Abstract
Recent advances suggest that precancerous lesions of pelvic serous carcinoma originate from tubal secretory cells. The purpose of our study was to determine if an increased number of secretory cells varies with age or location in the fallopian tube and to examine its association with serous neoplasia. Three groups (benign control, high-risk, and pelvic serous carcinoma) of age-matched patients were studied. The age data were stratified into 10-year intervals ranging from 20-29 to older than 80. The number of secretory and ciliated cells from both tubal fimbria and ampulla segments was counted by microscopy and immunohistochemical staining methods. The data were analyzed by standard contingency table and Poisson distribution methods after age justification. We found that the absolute number of tubal secretory cells increased significantly with age in all three groups. But a more dramatic increase of secretory cells was observed in high-risk and pelvic serous carcinoma patients. Secretory cell expansion is more prevalent than secretory cell outgrowth in both fimbria and ampulla tubal segments and is significantly associated with serous neoplasia (P < 0.001). Furthermore, age remained a significant risk factor for serous neoplasia after age adjustment. These findings suggest that secretory cell expansion could serve as a potential sensitive biomarker for early serous carcinogenesis within the fallopian tube. The study also supports a relationship between serous neoplasia and increased secretory to ciliated cell ratios, and the relationship between frequency of secretory cell expansion within the fallopian tube and increasing age and-more significantly-presence of high-risk factors or co-existing serous cancers.
Keywords: Fallopian tube, tubal secretory cells, pelvic serous carcinoma, ovarian cancer, pathogenesis, carcinogenesis
Introduction
Ovarian serous carcinoma, particularly high-grade serous cancers is the most common and most lethal type of mullerian malignancy, comprising more than 70% of all malignancies from the organs within the female pelvis [1-3]. Ovarian cancers (OvCa) present frequently in advanced stages leading to poor prognosis; therefore, investigators have emphasized the importance of understanding early phases of this disease, including detection of precursor lesions [4].
Important advances in recent years show that precancerous lesions of OvCa originate from the fallopian tube, rather than from the ovary [2,5-11]. This leads to a paradigm shift from ovary-centered studies and intervention to focus on the fallopian tube [12]. Within the fallopian tubal mucosa, there are two different cell types, ciliated and non-ciliated cells. The latter are also called secretory cells. It is the secretory cell that serves as the cell of origin for the majority of the OvCa [2,5,6,13,14]. The current carcinogenesis model for OvCa [15,16] is proposed as follows: 1) Initial loss of ciliated cells concurrent with expansion of secretory cells; 2) Subsequent TP53 gene mutations in secretory cells; 3) Development of a “p53 signature,” a precancerous lesion derived from a clonal population with altered p53 expression in the setting of DNA damage; 4) Progression to TIC; 5) Shedding of cancer cells from tubal fimbria that invade onto the ovary, implant on peritoneal surfaces or expand within the fallopian tube itself. The latent, p53 signature precancer shares attributes with serous carcinomas and has been demonstrated to exist in anatomic continuity with serous tubal intraepithelial carcinoma (TIC), the earliest morphologically identified form of serous type of OvCa [8,9,17-19].
With confidence that tubal secretory cells are the origin of OvCa, many investigators have focused attention on this particular group of cells within the fallopian tube. Prominent studies out of Brigham and Women’s Hospital at Harvard University have focused on a particular group of secretory cells called secretory cell outgrowths (SCOUTs), defined at the cellular level as non-interrupted growth of at least 30 secretory cells that are distinct against a heterogeneous background of intermixed secretory and ciliated cells within the tubal mucosa [13]. SCOUTs may represent an indirect precursor and may further signal alterations in gene function leading to pelvic serous carcinogenesis [13]. It has been recently described that SCOUTs stained strongly with BCL-2 and frequently have loss of PAX2, but showed no P53 gene mutation [6,13]. A compelling observation was that SCOUTs were found in a significantly higher frequency in tubal mucosa from patients with high-grade serous carcinoma in comparison with women without cancer [13]. Morphologically, SCOUTs are visible under light microscopy and, therefore, may be used as a surrogate biomarker for OvCa screening and potential cancer prevention without further molecular tests. However, the definition of SCOUTs, as described with 30 secretory cells, is arbitrary. It is unknown if the number of 30 secretory cells represents the optimal early biomarker for pelvic serous neoplasia. As a matter of fact, we have recently observed that secretory cell expansion (SCE), defined as 10 secretory cells in a row, is a common phenomenon within the tubal mucosa, particularly for those patients with OvCa [20]. Therefore, the aim of this study was to determine if the OvCa precursor model could be further scrutinized through the study of secretory cell expansion. We have studied the following: 1) the overall normal number of SCEs in the fallopian tubes with attention to differences in tubal location and patient age; 2) the extent of change in the number of SCEs in high-risk and OvCa patients; 3) whether these changes are independent of the aging process; and 4) if SCE requires more than 30 cells as an early morphologic marker for OvCa development.
Materials and methods
Case collection and grouping
A total of 426 consecutively identified cases that included fallopian tubes, all surgically removed from 2007 to 2015, were culled from pathology files of University of Arizona Medical Center in Tucson, Arizona. The study was approved by the institutional review board. There were three groups of patients: low-risk (n=270), high-risk (n=80), and patients with PSC (n=76). Low risk patients served as the control group and consisted of those post-hysterectomies and -salpingectomies performed for benign disease (adenomyosis, leiomyomata, endometriosis or uterine prolapse). Controls were further divided into age groups to determine normal distribution of tubal secretory and ciliated cells. High-risk patients were those with known BRCA mutations, breast cancer history, or first-degree family history of ovarian cancer. Typically, these patients underwent prophylactic bilateral salpingo-oophorectomy. Fallopian tubes from patients with OvCa were either intact or with only serosal involvement by the tumor. Patients were excluded if tumor involved the tubal lumen or extensive tubal mucosa. Patients were age-matched across the three groups. For controls, at least two representative sections of the fallopian tube, including ampulla (proximal) and fimbria, were submitted. For high-risk or OvCa patients, the entire fallopian tubes were submitted based on SEE-FIM protocol. At least 2 sections (one from fimbria and one from ampulla) from each case in the latter groups were examined via light microscopy by identified co-authors (YW, WZ).
Morphologic analysis
The number of secretory and ciliated cells within the tubal fimbria and ampulla epithelia was evaluated by 2 methods: light microscopy and immunostaining with PAX8 for secretory cells and tubulin for ciliated cells. In addition to the concept of secretory cell outgrowth (SCOUT), as defined by at least 30 secretory cells in a row, we counted all the secretory cells arranged more than 10 cells without interruption by any ciliated cells within the tubal mucosa. A stretch of more than 10 secretory cells in a row is defined as secretory cell expansion (SCE). From this perspective, SCOUTs fall into the category of SCE. The histologic diagnoses of serous tubal intraepithelial carcinoma (TIC) and ovarian carcinoma (OvCa) were made based on criteria described previously [21-23]. The majority of OvCa were high-grade serous carcinomas. To gain insight into the histogenesis of ovarian tumors based on the differential distribution (qualitative and quantitative) of SCEs and SCOUTs in the fallopian tube, we determined the numbers of these entities in the tubes from all three groups by both light microscopy and PAX8 immunostaining. The number of SCEs and SCOUTs was counted in the ampulla and fimbria of the fallopian tube in each case. Presence or absence of TIC was recorded. Authors performing slide reviews were blinded as to the group status of the tubal sections.
Immunohistochemical analyses
Immunohistochemistry (IHC) with antibody against PAX8 was performed. PAX8 is a member of the PAX gene family, consisting of nine well-described transcription factors (PAX 1-9) [24]. PAX8 has been considered a mullerian epithelial marker identifying tubal secretory cells [5]. It is helpful in the differential diagnosis of pelvic serous cancers from non-gynecologic cancers [25-29]. Proliferative endometrium and benign tubal section from young women without known risk for cancer served as positive controls for PAX8. Negative controls were carried out by replacing primary antibodies with class-matched mouse immunoglobulin G on parallel sections. The subcellular staining localization is nuclear for PAX8, and the number of secretory cells was counted based on the nuclear stained cells. TIC and ovarian cancer were confirmed by either consensus diagnosis by at least 2 pathologist co-authors or by staining with p53 and MIB1 [22,23].
Data evaluation and statistical analysis
Scoring was performed by two observers independently. When discrepancy was encountered, a third author was included for the evaluation. The following parameters were calculated for the cases studied: 1) The number of SCEs and SCOUTs and their distribution, calculated by both morphologic and immunohistochemical analyses, in different age groups using 10-year intervals; 2) The comparison of the number of SCEs in the ampulla and the fimbria within the fallopian tube; 3) The frequency of SCE and SCOUTs in cases and controls; 4) The frequency of TIC in cases and controls after correcting for age.
The data were analyzed by standard contingency table methods and nonparametric Mann-Whitney U-tests using the Eproliferative index LOG (Epicenter Software, Pasadena, CA, USA) and Stat View (SAS Institute, Cary, NC, USA) computer package programs. To adjust for age differences and the varying number of sections or microscopic fields examined for each case, the data were calculated on the assumption that the number of secretory cells, ciliated cells, SCEs, and SCOUTs, in each case follows a Poisson distribution, which is commonly used to model count data, with an offset term used to account for the microscopic fields examined. For comparisons of patient ages, the age data were stratified into 10-year intervals ranging from 20-29 to older than 80.
Results
As age increased, the number of secretory cell expansions increased
A total of 270 benign subjects, divided into 10 groups based on age (ie 20-29, 30-39, 70-79, and >80), were studied. There was a clear positive correlation between number of stretches of secretory cell expansion (SCE) in the fallopian tube and age. The number of SCE segments increased from not observed in the age group of 20-29, to an average of 18 segments of SCE/tube was present at age older than 70. This positive correlation of SCE increment with age was statistically significant (P < 0.001). The number of SCEs in the tubal ampulla and fimbriated end was calculated and compared. It was found that SCE segments in the tubal fimbria were significantly more numerous than that in the ampulla region. Both light microscopy and IHC methods were used to count the cells. There was no significant difference between the morphologic counting and the IHC calculation (data not shown). The detailed data about the overall number of SCEs in tubal segments by using microscopic counting are summarized in Table 1 and visualized as a bar graph in Figure 1.
Table 1.
The number of tubal secretory cell expansion changes among women in different age
| #SCE/Age | 20-29 (n=12) | 30-39 (n=25) | 40-49 (n=56) | 50-59 (n=68) | 60-69 (n=47) | 70-79 (n=37) | >80 (n=25) |
|---|---|---|---|---|---|---|---|
| Fimbria | 0.00 | 2.31 | 4.39 | 6.86 | 7.32 | 9.35 | 11.6 |
| Ampulla | 0.00 | 1.22 | 2.23 | 2.68 | 3.72 | 3.39 | 3.87 |
SCE: secretory cell expansion;
SCE represents the number of SCE/fallopian tube.
There is a clear trend that the number of SCE increases as a function of age in both fimbria and ampulla segments (P < 0.001). Compared to the number of SCE in tubal ampulla region, tubal fimbria has a significantly higher number of SCEs (P < 0.05). Standard deviations are shown in the corresponding bar graph in Figure 1.
Figure 1.
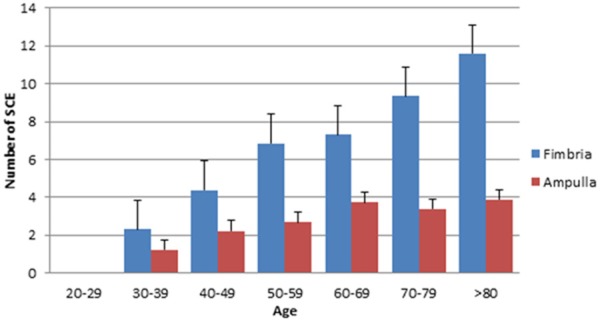
The number of secretory cell expansion increases with age. The figure corresponds to Table 1.
Increased number of secretory cell expansion is significantly associated with age, high-risk factors, and the status of ovarian cancer
Among 270 patients with benign gynecologic diseases, we selected 80 patients, whom we age-matched with the case group, for the comparison of overall number of SCEs in the fallopian tube by using both morphologic and IHC methods. Typically, the number of SCEs in benign tubes ranged from 0 to 9.90 with an average of 4.8/fallopian tube in the tubal fimbriated end. However, the number of SCEs was significantly increased in tubal segments from the patients with high-risk and OvCa. Compared to the benign group, the number of SCEs was about 2.5- and 3-fold greater in high-risk and OvCa patients, respectively, in tubal fimbria (P < 0.01). Interestingly, there was no change of SCE frequencies among the three groups in the tubal ampulla segments. The number of SCEs calculated with either microscopic direct counting (H&E) or IHC (PAX8/tubulin) method showed no statistical difference. The detailed data are summarized in Table 2. Representative pictures of the tubal secretory and ciliated cells are illustrated in Figure 2.
Table 2.
Increased number of secretory cell expansion significantly associated with age, high-risk factors, and the status of ovarian cancer
| Age | Benign (n=80) | High risk (n=80) | OvCa (n=76) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Fimbria | Ampulla | Fimbria | Ampulla | Fimbria | Ampulla | |
| 20-29 | 0.00 | 0.00 | - | - | - | - |
| 30-39 | 2.25 | 1.33 | 4.73 | 2.21 | - | - |
| 40-49 | 3.73 | 2.75 | 6.82 | 3.11 | 7.43 | 3.08 |
| 50-59 | 5.95 | 3.13 | 8.24 | 3.33 | 12.76 | 3.16 |
| 60-69 | 6.85 | 3.56 | 13.62 | 3.65 | 18.30 | 3.43 |
| 70-79 | 8.63 | 3.65 | 22.23 | 3.48 | 30.21 | 3.76 |
| >80 | 9.90 | 3.83 | - | - | 32.82 | 3.40 |
OvCa: ovarian cancer. There was no high-risk cases with age elder than 80 and no OvCa cases younger than 40 found in this study.
Figure 2.
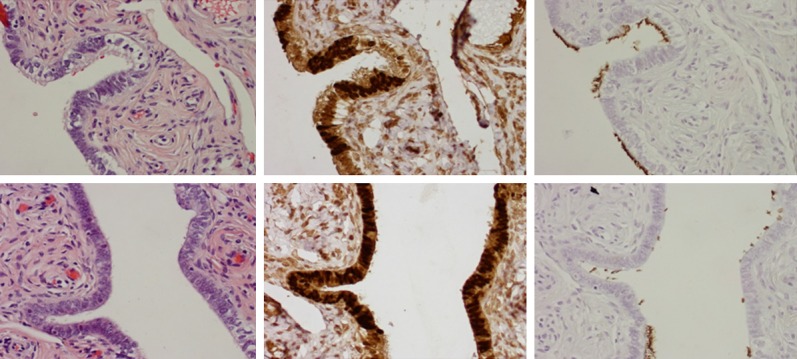
Secretory and ciliated cell distribution in tubal ampulla region. The tubal sections were from benign group (left panel), high-risk group (middle panel), and PSC (right panel). Low power (40×), light microscopic picture of the tubal sections (upper panel, A-C); Medium power (100×), light microscopy findings (the second row, D-F); PAX8 staining illustrates nuclear staining in the secretory cells (the third row, G-I); and tubulin staining shows apical brushes of the ciliated cells (bottom panel, J-L). Brown cytoplasmic stainings are nonspecific background (G).
Finding the results that increased number of SCEs in tubal fimbria is associated with increased age and is more frequent in high-risk and OvCa patients, we explored whether the increased number of SCEs in high-risk or OvCa patients are independent of age. We addressed this question in a regression model that adjusted for age, as well as the number of cross-sections examined for SCE by linear regression analysis. The three groups (benign control, high-risk, and OvCa) of patients were divided according to 10-year intervals and average frequencies for the intervals were compared. There were still significant differences in the increased number of SCEs as a function of increasing age in both case groups and in the control group. When all three groups were combined to increase n-size for age comparison, a significant correlation was observed with a determination coefficient of 0.163. This implies that approximately 16% of the increase in SCE could be attributed to age. Both high-risk and OvCa groups registered higher numbers of SCEs than in benign controls (P < 0.001), with an average increase of 0.78 log counts for high-risk cases vs. control and 0.86 log for OvCa cases vs control. Therefore, age, high-risk factors, and OvCa are all independent risk factors for increased number of SCEs. Presence of OvCa showed the strongest association with increased number of SCEs while age showed the weakest, but all three variables were statistically significant.
Compared with secretory cell outgrowth, secretory cell expansion was more frequently associated with patients showing high-risk or ovarian cancer
As we have defined earlier, SCE contains more than 10 secretory cells in a non-interrupted fashion, and this concept covers the SCOUTs entity. The results presented here represented pure secretory cells (confirmed by positive PAX8 stain) without ciliated cells (confirmed by lack of tubulin stain). For each case, a frequency distribution of SCE and SCOUTs expressed as a fraction of the total number of tubal segments examined was compiled. Considering that the overall number of TIC is much less than SCE or SCOUTs, we calculated the number of positive cases regardless of the number of foci of lesions found in a single case. Overall, SCE was a more sensitive marker for the process of serous carcinogenesis. The frequency of SCE was more common in tubal segments of patients with high-risk (4.8-fold) and those with OvCa (6.1-fold) than that in the control group (P < 0.0001). In contrast, the frequency of SCOUTs per case was 2.1-fold higher in high-risk patients and 3.5-fold higher in PSCs (P < 0.05).
The measurements of SCE and SCOUTs in the fallopian tube were parallel in either high risk or OvCa patients. However, compared with SCOUTs, presence of SCE in tubal segments was far more frequently associated with either high risk factors or presence of OvCa. The absolute frequency of TIC in tubal fimbria was 0% (0/80) in the benign group, 15% (12/80) in high-risk, and 33% (25/76) in OvCa. There was no TIC found in tubal ampulla segments from high-risk patients. There were only 3 ampulla foci of TIC present in OvCa cases, which also showed concurrent foci of TIC in the fimbriated end. The data of TIC are comparable to previous findings [22,23]. The distribution of individual frequencies of SCE, SCOUTs, and TIC in the control and study groups is summarized in Table 3.
Table 3.
Tubal fimbrial secretory cell expansion and tubal intraepithelial carcinoma in cases and controls
| Group | #case | Mean age | SCE | SCOUTs | TIC |
|---|---|---|---|---|---|
|
| |||||
| (mean#/case ± SD) | #of + cases (%) | ||||
| Benign | 80 | 46.5 | 4.8 ± 0.8 | 1.6 ± 0.2 | 0 (0) |
| HR | 80 | 45.8 | 22.6 ± 2.1 | 3.3 ± 0.5 | 12 (15) |
| PSC | 76 | 60.6 | 30.3 ± 2.6 | 5.6 ± 1.2 | 25 (33) |
HR: high risk; PSC: pelvic serous carcinoma; SCE: secretory cell expansion; SCOUT: secretory cell outgrowth; TIC: tubal intraepithelial carcinoma.
Discussion
In this study, we have examined the global change of tubal secretory and ciliated cells and its relationship with the aging process by using S/C ratio, SCE, SCOUTs in patients with low-risk (benign group), high-risk, and PSC. For patients in the low-risk category of pelvic serous cancer group (control), the S/C ratio increases with age, starting from less than 0.5 in the ages 20-29 range and peaking at more than 3 after age 70. The S/C ratio steadily increases as the age group of women increases, which is consistent with well-known epidemiologic findings that PSC increases with age and shows a peak incidence after menopause [16,30,31]. A more dramatic increase of S/C ratio is observed in patients with high-risk factors, such as BRCA mutations or family history of ovarian cancer, and in patients with PSC. This increased S/C ratio is also closely associated with age in both high-risk and PSC patients. Our findings further indicate that an increase in the absolute number of secretory cells within the fallopian tube is linked to pelvic serous neoplasia. In a recent study about the PAX2 null secretory cells (SCOUTs) in relation to serous neoplasia, Quick et al. also showed that the frequency of SCOUTs increases with age, but this was not an independent risk factor for serous neoplasia after age adjustment when compared with PSC patients [6]. We used a similar approach to address the age effect in the process of pelvic serous carcinogenesis and found that age alone is an independent risk factor, although it is less dramatic than the likely genetic alterations affecting high-risk and PSC populations [2,32,33]. Possible reasons to explain the discrepancy between studies includes our larger sample size and our defined secretory cell target. Quick’s study used SCOUTs with loss of PAX2 expression, which is more strict criteria, while we used global change of secretory versus ciliated cell ratio, which candidly reflects the overall growth of the secretory cells within the fallopian tube. Approximately 90% of women’s pelvic serous cancers, including tubal, ovarian, and peritoneal origins, are sporadic without identifiable genetic reasons [34], and the criteria in our study allow inclusion of this population of cells. The effect of age in the development of serous neoplasia has a significant social and medical impact. More studies are needed to address the age effect in the process of pelvic serous neoplasia.
Tubal secretory cells are currently believed to be the cellular origin for the majority PSCs. The current model for the serous carcinogenesis starts from the expansion of secretory cells or focal loss of tubal ciliated cells resulting in an increased S/C ratio. These stretches of SCEs are usually distributed evenly within the tubal fimbria and ampulla segments. In this study, we examined the number of secretory and ciliated cells and the frequency of SCE and SCOUTs in both tubal segments by using both morphologic and immunohistochemical methods. We found that there is no significant difference between the two tubal regions for either the S/C ratio or the frequency of SCEs or SCOUTs. Both counting methods are comparable. Assuming all the secretory cells are equally susceptible to serous neoplasia, the frequency of serous TIC or early invasive carcinoma should be equally distributed in those tubal regions. However, many studies in the last decade revealed that the majority of TICs with or without early invasions are located in the tubal fimbria, while only a small percentage of TICs are present in the tubal ampulla [15,35-37]. This supports the tubal fimbria as the main anatomic site for serous neoplasia. The mechanism of this fimbria-focused serous neoplasia has yet to be determined. Detailing the molecular changes in addition to p53 [15] and PAX2 [6] between the tubal fimbria and ampulla may shed light for our understanding.
The concept of SCOUTs is defined as the expansion of tubal secretory cells to 30 or more forming a morphologically distinct linear secretory cell population. This is a valid biomarker since it has been linked to the serous neoplasia [5,6,13,38]. However, tubal mucosa normally consists of both secretory and ciliated cells, arranged in a recurring pattern of alternating secretory cells with ciliated, which is typically less than 10 cells of one or the other cell type. We examined the concept of SCEs defining a linear stretch of more than 10 secretory cells without interrupting ciliated cells in between. In this study, we found that the frequency of SCE is more common than SCOUTs in all patients studied. Both SCEs and SCOUTs are more frequent in tubal segments of patients with either high-risk or PSC than in controls. However, SCE is more sensitive than SCOUTs in association with serous neoplasia. Though comparable to SCOUTs, SCE is morphologically distinct, and it can be used as an early, morphologically recognizable biomarker in the process of serous carcinogenesis. Genetic studies of SCE on a molecular level will likely facilitate strategies of PSC prevention and early intervention.
Acknowledgements
Dr. Yiying Wang was partially supported by Health Department, Henan Province, China. Dr. Li Li was partially supported by Postdoctoral International Exchange Program, China. Both Drs. Wang and Li were partially supported by Department of Pathology, University of Texas Southwestern Medical Center, USA. The project was also funded in part by Better Than Ever Grant, P30 CA23074 grant from University of Arizona Cancer Center and University of Texas Southwestern Medical Center startup fund to WZ.
Disclosure of conflict of interest
None.
Abbreviations
- OvCa
ovarian cancer
- SCE
secretory cell expansion
- SCOUTs
secretory cell outgrowths
- TIC
tubal intraepithelial carcinoma
References
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 1993;329:1550–1559. doi: 10.1056/NEJM199311183292108. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Fadare O, Xiang L, Kong B, Zheng W. Ovarian serous carcinoma: recent concepts on its origin and carcinogenesis. J Hematol Oncol. 2012;5:8. doi: 10.1186/1756-8722-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delair D, Soslow RA. Key features of extrauterine pelvic serous tumours (fallopian tube, ovary, and peritoneum) Histopathology. 2012;61:329–339. doi: 10.1111/j.1365-2559.2011.04167.x. [DOI] [PubMed] [Google Scholar]
- 4.Brewer MA, Johnson K, Follen M, Gershenson D, Bast R Jr. Prevention of ovarian cancer: intraepithelial neoplasia. Clin Cancer Res. 2003;9:20–30. [PubMed] [Google Scholar]
- 5.Li J, Abushahin N, Pang S, Xiang L, Chambers SK, Fadare O, Kong B, Zheng W. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol. 2011;24:1488–1499. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- 6.Quick CM, Ning G, Bijron J, Laury A, Wei TS, Chen EY, Vargas SO, Betensky RA, McKeon FD, Xian W, Crum CP. PAX2-null secretory cell outgrowths in the oviduct and their relationship to pelvic serous cancer. Mod Pathol. 2012;25:449–455. doi: 10.1038/modpathol.2011.175. [DOI] [PubMed] [Google Scholar]
- 7.Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, Muto MG, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J. Clin. Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 10.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J. Clin. Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 11.Crum CP. Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol Oncol. 2009;3:165–170. doi: 10.1016/j.molonc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Wei L, Li L, Yang B, Kong B, Yao G, Zheng W. Ovarian serous carcinogenesis from tubal secretory cells. Histol Histopathol. 2015;30:1295–302. doi: 10.14670/HH-11-645. [DOI] [PubMed] [Google Scholar]
- 13.Chen EY, Mehra K, Mehrad M, Ning G, Miron A, Mutter GL, Monte N, Quade BJ, McKeon FD, Yassin Y, Xian W, Crum CP. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J Pathol. 2010;222:110–116. doi: 10.1002/path.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levanon K, Ng V, Piao HY, Zhang Y, Chang MC, Roh MH, Kindelberger DW, Hirsch MS, Crum CP, Marto JA, Drapkin R. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehra K, Mehrad M, Ning G, Drapkin R, McKeon FD, Xian W, Crum CP. STICS, SCOUTs and p53 signatures; a new language for pelvic serous carcinogenesis. Front Biosci (Elite Ed) 2011;3:625–634. doi: 10.2741/e275. [DOI] [PubMed] [Google Scholar]
- 16.Crum CP, McKeon FD, Xian W. BRCA, the oviduct, and the space and time continuum of pelvic serous carcinogenesis. Int J Gynecol Cancer. 2012;22(Suppl 1):S29–34. doi: 10.1097/IGC.0b013e31824d7269. [DOI] [PubMed] [Google Scholar]
- 17.Colgan TJ. Challenges in the early diagnosis and staging of Fallopian-tube carcinomas associated with BRCA mutations. Int J Gynecol Pathol. 2003;22:109–120. doi: 10.1097/00004347-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Shaw PA, Rouzbahman M, Pizer ES, Pintilie M, Begley H. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol. 2009;22:1133–1138. doi: 10.1038/modpathol.2009.89. [DOI] [PubMed] [Google Scholar]
- 19.Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, Lee Y, Crum CP. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Ning Y, Abushahin N, Yuan Z, Wang Y, Wang Y, Yuan B, Cragun JM, Chambers SK, Hatch K, Kong B, Zheng W. Secretory cell expansion with aging: risk for pelvic serous carcinogenesis. Gynecol Oncol. 2013;131:555–560. doi: 10.1016/j.ygyno.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 22.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma f the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, Garber JE, Cramer DW, Crum CP. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 24.Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73:1–14. doi: 10.1016/j.bcp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, McDonald JF. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–337. doi: 10.1016/j.ygyno.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 26.Mittag J, Winterhager E, Bauer K, Grummer R. Congenital hypothyroid female pax8-deficient mice are infertile despite thyroid hormone replacement therapy. Endocrinology. 2007;148:719–725. doi: 10.1210/en.2006-1054. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008;32:1566–1571. doi: 10.1097/PAS.0b013e31816d71ad. [DOI] [PubMed] [Google Scholar]
- 28.Tong GX, Devaraj K, Hamele-Bena D, Yu WM, Turk A, Chen X, Wright JD, Greenebaum E. Pax8: a marker for carcinoma of Mullerian origin in serous effusions. Diagn Cytopathol. 2011;39:567–574. doi: 10.1002/dc.21426. [DOI] [PubMed] [Google Scholar]
- 29.Laury AR, Hornick JL, Perets R, Krane JF, Corson J, Drapkin R, Hirsch MS. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am J Surg Pathol. 2010;34:627–635. doi: 10.1097/PAS.0b013e3181da7687. [DOI] [PubMed] [Google Scholar]
- 30.Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiol Biomarkers Prev. 2009;18:132–139. doi: 10.1158/1055-9965.EPI-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crum CP, McKeon FD, Xian W. The oviduct and ovarian cancer: causality, clinical implications, and “targeted prevention”. Clin Obstet Gynecol. 2012;55:24–35. doi: 10.1097/GRF.0b013e31824b1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folkins AK, Jarboe EA, Roh MH, Crum CP. Precursors to pelvic serous carcinoma and their clinical implications. Gynecol Oncol. 2009;113:391–396. doi: 10.1016/j.ygyno.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J. Clin. Oncol. 2008;26:5284–5293. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55:3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- 35.Semmel DR, Folkins AK, Hirsch MS, Nucci MR, Crum CP. Intercepting early pelvic serous carcinoma by routine pathological examination of the fimbria. Mod Pathol. 2009;22:985–988. doi: 10.1038/modpathol.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vang R, Shih Ie M, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology. 2013;62:44–58. doi: 10.1111/his.12046. [DOI] [PubMed] [Google Scholar]
- 37.Tang S, Onuma K, Deb P, Wang E, Lytwyn A, Sur M, Daya D. Frequency of serous tubal intraepithelial carcinoma in various gynecologic malignancies: a study of 300 consecutive cases. Int J Gynecol Pathol. 2012;31:103–110. doi: 10.1097/PGP.0b013e31822ea955. [DOI] [PubMed] [Google Scholar]
- 38.Wen J, Shi JL, Shen DH, Chen YX, Song QJ. [Morphologic changes of fallopian tubal epithelium in ovarian serous tumors] . Zhonghua Bing Li Xue Za Zhi. 2012;41:433–437. doi: 10.3760/cma.j.issn.0529-5807.2012.07.001. [DOI] [PubMed] [Google Scholar]


