suppression of cell viability and induction of cell apoptosis as compared with the cells treated with Fisetin or Cisplatin alone, indicating that Fisetin made Cisplatin-resistant lung cancer cells vulnerable to the cytotoxicity of Cisplatin.
Figure 2.
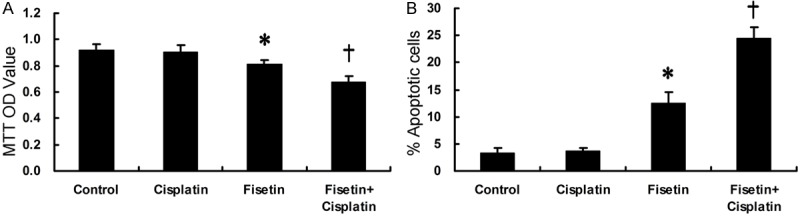
Cell viability (A) and apoptosis (B) in A549-CR assessed by MTT and apoptosis assay, respectively (*P<0.05 vs Control or †).
Fisetin reverses acquired Cisplatin-resistance of A549-CR cells through MAPK/Survivin/Caspase pathways
To explore the status of signaling pathways, we further detected the signaling proteins by western blot analysis. As shown in Figure 3, Cisplatin alone at a dose of 10 μM could not affect the protein expression of phosphorylation levels of MAPK as well as Survivin and apoptotic pathway-related molecules including Caspase-3, Caspase-8 and Cytochrome C. Treatment of Fisetin alone, or combined with Cisplatin, might lead to a decrease in the expression of p-MAPK, and Survivin protein. Accordingly, an increase in the expression of Caspase-3, Caspase-8 and Cytochrome C was also observed in these two groups, indicating that Fisetin might reverse Cisplatin-resistance of cancer cells via inactivation of MAPK pathway and repression of Survivin expression. Thus, the apoptotic signaling might be initiated in A549-CR cells.
Figure 3.

Expression of the MAPK, Survivin and apoptosis pathway-related proteins assessed by immunoblotting (C).
Inhibition of MAPK pathway might partially account for Fisetin-induced cisplatin-resistance reversion of A549-CR cells
To investigate the roles of MAPK pathways by which Fisetin reverses Cisplatin-resistance of A549-CR cells, we conducted further investigation.
A549-CR cells were divided into 6 groups as I, II, III, IV, V and VI, respectively. Group I was treated with DMEM for 48 h as a control. Group II, Group III and Group IV were treated with Cisplatin (10 μM), 10 µM U0126 (a specific MAPK inhibitor, Cell signaling), and Fisetin (40 μM) for 48 h, respectively. For group V and group VI, separately, cells were treated with a combination of Cisplatin (10 μM) with U0126 (10 µM) or Fisetin (40 μM) for 48 h. Cell viability and apoptosis were assessed by MTT and apoptosis assays, respectively.
As shown in Figure 4, when the cells were treated with any single reagent, we found that treatment with Cisplatin (II) alone could hardly suppress cell viability and induce cell apoptosis relative to those in the control group (I), while treatment with U0126 (III) could slightly induce cell apoptosis with the results insignificant. Notably, treatment with Fisetin (IV) or Cisplatin+U0126 (V) exhibited moderate inhibition of cell viability and induction of apoptosis compared with the controls (I). However, in group VI (Cisplatin+Fisetin), an evident effects on cell viability suppression and apoptosis induction were observed compared with those in group IV (Fisetin) or V (Cisplatin+U0126), respectively, suggesting that inhibition of MAPK pathways might play a role in the mechanisms by which Fisetin reverses Cisplatin-resistance. However, besides this, other unknown mechanisms might also be involved during this process. In other words, activation of MAPK pathways might play a partial role in the acquired Cisplatin-resistance.
Figure 4.
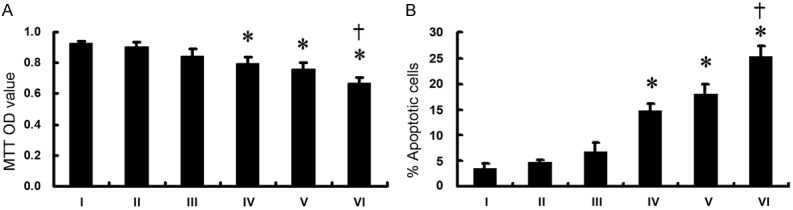
Cell viability (A) and apoptosis (B) of A549-CR cells treated with DMEM (I), Cisplatin (II), U0126 (III), Fisetin (IV), Cisplatin+U0126 (V), Cisplatin+Fisetin (VI) assessed by MTT and apoptosis assay, respectively. (*P<0.05 vs I or II; †P<0.05 vs V or IV).
Discussion
In the present study, we identified that Fisetin, a flavonoid widely found in fruits and vegetables, had a potential to reverse acquired Cisplatin-resistance of lung cancer cells possibly via inactivation of MAPK/Survivin pathways.
Fisetin is a flavonol, a structurally distinct chemical substance that belongs to the flavonoid group of polyphenols. It can be found in many plants, fruits and vegetables, such as parrot tree, honey locust, strawberries, apples, grapes and onions [7] and has been thought to have a wide variety of biological activities, such as anti-aging, anti-inflammatory [26] and anti-carcinogenic effects [27]. Evidence demonstrated that Fisetin had extensively anti-tumorigenic ability in a variety of cancers with complex mechanisms. Reports showed that Fisetin could induce p53 expression (a tumor suppressor) [14] and suppress cancer cell migration and invasion through inhibition of MMP and angiogenesis [28]. Moreover, Fisetin could reverse chemoresistance of cancer cells by inhibiting MAPK and NF-kappaB pathways [16,29].
In the present study, the data showed that Fisetin alone could repress cell viability and induce apoptosis of A549-CR cells in a time-and dose-dependent manner. Then, treatment with a combination of Fisetin and Cisplatin could exhibit strong inhibition effects on A549-CR cells compared with Fisetin or Cisplatin, respectively. These results demonstrated that Fisetin could not only induce cell apoptosis by itself, but also sensitize Cisplatin-resistant NSCLC cells to Cisplatin and enhance Cisplatin-induced apoptosis through a synergistic action, indicating that Fisetin might act as a potential agent for reversing Cisplatin-resistance in treating NSCLC patients. However, the mechanisms underlying this process remain little understood. To explore this, we further detected the possible signaling pathways involved in this process.
Ras/Raf/MAPK pathways have been indicated to play a role in multiple cellular processes, such as cell proliferation, apoptosis, transcription, and cell migration [30] and thus have been thought to have an association with genesis and progression of various malignancies [31] including NSCLC [32]. Evidence shows that hyperactive MAPKs pathway has been associated with Cisplatin-resistance of NSCLCs cells [33,34]. The results of the present study showed that single use of Cisplatin could hardly affect the phosphorylation of MAPK expression, with the cell apoptosis unchanged, whereas Fisetin alone could slightly decrease the level of p-MAPK in A549-CR cells, with a slight increase in cell apoptosis. However, combination of Fisetin with Cisplatin could markedly induce cell apoptosis, with inactivation of MAPKs pathways, implying that Fisetin might sensitize the Cisplatin-resistant cells to Cisplatin through inhibition of MAPK pathways. The activation of MAPK signaling pathway provided a survival signal for the Cisplatin-resistant A549 cells and the inhibition of this pathway presented an apoptotic signal. Nevertheless, we found that co-treatment of Fisetin and Cisplatin showed strong inhibition effects on cells compared with other groups, indicating that activation of the MAPK pathway might only play a partial role in the acquired Cisplatin-resistance of A549-CR cells. In addition to this pathway, other signaling pathways might also play important roles in this process.
Over-expression of Survivin has been widely detected in a variety of cancers and indicated to be associated with cancer development and drug resistance [35]. Reports showed that Survivin contributes to Cisplatin-resistance of NSCLC [36] and gastric cancer [37]. In the present study, the data showed that low-concentration of Cisplatin or Fisetin could scarcely reduce Survivin expression and failed to cause apoptosis in A549-CR cells, while co-treatment of Fisetin and Cisplatin presented strong inhibition of Survivin expression and induction of cell apoptosis compared with Fisetin or Cisplatin alone. Therefore, co-treatment of Fisetin and Cisplatin led to Survivin downregulation and increased cleavage of Caspase-3, -8 and release of Cytochrome C. The results suggested that down-regulation of Survivin might be involved in the mechanisms by which Fisetin reverses acquired Cisplatin-resistance of A549 cells.
Several limitations might be involved in the present study. First, only one cell line, A549, was used in this experiment. Future studies using other NSCLC cell lines might strengthen the significance of the results. Nevertheless, A549 is a well-established cell line that has been representative of NSCLC in a number of studies. Second, the present study had focused on in vitro study in order to explore the underlying signaling mechanisms by which Fisetin overcomes the Cisplatin-resistance of A549 cells. Future in vivo studies are needed to confirm the results. Third, only a small proportion of signaling pathways were evaluated in this study. Hence, other pathways that might also play critical roles in this process are needed to be determined in further investigations.
In conclusion, we found that Fisetin, a natural product, might be a potential agent that might reverse Cisplatin-resistance of lung adenocarcinoma cells, possibly via inactivating MAPKs pathways as well as suppressing Survivin expression.
Acknowledgements
This work was partly supported by the special foundation for the 1130 Project of Xinqiao Hospital of Third Military Medical University (2012).
Disclosure of conflict of interest
None.
References
- 1.Peng B, Wang YH, Liu YM, Ma LX. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med. 2015;8:3098–3106. [PMC free article] [PubMed] [Google Scholar]
- 2.Feng PH, Yu CT, Wu CY, Lee MJ, Lee WH, Wang LS, Lin SM, Fu JF, Lee KY, Yen TH. Tumorassociated macrophages in stage IIIA pN2 non-small cell lung cancer after neoadjuvant chemotherapy and surgery. Am J Transl Res. 2014;6:593–603. [PMC free article] [PubMed] [Google Scholar]
- 3.Artal Cortes A, Calera Urquizu L, Hernando Cubero J. Adjuvant chemotherapy in non-small cell lung cancer: state-of-the-art. Transl Lung Cancer Res. 2015;4:191–197. doi: 10.3978/j.issn.2218-6751.2014.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HY, Mohammed KA, Goldberg EP, Kaye F, Nasreen N. Cisplatin loaded albumin mesospheres for lung cancer treatment. Am J Cancer Res. 2015;5:603–615. [PMC free article] [PubMed] [Google Scholar]
- 5.Rose MC, Kostyanovskaya E, Huang RS. Pharmacogenomics of cisplatin sensitivity in non-small cell lung cancer. Genomics Proteomics Bioinformatics. 2014;12:198–209. doi: 10.1016/j.gpb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A, Castedo M, Kroemer G. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis. 2014;5:e1257. doi: 10.1038/cddis.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan N, Syed DN, Ahmad N, Mukhtar H. Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal. 2013;19:151–162. doi: 10.1089/ars.2012.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CM, Hsieh YH, Hwang JM, Jan HJ, Hsieh SC, Lin SH, Lai CY. Fisetin suppresses ADAM9 expression and inhibits invasion of glioma cancer cells through increased phosphorylation of ERK1/2. Tumour Biol. 2015;36:3407–3415. doi: 10.1007/s13277-014-2975-9. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Wu Q, Song L, He T, Li Y, Li L, Su W, Liu L, Qian Z, Gong C. Polymeric micelles encapsulating fisetin improve the therapeutic effect in colon cancer. ACS Appl Mater Interfaces. 2015;7:534–542. doi: 10.1021/am5066893. [DOI] [PubMed] [Google Scholar]
- 10.Kang KA, Piao MJ, Hyun JW. Fisetin induces apoptosis in human nonsmall lung cancer cells via a mitochondria-mediated pathway. In Vitro Cell Dev Biol Anim. 2015;51:300–309. doi: 10.1007/s11626-014-9830-6. [DOI] [PubMed] [Google Scholar]
- 11.Syed DN, Lall RK, Chamcheu JC, Haidar O, Mukhtar H. Involvement of ER stress and activation of apoptotic pathways in fisetin induced cytotoxicity in human melanoma. Arch Biochem Biophys. 2014;563:108–117. doi: 10.1016/j.abb.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan MI, Adhami VM, Lall RK, Sechi M, Joshi DC, Haidar OM, Syed DN, Siddiqui IA, Chiu SY, Mukhtar H. YB-1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget. 2014;5:2462–2474. doi: 10.18632/oncotarget.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li R, Zhao Y, Chen J, Shao S, Zhang X. Fisetin inhibits migration, invasion and epithelial-mesenchymal transition of LMP1-positive nasopharyngeal carcinoma cells. Mol Med Rep. 2014;9:413–418. doi: 10.3892/mmr.2013.1836. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Qu W, Cheng Y, Sun Y, Jiang Y, Zou T, Wang Z, Xu Y, Zhao H. The inhibitory effect of intravesical fisetin against bladder cancer by induction of p53 and down-regulation of NFkappa B pathways in a rat bladder carcinogenesis model. Basic Clin Pharmacol Toxicol. 2014;115:321–329. doi: 10.1111/bcpt.12229. [DOI] [PubMed] [Google Scholar]
- 15.Chung SY, Sung MK, Kim NH, Jang JO, Go EJ, Lee HJ. Inhibition of P-glycoprotein by natural products in human breast cancer cells. Arch Pharm Res. 2005;28:823–828. doi: 10.1007/BF02977349. [DOI] [PubMed] [Google Scholar]
- 16.Murtaza I, Adhami VM, Hafeez BB, Saleem M, Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappaB. Int J Cancer. 2009;125:2465–2473. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ash D, Subramanian M, Surolia A, Shaha C. Nitric oxide is the key mediator of death induced by fisetin in human acute monocytic leukemia cells. Am J Cancer Res. 2015;5:481–497. [PMC free article] [PubMed] [Google Scholar]
- 18.Jung CH, Kim H, Ahn J, Jeon TI, Lee DH, Ha TY. Fisetin regulates obesity by targeting mTORC1 signaling. J Nutr Biochem. 2013;24:1547–1554. doi: 10.1016/j.jnutbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Syed DN, Adhami VM, Khan MI, Mukhtar H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med Chem. 2013;13:995–1001. doi: 10.2174/18715206113139990129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30:300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Sousa RR, Queiroz KC, Souza AC, Gurgueira SA, Augusto AC, Miranda MA, Peppelenbosch MP, Ferreira CV, Aoyama H. Phosphoprotein levels, MAPK activities and NFkappaB expression are affected by fisetin. J Enzyme Inhib Med Chem. 2007;22:439–444. doi: 10.1080/14756360601162063. [DOI] [PubMed] [Google Scholar]
- 22.Tripathi R, Samadder T, Gupta S, Surolia A, Shaha C. Anticancer activity of a combination of cisplatin and fisetin in embryonal carcinoma cells and xenograft tumors. Mol Cancer Ther. 2011;10:255–268. doi: 10.1158/1535-7163.MCT-10-0606. [DOI] [PubMed] [Google Scholar]
- 23.Touil YS, Seguin J, Scherman D, Chabot GG. Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemother Pharmacol. 2011;68:445–455. doi: 10.1007/s00280-010-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M, Rachamalla SS, Sistla R. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-kappaB activation and antioxidant defence. PLoS One. 2014;9:e105070. doi: 10.1371/journal.pone.0105070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cetintas VB, Kucukaslan AS, Kosova B, Tetik A, Selvi N, Cok G, Gunduz C, Eroglu Z. Cisplatin resistance induced by decreased apoptotic activity in non-small-cell lung cancer cell lines. Cell Biol Int. 2012;36:261–265. doi: 10.1042/CBI20110329. [DOI] [PubMed] [Google Scholar]
- 26.Geraets L, Haegens A, Brauers K, Haydock JA, Vernooy JH, Wouters EF, Bast A, Hageman GJ. Inhibition of LPS-induced pulmonary inflammation by specific flavonoids. Biochem Biophys Res Commun. 2009;382:598–603. doi: 10.1016/j.bbrc.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 27.Khan N, Afaq F, Syed DN, Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–1056. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JH, Jang YJ, Choi YJ, Jang JW, Kim JH, Rho YK, Kim IJ, Kim HJ, Leem MJ, Lee ST. Fisetin inhibits matrix metalloproteinases and reduces tumor cell invasiveness and endothelial cell tube formation. Nutr Cancer. 2013;65:1192–1199. doi: 10.1080/01635581.2013.828090. [DOI] [PubMed] [Google Scholar]
- 29.Pal HC, Sharma S, Strickland LR, Katiyar SK, Ballestas ME, Athar M, Elmets CA, Afaq F. Fisetin inhibits human melanoma cell invasion through promotion of mesenchymal to epithelial transition and by targeting MAPK and NFkappaB signaling pathways. PLoS One. 2014;9:e86338. doi: 10.1371/journal.pone.0086338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandamme D, Herrero A, Al-Mulla F, Kolch W. Regulation of the MAPK pathway by raf kinase inhibitory protein. Crit Rev Oncog. 2014;19:405–415. doi: 10.1615/critrevoncog.2014011922. [DOI] [PubMed] [Google Scholar]
- 31.Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120:3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciuffreda L, Incani UC, Steelman LS, Abrams SL, Falcone I, Curatolo AD, Chappell WH, Franklin RA, Vari S, Cognetti F, McCubrey JA, Milella M. Signaling intermediates (MAPK and PI3K) as therapeutic targets in NSCLC. Curr Pharm Des. 2014;20:3944–3957. doi: 10.2174/13816128113196660763. [DOI] [PubMed] [Google Scholar]
- 33.Gadgeel SM, Wozniak A. Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer. Clin Lung Cancer. 2013;14:322–332. doi: 10.1016/j.cllc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Ercan D, Xu C, Yanagita M, Monast CS, Pratilas CA, Montero J, Butaney M, Shimamura T, Sholl L, Ivanova EV, Tadi M, Rogers A, Repellin C, Capelletti M, Maertens O, Goetz EM, Letai A, Garraway LA, Lazzara MJ, Rosen N, Gray NS, Wong KK, Janne PA. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2012;2:934–947. doi: 10.1158/2159-8290.CD-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung CH, Huang CC, Tsai FY, Lee JY, Cheng SM, Chang YC, Huang YC, Chen SH, Chang JY. Survivin - biology and potential as a therapeutic target in oncology. Onco Targets Ther. 2013;6:1453–1462. doi: 10.2147/OTT.S33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang HQ, Jin JJ, Wang J. Matrine induces mitochondrial apoptosis in cisplatinresistant non-small cell lung cancer cells via suppression of beta-catenin/survivin signaling. Oncol Rep. 2015;33:2561–2566. doi: 10.3892/or.2015.3844. [DOI] [PubMed] [Google Scholar]
- 37.Sun XP, Dong X, Lin L, Jiang X, Wei Z, Zhai B, Sun B, Zhang Q, Wang X, Jiang H, Krissansen GW, Qiao H, Sun X. Up-regulation of survivin by AKT and hypoxia-inducible factor 1alpha contributes to cisplatin resistance in gastric cancer. FEBS J. 2014;281:115–128. doi: 10.1111/febs.12577. [DOI] [PubMed] [Google Scholar]


