Summary
Background
Couples HIV testing and counselling (CHTC) is encouraged but is not widely done in sub-Saharan Africa. We aimed to compare two strategies for recruiting male partners for CHTC in Malawi’s option B+ prevention of mother-to-child transmission programme: invitation only versus invitation plus tracing and postulated that invitation plus tracing would be more effective.
Methods
We did an unblinded, randomised, controlled trial assessing uptake of CHTC in the antenatal unit at Bwaila District Hospital, a maternity hospital in Lilongwe, Malawi. Women were eligible if they were pregnant, had just tested HIV-positive and therefore could initiate antiretroviral therapy, had not yet had CHTC, were older than 18 years or 16–17 years and married, reported a male sex partner in Lilongwe, and intended to remain in Lilongwe for at least 1 month. Women were randomly assigned (1:1) to either the invitation only group or the invitation plus tracing group with block randomisation (block size=4). In the invitation only group, women were provided with an invitation for male partners to present to the antenatal clinic. In the invitation plus tracing group, women were provided with the same invitation, and partners were traced if they did not present. When couples presented they were offered pregnancy information and CHTC. Women were asked to attend a follow-up visit 1 month after enrolment to assess social harms and sexual behaviour. The primary outcome was the proportion of couples who presented to the clinic together and received CHTC during the study period and was assessed in all randomly assigned participants. This study is registered with ClinicalTrials.gov, number NCT02139176.
Findings
Between March 4, 2014, and Oct 3, 2014, 200 HIV-positive pregnant women were enrolled and randomly assigned to either the invitation only group (n=100) or the invitation plus tracing group (n=100). 74 couples in the invitation plus tracing group and 52 in the invitation only group presented to the clinic and had CHTC (risk difference 22%, 95% CI 9–35; p=0·001) during the 10 month study period. Of 181 women with follow-up data, two reported union dissolution, one reported emotional distress, and none reported intimate partner violence. One male partner, when traced, was confused about which of his sex partners was enrolled in the study. No other adverse events were reported.
Interpretation
An invitation plus tracing strategy was highly effective at increasing CHTC uptake. Invitation plus tracing with CHTC could have many substantial benefits if brought to scale.
Introduction
In 2011, Malawi was the first country to implement option B+, a prevention of mother-to-child transmission (PMTCT) programme designed to provide all pregnant and breastfeeding HIV-positive women with free lifelong antiretroviral therapy from the time of HIV diagnosis, irrespective of CD4 cell count or clinical stage.1 Many other countries in the region have followed suit2 and WHO has endorsed this approach. Option B+ was designed to reduce mother-to-child transmission, morbidity, and mortality in HIV-positive women, and HIV transmission to HIV-negative sex partners.3 Since option B+ implementation, antenatal HIV testing and counselling (HTC) and provision of immediate antiretroviral therapy has expanded rapidly4 with corresponding reductions in mother-to-child transmission rates.5
Despite these advances, important challenges remain in option B+. Most loss of women from the option B+ programme occurs immediately after initiation.6 The odds of no follow-up are five times higher in women initiating antiretroviral therapy for pregnancy than in those initiating antiretroviral therapy for clinical indication.6 Difficulties with HIV status disclosure to sex partners contributes to early loss from option B+; women who cannot disclose often default (ie, do not return for antiretroviral therapy pickup <60 days after their scheduled appointment).7
Couples HTC (CHTC) is an effective strategy for supporting HIV status disclosure in HIV-positive women. During CHTC, both members of a couple learn their own and their partner’s HIV status from a counsellor. WHO has encouraged CHTC for mutual awareness of HIV status,8 treatment as prevention,3 behavioural HIV prevention,9,10 and prevention of mother-to-child transmission.11,12
Despite the many known benefits of CHTC, uptake remains low in antenatal settings throughout the region, including Bwaila Hospital,13 suggesting a need for partner recruitment strategies. Provider-supported strategies have been more effective than patient-led strategies for partner recruitment.14–17 However, provider-supported partner-recruitment strategies have not been assessed within the context of an option B+ programme, nor have they been assessed with CHTC as the primary outcome. Typically they have been used to promote individual testing.
We aimed to compare two strategies for recruiting male partners for CHTC: invitation only versus invitation plus tracing. We hypothesised that invitation plus tracing would be the most effective. We also assessed whether each strategy was associated with 1 month option B+ retention, sexual behaviour changes, HIV test results for men (known HIV-positive, new HIV-positive, or HIV-negative), and linkage of men to care.
Methods
Study design and participants
We did an unblinded, randomised, controlled trial comparing an invitation only strategy of recruitment with an invitation plus tracing strategy in the antenatal unit at Bwaila District Hospital, a maternity hospital in Lilongwe, Malawi, providing antenatal care to 13 500 pregnant women annually. Bwaila has offered option B+ services since 2011. During this period, the clinic was staffed with two counsellors who provided opt-out HTC to all pregnant women at their first antenatal visit unless they were already taking antiretroviral therapy or had a documented HIV-positive test or HIV-negative test (<3 months ago) in their health passport. HIV-positive women were routinely offered antiretroviral therapy at the time of diagnosis. Immediately before the study, at the first antenatal visit, most women presented alone and received individual HTC; about 10% presented with a partner and received CHTC. Women were typically encouraged to return for a subsequent antenatal visit with a male partner, but this was uncommon (about 1%).
All women presenting to Bwaila antenatal care clinic for their first antenatal visit without a documented HIV status were tested for HIV with routine opt-out HTC clinical procedures. Many HIV-positive women with CD4 counts of more than 500 cells per µL enrolled in another study (PROMISE, NCT01253538) and these women were not eligible for participation in this study. Study screening of HIV-infected women occurred after HTC post-test counselling. Women were eligible if they were pregnant, older than 18 years or 16–17 years and married, had not received CHTC at that visit, intended to remain in Lilongwe for 1 month, and had a locatable sex partner in Lilongwe. Women who were already receiving antiretroviral therapy were not eligible. Eligible women who refused to participate in this study were asked to fill out a refusal form with reasons for refusal. Eligible women who wished to participate provided written informed consent and locator information and responded to an interviewer-administered questionnaire about demographics, HTC history, and sexual behaviour. They then received their randomised assignment; those assigned to invitation plus tracing signed a contract permitting partner tracing. Approval was provided by National Health Sciences Research Committee of Malawi and UNC School of Medicine Institutional Review Board. The protocol is available online.
Randomisation and masking
Women were randomly assigned (1:1) to either the invitation only group or the invitation plus tracing group with block randomisation (block size=4) by a biostatistician. Randomised assignments were placed in sealed envelopes and numbered consecutively. A study nurse opened the envelopes and informed participants of assignment. Study staff were aware of randomisation assignment; clinic staff were not informed of randomisation assignment, but might have learned of it from participants or colleagues.
Procedures
In both groups, female participants (indexes) were given one invitation inviting a male partner with the message: “At Bwaila Hospital we are providing family-focused services for pregnant women and their male partners. We ask you to accompany your partner to the antenatal clinic so that we can provide you with important health information.” The invitation did not disclose the index’s HIV-positive status or mention CHTC, as formative work suggested a general health message would enhance acceptability. A trained research nurse worked with each index to identify an appointment date within 1 week and wrote this on the invitation, but indexes were told they could present at any time. Indexes were told they could disclose their HIV status on their own or through cHCT.
In the invitation plus tracing group, women signed a contract granting permission for a community health worker to trace the partner if he did not present within 1 week. Female partners provided male partner locator information, including phone numbers, physical addresses, and directions. Men were traced by a community health worker employed full-time as part of the research team. During phone and community encounters, the community health worker used messages consistent with the invitation. He did not disclose the index’s HIV status or mention CHTC. He discussed strategies to help participants overcome barriers to attendance and then agree on a day and time to attend. These encounters were about 5 min. The community health worker was supposed to make three phone attempts followed by three community attempts. Phone tracing was supposed to be initiated just after the couple appointment date, and physical tracing just after this, although in practice phone tracing often extended for several weeks with corresponding delays in physical tracing.
About 1 week after the initial visit, women were asked to present with partners for a couple visit. Both partners received information on pregnancy topics, including nutrition, tobacco, alcohol, malaria, antenatal care-seeking, facility delivery, and the importance of CHTC by a research nurse. They were then offered opt-out CHTC by clinic counsellors, consisting of couple pre-test counselling, HIV testing, and couple post-test counselling. Male partners then provided informed consent and completed an interviewer-administered enrolment questionnaire. If one or both partners had already tested HIV-positive and disclosed to their partner, they were typically not retested. For HIV-positive male partners not receiving treatment, a Pima (Alere, Waltham, MA, USA) CD4 cell count test was done, and they were referred for HIV care and treatment.
1 month after enrolment, indexes were asked to attend a study follow-up visit, coinciding with their first antiretroviral therapy refill appointment. At this visit, women were asked about their experience with HIV prevention and treatment behaviour and social harms. Consistent with other studies in Lilongwe, a transport allowance was provided for each study visit.
Data were obtained on paper forms by trained research staff in Chichewa and double entered into a password-protected Microsoft Access database on a secure server. Entries were compared and discrepancies were reconciled. The study database was merged with electronic medical records from the antenatal option B+ programme at Bwaila Hospital using unique identifiers. A trained research assistant linked men to the electronic medical records of an adjacent antiretroviral therapy clinic, Martin Preuss Centre, using names, addresses, dates, and ages. If all information was concordant, they were regarded as linked to care. The principal investigator adjudicated a few entries that were similar, but not identical. Electronic records were administered by Lighthouse Trust.
Outcomes
The primary outcome was the proportion of couples who presented to the clinic together and received CHTC from enrolment through to the study closure date, which was Jan 2, 2015. For this analysis, CHTC refers to counsellor-assisted mutual disclosure, irrespective of whether both partners received an HIV test. We also assessed HIV status of men, proportion of HIV-positive men newly diagnosed, and proportion of newly diagnosed HIV-positive men linked to care as secondary outcomes. The proportion of indexes who defaulted was assessed by use of clinic records. The proportion of women who reported unprotected sex at baseline and no unprotected sex (either through abstinence or consistent condom use) at follow-up was used to assess initiation of safe sex.
Statistical analysis
To have 96% power to detect a difference of 25% CHTC uptake in the invitation only group and 50% CHTC uptake in the invitation plus tracing group, with a two-tailed test and an α level of 0·05, we decided on a sample size of 100 women per group. We calculated risk differences comparing outcomes between the two groups using generalised linear models with identity links and binomial distributions. Time to CHTC was calculated with the Kaplan-Meier estimator. These analyses were done on an intention-to-treat basis on the basis of study group assignment. We calculated risk ratios assessing factors associated with 3 month CHTC with log-binomial regression models. The primary outcome was assessed 3 months after enrolment because all participants had at least 3 months of follow-up. An α level of 0·05 was deemed statistically significant and an α level of 0·1 was regarded as a statistical trend. We analysed data with Stata version 12.1. This trial is registered with ClinicalTrials.gov, number NCT02139176.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 4, 2014, and Oct 3, 2014, 576 women were tested individually and diagnosed with HIV at Bwaila antenatal care clinic. Of these women, 175 (30%) were referred to the PROMISE trial (NCT01253538) and 65 (11%) were not screened at all because of study staffing issues. Of 336 women screened, 220 (65%) were eligible for enrolment in this study. The main reason for ineligibility was not having a sex partner in Lilongwe (figure 1). 200 (91%) women provided consent and were enrolled in the study and 20 (9%) women refused participation. Of these, only four (20%) filled out a refusal form. They described not having time (n=2), not believing the partner would present, and wanting to consult a partner before research participation as reasons for refusal. All four reported that they had disclosed or expected to disclose their HIV-positive status to their partner; none reported fear of abandonment or violence. The median age of participants was 26 years (IQR 22–30 years). Before enrolment, 22 (11%) women of the 200 enrolled had never been tested, 141 (71%) had previously tested HIV-negative, and 35 (18%) had previously tested HIV-positive, based on self-report. Baseline characteristics had a similar distribution between groups (table 1).
Figure 1. Trial profile.
CHTC=couple HIV testing and counselling.
Table 1.
Baseline characteristics
| Invitation only group (n=100) |
Invitation plus tracing group (n=100) |
|
|---|---|---|
| Age (years) | ||
| 16–17 | 1 (1%) | 1 (1%) |
| 18–25 | 43 (43%) | 43 (43%) |
| 26–35 | 52 (52%) | 52 (52%) |
| ≥36 | 4 (4%) | 4 (4%) |
| Education | ||
| Primary not completed | 44 (44%) | 44 (44%) |
| Primary completed | 14 (14%) | 16 (16%) |
| Secondary not completed | 27 (27%) | 25 (25%) |
| Secondary completed | 15 (15%) | 15 (15%) |
| Material floor of house is made of | ||
| Dirt or dung | 29 (29%) | 22 (22%) |
| Cement or tile | 71 (71%) | 78 (78%) |
| Hunger in the past month | ||
| No | 84 (84%) | 85 (85%) |
| Yes | 16 (16%) | 15 (15%) |
| Earns a salary | ||
| No | 96 (96%) | 92 (92%) |
| Yes | 4 (4%) | 8 (8%) |
| Trimester of pregnancy | ||
| First | 13 (13%) | 16 (16%) |
| Second | 72 (72%) | 72 (72%) |
| Third | 15 (15%) | 12 (12%) |
| Primiparous | ||
| No | 82 (82%) | 85 (85%) |
| Yes | 18 (18%) | 15 (15%) |
| Duration with partner (years) | ||
| ≤1 | 28 (28%) | 32 (32%) |
| 1–5 | 23 (23%) | 26 (26%) |
| >5 | 49 (49%) | 42 (42%) |
| Female HTC history* | ||
| Never tested | 12 (12%) | 10 (10%) |
| Previously negative | 69 (70%) | 72 (73%) |
| Previously positive | 18 (18%) | 17 (17%) |
| Previous CHTC | ||
| No | 80 (80%) | 78 (78%) |
| Yes | 20 (20%) | 22 (22%) |
| Previous disclosure to a partner† | ||
| No | 19 (22%) | 16 (18%) |
| Yes | 69 (78%) | 74 (82%) |
| Female perception of partner HTC history | ||
| No test or does not know | 64 (64%) | 61 (61%) |
| Negative | 27 (27%) | 28 (28%) |
| Positive | 8 (8%) | 11 (11%) |
| Indeterminate | 1 (1%) | 0 |
| Number of sex acts in past month‡ | ||
| None | 3 (3%) | 2 (2%) |
| 1–7 | 43 (43%) | 37 (37%) |
| 8–14 | 38 (38%) | 40 (40%) |
| ≥15 times | 16 (16%) | 21 (21%) |
| Condom use in last three sex acts‡ | ||
| Never | 95 (95%) | 98 (98%) |
| 1–2 times | 2 (2%) | 1 (1%) |
| 3 times | 3 (3%) | 1 (1%) |
| More than one sex partner in the past year | ||
| No | 76 (76%) | 85 (85%) |
| Yes | 24 (24%) | 15 (15%) |
| Believes partner has had other partners in the past year‡ | ||
| No | 19 (19%) | 14 (14%) |
| Uncertain | 42 (42%) | 46 (46%) |
| Yes | 39 (39%) | 40 (40%) |
| Participant ever yelled at or threatened by partner‡ | ||
| No | 51 (51%) | 52 (52%) |
| Yes | 49 (49%) | 48 (48%) |
| Participant ever physically hurt by partner‡ | ||
| No | 86 (86%) | 89 (89%) |
| Yes | 14 (14%) | 11 (11%) |
Data are n (%). Responses are based on self-report by female participants.
HTC=HIV testing and counselling. CHTC=couple HIV testing and counselling.
Responses missing for two women; the denominator is 99 in each column.
Only in those who previously tested; the denominator is 88 in the invitation only group and 90 in the invitation plus tracing group.
Restricted to the partner that the woman planned to invite to the clinic.
Nearly all women (99%) planned to give the invitation to a man they were married to, living with, and believed to be the father of their current pregnancy. Median relationship length was 4 years (IQR 1–8 years). 125 women (63%) had never learned their partner’s HIV status. With respect to partner HIV status disclosure, 81 (46%) of 175 feared partner anger, 19 (37%) feared abandonment, and 64 (11%) feared being physically hurt.
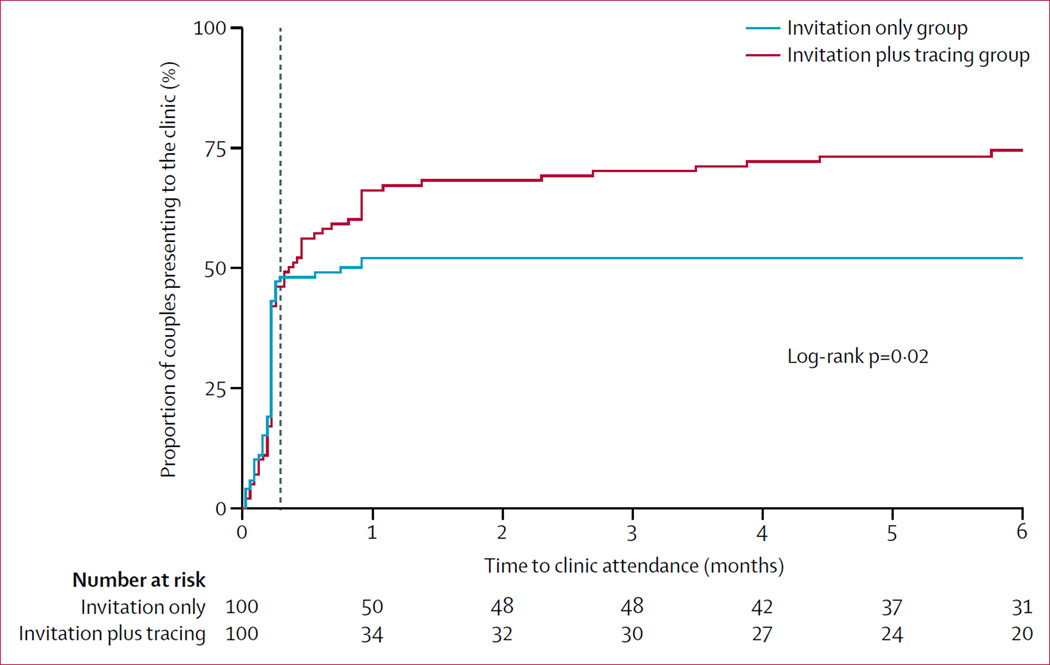
In the invitation only group, 52 of 100 couples presented for CHTC compared with 74 of 100 in the invitation plus tracing group (p=0·001). In the first week, presentation was nearly identical: 43 from the invitation only group and 42 from the invitation plus tracing group. After initiation of tracing, presentation was substantially higher in the invitation plus tracing group (figure 2). Of the 100 men in the invitation plus tracing group, 51 presented without tracing, 18 presented after phone tracing, three presented after community tracing, and two presented after both phone and community tracing. 12 men were not traceable with no phone number or no locatable physical address and 14 did not present despite tracing (nine by phone, one in the community, and four by both). From the 18 men who presented after phone tracing, 15 presented before or at the female partner’s antiretroviral therapy refill visit. Of the five men who needed home tracing, only one presented within this period.
Figure 2. Kaplan-Meier curve of time to presentation of male partners at couples HIV testing and counselling.
The vertical line at 8 days shows the time when tracing was supposed to be initiated.
From the 126 men who presented, 122 (97%) presented within 3 months of index enrolment. Those in the invitation plus tracing group were 1·35 times (95% CI 1·07–1·69) more likely to present within 3 months than those in the invitation only group. This association was not confounded by any baseline factors. Few other baseline factors were associated with partner presentation within 3 months (table 2). A partner was more likely to present if the index already knew he was HIV-infected, but this was a minority of partners (table 2). A partner was less likely to present if the index was afraid of being hurt, abandoned, or yelled at (table 2).
Table 2.
Factors associated with male presentation within a 3 month period
| CHTC | Total | Unadjusted risk ratio (95% CI) |
p value | |
|---|---|---|---|---|
| Invitation only group | 52 | 100 | 1 | ·· |
| Invitation plus tracing group | 70 | 100 | 1·35 (1·07–1·69) | 0·01 |
| Age (years) | ||||
| 16–25 | 51 | 88 | 1 | ·· |
| 26–35 | 66 | 104 | 1·10 (0·87–1·38) | 0·4 |
| ≥36 | 5 | 8 | 1·08 (0·61–1·90) | 0·8 |
| Education | ||||
| No secondary | 65 | 118 | 1 | ·· |
| At least some secondary | 57 | 82 | 1·26 (1·02–1·57) | 0·04 |
| Material floor of house is made of | ||||
| Dirt or dung | 29 | 51 | 1 | ·· |
| Cement or tile | 93 | 149 | 1·10 (0·84–1·44) | 0·5 |
| Hunger in the past month | ||||
| No | 104 | 169 | 1 | ·· |
| Yes | 18 | 31 | 0·94 (0·68–1·30) | 0·7 |
| Earns salary | ||||
| No | 114 | 188 | 1 | ·· |
| Yes | 8 | 12 | 1·10 (0·73–1·67) | 0·7 |
| Trimester | ||||
| First | 19 | 29 | 1 | ·· |
| Second | 90 | 144 | 0·95 (0·71–1·28) | 0·8 |
| Third | 13 | 27 | 0·73 (0·46–1·18) | 0·2 |
| Primiparous | ||||
| No | 101 | 167 | 1 | ·· |
| Yes | 21 | 33 | 1·05 (0·79–1·40) | 0·7 |
| Duration with partner (years) | ||||
| <1 year | 38 | 60 | 1 | ·· |
| 1–5 years | 32 | 49 | 1·03 (0·78–1·37) | 0·8 |
| >5 years | 52 | 91 | 0·90 (0·69–1·17) | 0·4 |
| Female HTC history* | ||||
| No previous results | 14 | 22 | 1 | ·· |
| Previously negative | 79 | 141 | 0·88 (0·62–1·25) | 0·5 |
| Previously positive | 28 | 35 | 1·26 (0·88–1·80) | 0·2 |
| Previous CHTC | ||||
| No | 97 | 158 | 1 | ·· |
| Yes | 25 | 42 | 0·97 (0·73–1·28) | 0·8 |
| Previous disclosure to a partner* | ||||
| No | 18 | 35 | 1 | ·· |
| Yes | 90 | 143 | 1·22 (0·87–1·73) | 0·3 |
| Female perception of partner HTC history† | ||||
| No test or does not know | 69 | 125 | 1 | ·· |
| Negative | 36 | 55 | 1·19 (0·92–1·52) | 0·2 |
| Positive | 16 | 19 | 1·53 (1·19–1·96) | 0·001 |
| Number of sex acts with main partner in past month | ||||
| ≤7 | 50 | 85 | 1 | ·· |
| ≥8 | 72 | 115 | 1·06 (0·85–1·34) | 0·6 |
| Condom use in past three sex acts with main partner | ||||
| Never | 116 | 193 | 1 | |
| 1–3 times | 6 | 7 | 1·43 (1·03–1·97) | 0·03 |
| >1 sex partner in past year | ||||
| No | 94 | 161 | 1 | ·· |
| Yes | 28 | 39 | 1·23 (0·97–1·56) | 0·09 |
| Believes partner has had other partners in the past year | ||||
| No | 21 | 33 | 1 | ·· |
| Uncertain | 55 | 88 | 0·98 (0·72–1·33) | 0·9 |
| Yes | 46 | 79 | 0·92 (0·67–1·26) | 0·6 |
| Participant yelled at or threatened by partner | ||||
| No | 66 | 103 | 1 | ·· |
| Yes | 56 | 97 | 0·90 (0·72–1·13) | 0·4 |
| Participant physically hurt by partner | ||||
| No | 106 | 175 | 1 | ·· |
| Yes | 16 | 25 | 1·06 (0·77–1·45) | 0·7 |
| Fear of partner being angry† | ||||
| No | 61 | 94 | 1 | ·· |
| Yes | 41 | 81 | 0·78 (0·60–1·01) | 0·06 |
| Fear of being left‡ | ||||
| No | 71 | 111 | 1 | ·· |
| Yes | 31 | 64 | 0·76 (0·57–1·01) | 0·06 |
| Fear of being hurt† | ||||
| No | 95 | 156 | 1 | ·· |
| Yes | 7 | 19 | 0·60 (0·33–1·10) | 0·1 |
| Fear of partner telling others† | ||||
| No | 4 | 9 | 1 | ·· |
| Yes | 98 | 166 | 1·33 (0·63–2·79) | 0·5 |
For this analysis, we restricted the primary outcome to those partners who presented within 3 months of the initial female visit. All participants were capable of having at least 3 months of follow-up. 4 of 126 men presented after 3 months and were deemed non-presenters in this analysis. All characteristics are based on reports by the female index at baseline. CHTC=couple HIV testing and counselling.
CHTC=couple HIV testing and counselling.
In patients who had a previous HIV test.
Numbers do not add up to 200 because of missing data.
Numbers do not add up to 200 because of skip patterns (certain questions are only answered by certain persons based on responses to previous questions, which was intentional).
Of the 126 couples who presented, in 16 (13%) both partners tested for HIV, in 90 (71%) only the male partner tested for HIV, and in 20 (16%) neither partner tested for HIV. Those who did not test already had documented HIV test results. Of the 126 male partners, 36 (29%) were HIV-negative, 59 (47%) were HIV-positive and newly diagnosed, and 31 (25%) were HIV-positive and previously diagnosed with no difference between groups (p=0·7). Of the 90 HIV-positive men, 15 (17%) were already on antiretroviral therapy with no differences between groups. Four HIV-positive men not receiving antiretroviral therapy had missing CD4 cell count results. Of the remaining 71 HIV-positive men, 14 (20%) had CD4 counts of more than 500 cells per µL, 13 (18%) had CD4 counts of 350–499 cells per µL, 24 (34%) had CD4 counts of 200–349 cells per µL, and 20 (28%) had CD4 counts of less than 200 cells per µL, with no difference between groups (p=0·354). Newly diagnosed HIV-positive men in the invitation plus tracing group were more likely to be linked to care than were those in the invitation only group (table 3).
Table 3.
Trial outcomes
| Invitation only group |
Invitation plus tracing group |
Risk difference (95% CI) | |
|---|---|---|---|
| Men who presented to clinic and received CHTC | 52/100 (52%) | 74/100 (74%) | 22·0% (8·9 to 35·1) |
| Proportion who were HIV-positive | 37/52 (71%) | 53/74 (72%) | 0·5% (–15·7 to 16·6) |
| New HIV-positive diagnosis (of all positives) | 26/37 (70%) | 33/53 (62%) | –8·0% (–28·1 to 12·1) |
| New HIV-positive men linked to care | 5/26 (19%) | 15/33 (46%) | 26·2% (2·4 to 50·0) |
| CD4 count <500 cells per µL* | 25/31 (81%) | 32/40 (80%) | –0·6% (–19·6 to 18·3) |
| Female had 1 month default | 17/100 (17%) | 9/100 (9%) | –8·0% (–17·3 to 1·3) |
| Uptake of abstinence or consistent condom use | 42/88 (48%) | 57/94 (61%) | 12·9% (–1·5% to 27·3%) |
| Social harms | 0/87 | 3/94 (3%) | 3·2% (–2·1% to 8·4%) |
Data are n/N (%), unless otherwise specified.
In patients not receiving antiretroviral therapy.
Data from clinic records suggest that women in the invitation only group were more likely to have defaulted from the option B+ programme at 1 month than were women in the invitation plus tracing group (table 3). Of the 74 women whose partners did not present, 17 (23%) defaulted, and of the 126 women whose partners did present nine (7%) defaulted (p=0·001).
182 women (91%) presented for a 1 month study visit. 58 women had not presented with a partner. Reported reasons for non-presentation of partners were 24 (41%) not having time, 14 (24%) not wanting to come to antenatal care, 11 (19%) not interested in health information, five (9%) being away, and three (6%) being sick or injured. 55 (95%) of these women reported giving their partner the invitation. 48 (83%) reported disclosing their HIV status. 29 (62%) reported no reaction from the partner, ten (21%) reported support, six (13%) sadness, one (2%) worry, and one (2%) anger. One response was missing.
None of the women in the invitation only group and three women (3%) in the invitation plus tracing group reported social harms (p=0·2): two reported that their partners left them, one felt blamed for bringing HIV into the relationship, even though the man’s HIV status was unknown. These social harms occurred before tracing was initiated. No women reported physical violence as a result of the study. Of 181 women with follow-up data, two reported union dissolution, one reported emotional distress, and none reported intimate partner violence. One male partner, when traced, was confused about which of his sex partners was enrolled in the study, and this led to a dispute with a partner, who was not in the study. No other adverse events were reported.
Sexual behaviour between study partners became safer in both groups after study initiation. At enrolment, 191 (96%) of 200 women reported at least one unprotected sex act in the past month; this decreased to 76 (42%) of 182 at follow-up and differed by couple’s HIV status: nine (25%) of 36 serodiscordant couples, 40 (45%) of 88 concordant positive couples, and 27 (47%) of 58 status unknown couples (p=0·07). Of the 182 indexes with baseline and follow-up data, safer sex initiation was reported by 57 (61%) of 94 in the invitation plus tracing compared with 42 (48%) of 88 in the invitation only are (p=0·08).
Discussion
More than half of women were able to recruit male partners to CHTC with invitation alone; with the addition of tracing, nearly three-quarters were able to recruit male partners for CHTC. Women who were afraid of their partners’ reactions were less likely to present for CHTC and women who already knew of their partners’ HIV-positive status were more likely to present. Women in the invitation plus tracing group were less likely to default early from the option B+ programme and more likely to initiate safer sex practices. Both strategies identified a substantial number of HIV-positive men previously unaware of their HIV status and most of them were in need of treatment.
To our knowledge, this is the first assessment of a provider-based recruitment strategy (invitation plus tracing) within an option B+ setting. Provider-based recruitment strategies are more effective than patient-based strategies,17–19 although more costly.20 We attempted to minimise costs in the provider-based tracing group by first having patients trace partners (free), by next using phone tracing (low-cost), and by finally using community-based tracing (higher-cost). Our finding that the most successful provider tracing was phone-based is encouraging because this is a low-cost replicable method. Furthermore, our intervention was done within a setting in which provider-based strategies are highly cost-effective: a high prevalence epidemic with low awareness of HIV status in men.21,22
Our interventions had several unique features. First, the invitations and tracing messages focused on health information in the context of pregnancy, and male partners were not informed of HIV status exposure by the clinic until CHTC. This message choice allowed women to decide whether to disclose on their own, or use CHTC as a disclosure strategy, which probably improved acceptability and ultimately uptake. Another unique feature was use of CHTC, rather than individual HTC as part of this partner notification programme; CHTC is associated with important prevention, treatment, and PMTCT benefits,9,11,12,23 and has been promoted by WHO.8 Next, giving women an initial HIV test during the first antenatal encounter meant that all women learned their own HIV status, even if they did not present with a partner. Not testing women at an initial encounter and waiting for CHTC lowers overall maternal testing.24 Finally, clinic-based CHTC was used, rather than home-based CHTC,25 because of evidence that this would help link men to care.
Recruitment enhancements might have increased uptake of CHTC further. Many men expressed willingness to present to the clinic, but did not attend because of work commitments. Offering periodic weekend clinics would potentially increase CHTC uptake further. In a large urban catchment area, the community health worker was not able to locate many physical addresses. Having the community health worker obtain tracing information from participants, rather than relying on a research nurse, might have resulted in being able to locate more partners in the community. Use of a computer mapping programme with a street view might also have helped identify physical addresses. Finally, physical tracing was often initiated later than indicated, and earlier physical tracing might have been more effective.
Partner presentation in the invitation groups was higher than partner presentation rates reported in similar antenatal-based invitation programmes.14–16 Increased uptake might be due to the decision to restrict the population to women with partners living in Lilongwe. Another explanation for increased uptake is increased acceptance of HIV status in a maturing HIV epidemic with a strong antiretroviral therapy programme. Another factor might have been the provision of a research-related transport reimbursement, which could have served to incentivise participation.
Invitation plus tracing has great potential as an intervention to achieve WHO 90-90-90 targets: 90% of HIV-positive people aware of their HIV status, 90% of eligible people retained in care, and 90% virally suppressed. Nearly half of the men we identified were HIV-positive and previously unaware of their HIV status. Furthermore, two-thirds of these HIV-infected men were treatment-eligible, but not on treatment, and many were linked to care after CHTC. However, linkage to care was low, and additional research is needed to explore how to improve this outcome.
Women randomly assigned to the invitation plus tracing group had half the rate of option B+ early default seen in the invitation only group. Additionally, women in the invitation only group had lower 1 month default than did women in the general clinic population in the period just before to the study. The mechanism underlying these findings needs to be better understood. The invitation or CHTC process might have encouraged HIV status disclosure, eliminating the need to hide pills or care-seeking, and thus facilitating retention in care. A related possibility is that men provided motivation, social support, or reminders once they knew their partners were HIV-positive. Research exploring these mechanisms is underway, and is needed to help improve CHTC counselling. Irrespective of mechanism, the finding that CHTC is associated with increased retention at 1 month is noteworthy because the option B+ initiation period has been associated with very high loss, and male involvement could enhance retention.6
Our study has important implications for HIV prevention. Improved care-seeking is likely to result in “treatment as prevention” for HIV-discordant couples.3 Additionally, unprotected sex decreased markedly after CHTC, especially in HIV-discordant couples, a finding reported in many sub-Saharan African settings.9,10,23 Furthermore uptake of safer sexual behaviours improved more in the invitation plus tracing group, an important benefit of the intervention. The reports of condom use might be over-estimates because of socially desirable reporting, especially since we encouraged condom use throughout the study period. However, we are sceptical that this is the main explanation because condom distribution records correlated well with self-reported condom use.
Few social harms were reported, a finding consistent with other research.18 Additionally, those concerned about partner anger, violence, or abandonment were less inclined to return with a partner. These findings suggest women can judge whether partner recruitment is safe. A related observation is that HIV-positive women who already knew their partners’ HIV status were more likely to return with a partner than were women who did not because these relationships were probably considered safer. Male partner recruitment should be encouraged, but not mandated.
Our study population was highly representative of HIV-positive women with partners in Lilongwe, especially those with CD4 counts of less than 500 cells per µL, as enrolment and retention both exceeded 90%. However, our study is not representative of women who have no partners, partners outside of Lilongwe, or non-primary partners. Research is needed to explore how to recruit these men who might be more difficult to reach. Additionally, our study was done in a small population in one setting. Replication in a range of clinics is needed to assess robustness in other settings and outside of the research context.
Assessment of the long-term effects in a larger population is the next step. We postulate that invitation plus tracing plus CHTC would improve long-term retention and virological outcomes in HIV-positive women; linkage to care, retention, and virological outcomes in HIV-positive men; HIV prevention outcomes in HIV-negative men; and HIV acquisition outcomes in infants. A planned randomised controlled trial will explore these essential questions.
As option B+ is brought to scale in sub-Saharan Africa, male partner engagement is a crucial opportunity to improve a range of behaviour that could ultimately improve maternal, infant, and male partner health. These family-oriented option B+ approaches, such as CHTC, could have profound public health benefits.
Research in context.
Evidence before this study
We searched PubMed on March 28, 2015, using the search terms “partner notification”, “contract notification”, “provider referral”, or “case finding” and “HIV”, and “Africa”, with no language restrictions, yielding 183 references. Articles were included if they were done in sub-Saharan Africa, included provider-based partner tracing of an HIV-positive index in a clinical setting, and reported the proportion of partners who presented. Bibliographies of articles meeting these criteria, and two systematic reviews, were searched and the same criteria were applied. The search yielded two articles. A single-site randomised controlled trial was done in a sexually transmitted infection clinic in Malawi from 2008 to 2009 in which 240 participants were randomly assigned to passive referral, provider referral, or contract referral. The proportions of locatable partners who presented were 24% (passive referral), 51% (provider referral), and 51% (contract referral) and a multisite programme assessment that was done in antenatal care, voluntary counselling and testing, and inpatient facilities in Cameroon from 2009 to 2010. In the multisite study, 1462 participants selected the type of partner referral they preferred for each named partner: passive referral (20%), provider referral (60%), contract referral (14%), or no referral (7%). Overall, 84% of partners were notified, and 67% of these were tested for HIV; findings were not disaggregated by notification method. Additionally, one article was identified describing a cluster randomised controlled trial comparing passive referral to provider referral in 18 primary care facilities in Kenya. This study commenced in 2012 and excluded pregnant women.
Added value of this study
To our knowledge, this is the first randomised assessment of a contract referral strategy (invitation plus tracing) within an antenatal setting in sub-Saharan Africa. It is also the first assessment of any contract referral strategy within an option B+ antenatal setting and the first to assess antiretroviral therapy retention as an outcome. Additionally, previous interventions did not integrate a highly effective recruitment strategy (contract referral) with a highly effective HIV counselling and testing strategy (couple HIV counselling and testing). Our assessment of a contract referral strategy plus couple HIV counselling and testing in an option B+ setting is a novel and important contribution. Partner testing increases when provider-based strategies are available. This reported increase is apparent irrespective of whether the provider-based strategy is assigned through randomisation or selected by the patient, and irrespective of whether the testing modality is individual HIV counselling and testing or couple HIV counselling and testing.
Implications of all the available evidence
As option B+ is brought to scale in sub-Saharan Africa, male partner engagement is a crucial opportunity to improve a range of behaviours that could ultimately improve maternal, infant, and male partner health. Family-oriented option B+ approaches could have profound public health benefits. Invitation plus tracing strategies could play a crucial part in engaging male partners.
Acknowledgments
This research was funded by a 2014 developmental award from the University of North Carolina at Chapel Hill Center for AIDS Research, an NIH funded programme (grant numbers P30-AI50410, R25-TW009340, and K99-MH104154-01A1). NER was supported by the UNC Hopkins Morehouse Tulane Fogarty Global Health Fellows Program (R25 TW009340) and The National Institute of Mental Health (K99 MH104154-01A1). We thank Sarah Chirwa and Sophie Mtombosola for providing HIV testing and counselling, the staff at Bwaila District Hospital antenatal unit, and the District Health Office. We are grateful to the participants for allowing us to report their experiences in option B+.
Funding National Institutes of Health.
Footnotes
For the protocol see https://clinicaltrials.gov/ct2/show/NCT02139176
Contributors
NER designed the study in collaboration with FS, WCM, IH, IM, and MH. Randomisation was done by CS. TKM, EJ, LM, and this was managed by CS and WN. TKM supervised the study. NER did all analyses and wrote the first draft. All authors revised the manuscript and approved the final draft.
Declaration of interests
We declare no competing interests.
References
- 1.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 2.Thyssen A, Lange JH, Thyssen E, Reddi A. Toward an AIDS-free generation with option B+: reconceptualizing and integrating prevention of mother to child transmission (PMTCT) with pediatric antiretroviral therapy initiatives. J Acquir Immune Defic Syndr. 2013;62:127–128. doi: 10.1097/QAI.0b013e3182749994. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Impact of an innovative approach to prevent mother-to-child transmission of HIV—Malawi, July 2011–September 2012. MMWR Morb Mortal Wkly Rep. 2013;62:148–151. [PMC free article] [PubMed] [Google Scholar]
- 5.Government of Malawi Ministry of Health. Integrated HIV program report: July–September 2014. [(accessed Sept 30, 2015)]; http://www.who.int/hiv/pub/guidelines/9789241501972/en/
- 6.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS. 2014;28:589–598. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyondo A, Chimwaza A, Muula A. Exploring the relevance of male involvement in the prevention of mother to child transmission of HIV services in Blantyre, Malawi. BMC Int Health Hum Rights. 2014;14:30. doi: 10.1186/s12914-014-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Couples HIV testing and counselling including antiretroviral therapy for treatment and prevention in serodiscordant couples: recommendations for a public health approach 2012. [PubMed]
- 9.Rosenberg NE, Pettifor AE, Bruyn GD, et al. HIV testing and counseling leads to immediate consistent condom use among South African stable HIV-discordant couples. J Acquir Immune Defic Syndr. 2013;62:226–233. doi: 10.1097/QAI.0b013e31827971ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy CE, Medley AM, Sweat MD, O’Reilly KR. Behavioural interventions for HIV positive prevention in developing countries: a systematic review and meta-analysis. Bull World Health Organ. 2010;88:615–623. doi: 10.2471/BLT.09.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2011;56:76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalembo FW, Zgambo M, Mulaga AN, Yukai D, Ahmed NI. Association between male partner involvement and the uptake of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Mwanza District, Malawi: a retrospective cohort study. PLoS One. 2013;8:e66517. doi: 10.1371/journal.pone.0066517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mphonda S, Rosenberg NE, Kamanga E, et al. Assessment of peer-based and structural strategies for increasing male participation in an antenatal setting in Lilongwe, Malawi. Afr J Reprod Health. 2014;18:97–104. [PMC free article] [PubMed] [Google Scholar]
- 14.Nyondo AL, Choko AT, Chimwaza AF, Muula AS. Invitation cards during pregnancy enhance male partner involvement in prevention of mother to child transmission (PMTCT) of human immunodeficiency virus (HIV) in Blantyre, Malawi: a randomized controlled open label trial. PLoS One. 2015;10:e0119273. doi: 10.1371/journal.pone.0119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byamugisha R, Astrom AN, Ndeezi G, Karamagi CA, Tylleskar T, Tumwine JK. Male partner antenatal attendance and HIV testing in eastern Uganda: a randomized facility-based intervention trial. J Int AIDS Soc. 2011;14:43. doi: 10.1186/1758-2652-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohlala BK, Boily MC, Gregson S. The forgotten half of the equation: randomized controlled trial of a male invitation to attend couple voluntary counselling and testing. AIDS. 2011;25:1535–1541. doi: 10.1097/QAD.0b013e328348fb85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landis SE, Schoenbach VJ, Weber DJ, et al. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N Engl J Med. 1992;326:101–106. doi: 10.1056/NEJM199201093260205. [DOI] [PubMed] [Google Scholar]
- 18.Brown LB, Miller WC, Kamanga G, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56:437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henley C, Forgwei G, Welty T, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex Transm Dis. 2013;40:909–914. doi: 10.1097/OLQ.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutstein SE, Brown LB, Biddle AK, et al. Cost-effectiveness of provider-based HIV partner notification in urban Malawi. Health Policy Plan. 2014;29:115–126. doi: 10.1093/heapol/czs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armbruster B, Helleringer S, Kalilani-Phiri L, Mkandawire J, Kohler HP. Exploring the relative costs of contact tracing for increasing HIV case finding in sub-Saharan countries. J Acquir Immune Defic Syndr. 2011;58:e29–e36. doi: 10.1097/QAI.0b013e31822a9fa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Statistical Office Zomba, Malawi. Malawi Demographic and Health Survey 2010. [(accessed Sept 30, 2015)]; http://dhsprogram.com/pubs/pdf/FR247/FR247.pdf. [Google Scholar]
- 23.LaCroix JM, Pellowski JA, Lennon CA, Johnson BT. Behavioural interventions to reduce sexual risk for HIV in heterosexual couples: a meta-analysis. Sex Transm Infect. 2013;89:620–627. doi: 10.1136/sextrans-2013-051135. [DOI] [PubMed] [Google Scholar]
- 24.Becker S, Mlay R, Schwandt HM, Lyamuya E. Comparing couples’ and individual voluntary counseling and testing for HIV at antenatal clinics in Tanzania: a randomized trial. AIDS Behav. 2010;14:558–566. doi: 10.1007/s10461-009-9607-1. [DOI] [PubMed] [Google Scholar]
- 25.Osoti AO, John-Stewart G, Kiarie J, et al. Home visits during pregnancy enhance male partner HIV counselling and testing in Kenya: a randomized clinical trial. AIDS. 2014;28:95–103. doi: 10.1097/QAD.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]