Abstract
Objectives
To investigate the frequency and risk factors affecting the incidence of post-transplantation glomerulonephritis (GN) and the impact of GN on the survival of the graft and the patient.
Patients and methods
Patients were classified based on histological findings into three groups. Graft survival was ascertained using the Kaplan–Meier method and significance calculated using log-rank tests. For multivariate analysis the Cox model was used.
Results
Transplant glomerulopathy was the most prevalent glomerular disease in our series followed by recurrent GN and lastly de novo GN. In all, 50% of the de novo GN group had diabetes. The worst graft outcomes were in the recurrent GN group (P = 0.044). Multivariate analysis revealed ageing of the graft and mammalian target of rapamycin (mTOR) immunosuppression as risk factors for development of GN. While, the age of the recipient and donor, anti-lymphocyte globulin induction therapy, and acute rejection were risk factors for poor graft outcomes.
Conclusions
GN is an important issue after transplantation. Tracking the incidence and progression of histological findings in the graft may help to guide proper management and improve graft outcome.
Abbreviations: ESRD, end-stage renal disease; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; HCV, hepatitis C virus; HR, hazard ratio; MPGN, membrano-proliferative GN; PTGN, post-transplantation GN
Keywords: Post-transplantation glomerulonephritis (GN), Renal transplantation, Long-term survival, Recurrent GN, De novo GN
Introduction
Transplantation has proven to be the best therapy for end-stage renal disease (ESRD), being superior to maintenance dialysis therapy with better quality of life and lower mortality risk [1]. The impact of glomerulonephritis (GN) on graft outcome is not fully understood [2].
GN has been reported to recur in renal grafts at different rates depending on histological type [3]. Impairment of graft function and even loss has mostly been reported with recurrent GN [4]. Exact diagnosis of GN before transplantation is not easy, as most of the patients present with ESRD without an available histological diagnosis for varying reasons. Thus, most reported diagnoses of GN are based on clinical judgement rather than histological evidence, leading to an incorrect estimation of the true incidence of GN [5]. Another problem is the difficultly in differentiating between GN histological findings and calcineurin-inhibitor nephrotoxicity and chronic allograft nephropathy [6].
Recurrent GN is clinically relevant, as it can result in long-term graft loss; it was reported to be the third most common cause of graft loss during the 10-year period after transplantation. The negative impact of recurrent GN increased from 0.6% during the first year after transplantation to 8.4% after 10 years [3]. In the present study, we analysed the incidence of different types of GN reported after transplantation, potential precipitating factors, and their potential risk on graft survival.
Patients and methods
This study comprised 2000 transplant recipients who received their grafts between March 1976 and February 2010 at Mansoura Urology and Nephrology Center. In all, 1648 patients received their grafts from related donors, while the other 352 received their grafts from unrelated donors. Among the unrelated group, 122 were spouses. The procedures were approved by the ethics committee of human experimentation in our centre and in accordance with the Helsinki declaration of 1975.
Exclusion criteria included: couples with historical positive lymphocytotoxic cross match, recent malignancy, addiction, psychiatric disorders, type I diabetes mellitus, significant extra-renal organ failure (pulmonary, hepatic, or cardiac), other exclusion criteria for donors included: unwilling donors, diabetes mellitus, hypertension, positive hepatitis B surface antigen (HBsAg), anti-hepatitis C virus (HCV) antibodies, anti-HIV and anti-cytomegalovirus (CMV) IgM antibodies. All clinical records of all kidney transplant recipients were entered prospectively in our computer network transplant database.
Study design
This is a retrospective study in which the patients were divided according to graft biopsy results into two groups: Group I (No GN) included patients who did not have post-transplantation GN (PTGN). Group II (PTGN) included patients who developed PTGN. This group was further divided according to the nature of GN into:
-
•
de novo GN, which included patients who did not have biopsy confirmed GN before transplantation or had a different type of GN than the one discovered after transplantation;
-
•
recurrent GN, which included patients with PTGN of the same histopathological type as that before transplantation;
-
•
transplant glomerulopathy, which included patients with glomerular injury with unique pathological and pathogenic entity distinct from other forms of chronic allograft injury.
GN management was according to the international protocols valid at the time of graft biopsy. The protocol table is provided in the Appendix.
Statistical analysis
Qualitative data are presented in cross tabulation and quantitative data are presented as the mean (standard deviation, SD). Univariate analyses were used for initial evaluation of differences using the chi-square and Fisher’s exact tests. A P < 0.05 was considered to indicate statistical significance. Graft and patient survival rates were evaluated by means of Kaplan–Meier survival curves. Significant variables in the univariate analysis were further analysed by multivariate analysis to determine those that acted independently (P < 0.05) using the Cox model. All analyses were carried out using the computer package SPSS for windows, release 16 SPSS Inc. Chicago, IL, USA.
Results
The patients’ demographic data show that there was a higher frequency of PTGN in younger recipients. Focal segmental glomerulosclerosis (FSGS) was the predominant original cause of ESRD among group II, whereas in group I chronic pyelonephritis was the most common original kidney disease (Table 1).
Table 1.
The demographic characteristics of the 2000 renal transplants.
| Characteristic | No GN | PTGN | P |
|---|---|---|---|
| Number of patients | 1897 | 103 | |
| Mean (SD) recipient age, years | 29.84 (10.62) | 27.56 (10.55) | 0.034 |
| N (%): | |||
| Recipients age range, years | |||
| <20 | 363 (19.1) | 30 (29.1) | 0.042 |
| 20–30 | 595 (31.4) | 31 (30.1) | |
| 30–40 | 572 (30.2) | 28 (27.2) | |
| 40–50 | 295 (15.6) | 11 (10.7) | |
| >50 | 72 (3.8) | 3 (2.9) | |
| Sex | |||
| Male | 1413 (74.5) | 78 (75.7) | 0.778 |
| Female | 484 (25.5) | 25 (24.3) | |
| Pre-transplant hypertension | |||
| No | 799 (42.1) | 42 (40.7) | 0.788 |
| Yes | 1098 (57.9) | 61 (59.3) | |
| Type of blood transfusion | |||
| Donor specific | 11 (0.6) | 1 (0.97) | 0.273 |
| Third party | 852 (44.9) | 54 (52.4) | |
| No transfusion | 1034 (54.5) | 48 (46.6) | |
| Type of dialysis | |||
| Pre-emptive | 79 (4.2) | 1 (0.9) | 0.280 |
| Haemodialysis | 1797 (94.7) | 102 (99.1) | |
| Peritoneal dialysis | 21 (1.1) | – | |
| Mean (SD) donors age, years | 35.47 (10.1) | 36 (10.7) | 0.605 |
| N (%) | |||
| Donors age range, years | |||
| <30 | 745 (39.3) | 37 (35.9) | 0.789 |
| 30–40 | 604 (31.8) | 33 (32) | |
| 40–50 | 379 (20) | 20 (19.4) | |
| >50 | 169 (8.9) | 13 (12.6) | |
| Donors sex | |||
| Male | 903 (47.6) | 50 (48.5) | 0.881 |
| Female | 994 (52.4) | 53 (51.5) | |
| Consanguinity | |||
| Parent | 542 (28.6) | 35 (34) | 0.238 |
| Sibling | 890 (46.9) | 46 (44.7) | 0.655 |
| Off-spring | 29 (1.5) | 1 (0.97) | 0.650 |
| Emotionally related | 116 (6.1) | 6 (5.8) | 0.905 |
| Other relative | 101 (5.3) | 4 (3.9) | 0.523 |
| Unrelated | 219 (11.5) | 11 (10.7) | 0.789 |
| Blood groups, recipient/donor | |||
| Same | 1527 (80.5) | 80 (77.7) | 0.482 |
| Different (but compatible) | 370 (19.5) | 23 (22.3) | |
| HLA Class I mismatch | |||
| Zero | 148 (7.8) | 6 (5.8) | 0.181 |
| One | 215 (11.3) | 16 (15.5) | |
| Two | 952 (50) | 58 (56.3) | |
| Three | 294 (15.5) | 16 (15.5) | |
| Four | 129 (6.8) | 4 (3.9) | |
| Undetermined | 159 (8.4) | 3 (2.9) | |
| HLA Class II (DR) mismatch | |||
| Zero | 195 (10.3) | 10 (9.7) | 0.452 |
| One | 1656 (87.3) | 93 (90.3) | |
| Two | 2 (0.1) | – | |
| Undetermined | 44 (2.3) | – | |
| Transplant received | |||
| First | 1821 (96) | 101 (98.1) | 0.562 |
| Second | 73 (3.8) | 2 (1.9) | |
| Third | 3 (0.2) | – | |
| Ischaemia time, min | |||
| <30 | 217 (11.4) | 9 (8.7) | 0.672 |
| 30–60 | 1378 (72.6) | 76 (73.8) | |
| >60 | 302 (15.9) | 18 (17.5) | |
| Time to diuresis | |||
| Immediate | 1745 (92) | 94 (91.3) | 0.792 |
| Delayed | 152 (8) | 9 (8.7) | |
| Number of renal arteries | |||
| One | 1681 (88.6) | 95 (92.2) | 0.975 |
| Two | 193 (10.2) | 8 (7.8) | |
| Three | 21 (1.1) | – | |
| Four | 1 (0.05) | – | |
| Five | 1 (0.05) | – | |
HLA, human leucocyte antigen.
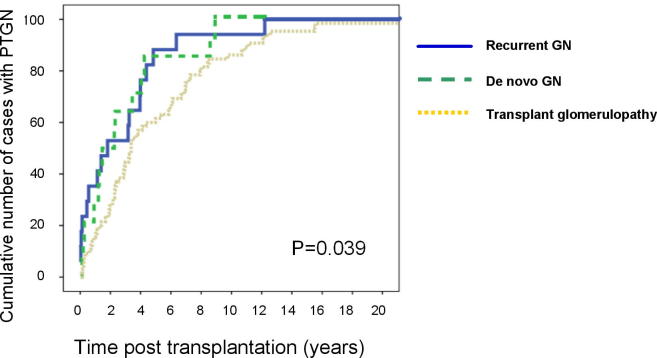
Most of our ESRD recipients with GN were children, so parents constituted most of the donors in group II. The donors’ gender was comparable in both groups. The percentage of parent donors was higher in group I. The incidence of recurrent GN was higher in the first 3 months after transplant compared with de novo GN. While transplant glomerulopathy incidence was higher than recurrent and de novo GN at 5 years after transplantation (P = 0.039; Fig. 1).
Figure 1.
Incidence of GN after transplantation. Post-transplantation incidence of recurrent GN (solid line) and de novo GN (dashed line) were significantly higher than transplant glomerulopathy (pointed line) (P = 0.039).
Table 2 shows that either de novo GN or transplant glomerulopathy most commonly occurred in patients with uncertain original kidney disease. Recurrent FSGS was the most common histopathological type of GN, accounting for 44.4% of recurrent and 6.7% of de novo GNs, membrano-proliferative glomerulonephritis (MPGN) was the second most common histological type, systemic lupus GN was the most common original kidney disease associated with transplant glomerulopathy after transplantation. There were no significant differences for induction immunosuppression and primary immunosuppression in patients who had PTGN (P = 0.881, P = 0.197, respectively). Acute cellular rejection episodes were higher, but not significantly so, among patients who developed de novo GN (P = 0.566). Chronic rejection was significantly higher among patients who developed transplant glomerulopathy (P < 0.001). A significantly higher percentage of recipients had post-transplantation hypertension in group II than in group I. The percentage of other medical complications was comparable between the groups (Table 3).
Table 2.
Original kidney disease and immunosuppression of patients with PTGN.
| Recurrent GN, n (%) | De novo GN, n (%) | Transplant glomerulopathy, n (%) | P | |
|---|---|---|---|---|
| Number of patients | 18 | 15 | 70 | |
| Patients with pre-transplant GN | ||||
| Mesangial | 1 (5.6) | – | – | <0.001 |
| Membranous nephropathy | 1 (5.6) | – | 1 (1.4) | |
| FSGS | 8 (44.4) | 1 (6.7) | 5 (7.1) | |
| Membrano-proliferative | 4 (22.2) | 2 (13.3) | 2 (2.8) | |
| Crescentic GN | 1 (5.6) | 1 (6.7) | – | |
| Hereditary nephritis | – | 2 (13.3) | 2 (2.8) | |
| Amyloidosis | 2 (11.1) | – | 2 (2.8) | |
| SLE⁎ | 1 (5.6) | 1 (6.7) | 8 (11.4) | |
| Patients with no pre-transplant GN | ||||
| Polycystic kidney | – | 1 (6.7) | 1 (1.4) | <0.001 |
| Hypoplasia | – | – | 2 (2.8) | |
| Chronic pyelonephritis | – | 1 (6.7) | 6 (8.6) | |
| Nephrosclerosis | – | – | 2 (2.8) | |
| ESRD | – | 3 (20) | 27 (38.6) | |
| Congenital | – | – | 1 (1.4) | |
| Obstructive uropathy | – | – | 1 (1.4) | |
| Inapplicable | – | 3 (20) | 10 (14.3) | |
| Induction therapy | ||||
| Polyclonal antibodies | 2 (11.1) | 3 (20) | 8 (11.4) | 0.881 |
| Monoclonal antibodies | 5 (27.8) | 4 (26.7) | 15 (21.5) | |
| No induction | 11 (61.1) | 8 (53.3) | 47 (67.1) | |
| Primary immunosuppression | ||||
| Conventional based | 2 (11.1) | 2 (13.3) | 22 (31.4) | 0.197 |
| Triple based | 14 (77.7) | 9 (60) | 38 (54.4) | |
| Tacrolimus based | 1 (5.6) | – | – | |
| Sirolimus based | 1 (5.6) | 3 (20) | 8 (11.4) | |
| Steroid avoidance | – | 1 (6.7) | 1 (1.4) | |
| Alemtuzumab | – | – | 1 (11.4) | |
Systemic lupus erythematosus.
Table 3.
Medical complications after transplantation in patients with PTGN.
| Recurrent GN, n (%) | De novo GN, n (%) | Transplant glomerulopathy, n (%) | P | |
|---|---|---|---|---|
| Number of patients | 18 | 15 | 70 | |
| Acute tubular necrosis | ||||
| No | 16 (88.9) | 15 (100) | 67 (95.7) | 0.310 |
| Yes | 2 (11.1) | – | 3 (4.3) | |
| Hypertension | ||||
| No | 6 (33.3) | 2 (13.3) | 18 (25.7) | 0.415 |
| Yes | 12 (66.7) | 13 (86.7) | 52 (74.3) | |
| Post-transplantation DM | ||||
| No | 16 (88.9) | 9 (60) | 56 (80) | 0.116 |
| Yes | 2 (11.1) | 6 (40) | 14 (20) | |
| Medical infection | ||||
| No | 14 (77.8) | 13 (86.7) | 47 (67.1) | 0.258 |
| Yes | 4 (22.2) | 2 (13.3) | 23 (32.9) | |
| Hepatic impairment | ||||
| No | 17 (94.4) | 13 (86.7) | 64 (91.4) | 0.730 |
| Yes | 1 (5.6) | 2 (13.3) | 6 (8.6) | |
| Acute rejection | ||||
| No | 12 (66.7) | 7 (46.7) | 36 (51.4) | 0.566 |
| Acute cellular | 6 (33.3) | 8 (53.3) | 31 (44.3) | |
| Acute vascular | – | – | 3 (4.3) | |
| Chronic rejection | ||||
| No | 16 (88.9) | 14 (93.3) | 36 (51.4) | <0.001 |
| Yes | 2 (11.1) | 1 (6.7) | 34 (48.6) | |
| Malignancy | ||||
| No | 17 (94.4) | 13 (86.7) | 68 (97.1) | 0.228 |
| Yes | 1 (5.6) | 2 (13.3) | 2 (2.9) | |
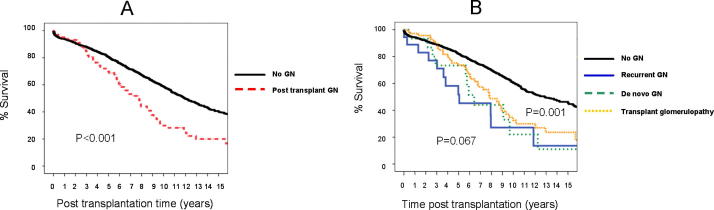
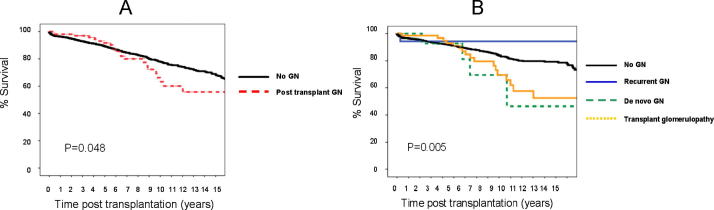
Fig. 2 shows that long-term graft survival was significantly lower in group II vs group I within the first 2 years after transplantation (P < 0.001). While recurrent GN negatively impacted graft survival significantly compared with de novo and transplant glomerulopathy in the first 5 years. These differences equalised after 10 years (P = 0.067). There were significant differences between groups I and II for patient survival (P = 0.048). De novo GN and transplant glomerulopathy had a significantly negative impact on patient survival compared with recurrent GN and group I (P = 0.005; Fig. 3).
Figure 2.
Graft survival of the recipients who did not develop PTGN vs recipients who developed PTGN. (A) Graft survival in the recipients who developed PTGN (dashed line) was comparable to those with no PTGN (solid line) in the first 2 years. Thereafter, there was a significant drop in graft survival in the group of recipients who had PTGN vs those who did not develop PTGN (P < 0.001). (B) Graft survival in the recipients who developed de novo GN (dashed line) and transplant glomerulopathy (pointed line) was comparable to those who did not develop PTGN (solid bold line) in the first 2 years (P = 0.067). While there was a significant drop in graft survival in recipients with recurrent GN (solid thin line) vs other groups in the first 2 years. Thereafter, there was a significant drop in graft survival in the group of recipients who had PTGN (whatever the type) vs those who did not develop PTGN (P = 0.001).
Figure 3.
Patient survival of the recipients who did not develop GN vs recipients who developed PTGN. (A) Patient survival in the recipients who developed PTGN (dashed line) was comparable to those with no PTGN (solid line) in the first 5 years. Thereafter, there was a significant drop in patient survival in the group of recipients who had PTGN vs those who did not develop PTGN (P = 0.048). (B) patient survival in the recipients who developed PTGN (whatever the cause) was comparable to those who did not develop PTGN in the first 5 years. Thereafter, there was a significant drop in patient survival in the group of recipients who had de novo GN (dashed line) and transplant glomerulopathy (solid line) vs those who did not develop PTGN (P = 0.005).
Table 4 shows Cox multivariate analysis for the identification of possible independent risk factors for the development of PTGN, which showed that middle-age donors (aged 31–40 years) carried 1.76 risk (95% CI 1.04–3) compared with other donor grafts (P = 0.035). A Sirolimus-based immunosuppression protocol was associated with 4.6-fold risk (95% CI 2.14–10) for the development of PTGN (P < 0.001). While, a different blood group between the donor and recipient carried a favourable significant delay in the development of PTGN (P = 0.005).
Table 4.
Multivariate analysis of the risk factors for developing PTGN.
| Regression estimate (B) | Relative risk (95% CI), Exp B | P | |
|---|---|---|---|
| Recipient age, years | |||
| <20 | – | 1 | |
| 20–30 | –0.929 | 0.40 (0.152–1.03) | 0.057 |
| 31–40 | –0.512 | 0.60 (0.26–1.39) | 0.231 |
| 41–50 | –0.330 | 0.72 (0.25–2.1) | 0.540 |
| >50 | 0.793 | 0.45 (0.039–5.23) | 0.525 |
| Original kidney disease | |||
| No GN | – | 1 | |
| GN | 0.015 | 1.01 (0.566–1.821) | 0.959 |
| Donor age, years | |||
| <30 | – | 1 | |
| 31–40 | 0.570 | 1.76 (1.04–3) | 0.035 |
| 41–50 | 0.602 | 1.82 (0.977–3.41) | 0.059 |
| >50 | 0.289 | 1.33 (0.657–2.71) | 0.424 |
| Recipient sex match | |||
| Male–male | – | 1 | |
| Male–female | –0.228 | 0.796 (0.319–1.98) | 0.625 |
| Female–male | 0.397 | 1.48 (0.743–2.97) | 0.263 |
| Female–female | 0.451 | 1.57 (0.576–4.27) | 0.378 |
| Consanguinity | |||
| Parent | – | 1 | |
| Sibling | 0.518 | 1.67 (0.47–5.97) | 0.424 |
| Off-spring | 1.261 | 3.52 (0.1–125) | 0.489 |
| Other relative | 0.898 | 2.45 (0.57–10.6) | 0.231 |
| Unrelated | 1.220 | 3.38 (0.82–14.1) | 0.093 |
| Donor/recipient blood group match | |||
| Same | – | 1 | |
| Different | –0.869 | 0.42 (0.23–0.77) | 0.005 |
| Blood transfusion | |||
| No | – | 1 | |
| Yes | –0.451 | 0.64 (0.39–1.05) | 0.075 |
| Ischaemia time, min | |||
| <30 | – | 1 | |
| 30–60 | –0.711 | 0.491 (0.194–1.27) | 0.134 |
| >60 | –0.437 | 0.65 (0.196–2.13) | 0.473 |
| Time to diuresis | |||
| Immediate | – | 1 | |
| Delayed | 0.473 | 1.60 (0.58–4.47) | 0.366 |
| Induction therapy | |||
| No | – | 1 | |
| Polyclonal | 0.217 | 1.24 (0.361–4.28) | 0.731 |
| Monoclonal | –0.271 | 0.763 (0.262–2.22) | 0.619 |
| Maintenance immunosuppression | |||
| Conventional | – | 1 | |
| CsA based | 0.278 | 1.32 (0.74–2.34) | 0.343 |
| Tacrolimus | 1.855 | 6.39 (0.76–54) | 0.089 |
| Sirolimus | 1.536 | 4.64 (2.14–10) | <0.001 |
| Steroid avoidance | 0.589 | 1.80 (0.386–8.4) | 0.454 |
| Steroids in first 3 months, g | |||
| <5 | – | 1 | |
| 5–10 | −0.088 | 0.92 (0.45–1.89) | 0.812 |
| >10 | 0.631 | 1.87 (0.78–4.5) | 0.158 |
CsA, cyclosporin A.
Table 5 shows the Cox multivariate analysis for risk factors for graft loss, which showed that transplant recipients aged 40–50 years have a 1.82-fold risk (95% CI 1.24–2.67) of losing their graft after 10 years vs the other transplant recipients (P = 0.002). Induction therapy with polyclonal antibody (anti-thymocyte globulin, ATG) doubled the risk of graft loss [hazard ratio (HR) 1.99, 95% CI 1.53–2.6] than induction therapy with monoclonal antibodies (P < 0.001). Acute rejection episodes carry an independent negative impact on long-term graft survival. One episode of acute rejection had a 1.35-fold risk (95% CI 1–1.8) of graft loss (P = 0.049). Two or more acute rejection episodes increased the risk of graft loss by 1.97 times (95% CI 1.45–2.7) (P < 0.001). Development of chronic rejection increased the risk to 2.41 times (95% CI 1.9–3) of long-term graft loss (P < 0.001). De novo GN had an independent negative risk on long-term graft loss of 3.33-times (95% CI 1.5–7.4) that of the other histopathological types (P = 0.003). Middle-aged donor grafts carried a favourable significant effect on graft survival (HR 0.77, 95% CI 0.61–0.99). Patients receiving their grafts from their offspring were at less risk of losing their graft after 10 years (HR 0.74, 95% CI 0.56–0.97) (P = 0.028). A different blood group had a favourable effect on graft survival (HR 0.75, 95% CI 0.59–0.95) (P = 0.016).
Table 5.
Multivariate analysis of the risk factors of graft survival.
| Regression estimate (B) | Relative risk (95% CI), Exp B | P | |
|---|---|---|---|
| Recipient age, years | |||
| <20 | – | 1 | |
| 20–30 | 0.070 | 1.07 (0.80–1.44) | 0.640 |
| 31–40 | 0.262 | 1.30 (0.93–1.81) | 0.120 |
| 41–50 | 0.601 | 1.82 (1.24–2.67) | 0.002 |
| >50 | 0.562 | 1.75 (0.95–3.22) | 0.070 |
| Original kidney disease | |||
| No GN | – | 1 | |
| GN | –0.071 | 0.93 (0.68–1.27) | 0.652 |
| Donor age, years | |||
| <30 | – | 1 | |
| 31–40 | –0.257 | 0.77 (0.61–0.99) | 0.038 |
| 41–50 | 0.245 | 1.27 (0.94–1.74) | 0.118 |
| >50 | 0.109 | 1.12 (0.73–1.71) | 0.615 |
| Recipient sex match | |||
| Male–male | – | 1 | |
| Male–female | 0.127 | 1.14 (0.82–1.56) | 0.439 |
| Female– male | 0.018 | 1.02 (0.67–1.54) | 0.931 |
| Female–female | 0.059 | 1.06 (0.78–1.44) | 0.705 |
| Consanguinity | |||
| Parent | – | 1 | |
| Sibling | –0.036 | 0.97 (0.65–1.43) | 0.858 |
| Off-spring | –0.303 | 0.74 (0.56–0.97) | 0.028 |
| Other relative | 0.771 | 2.16 (0.95–4.92) | 0.066 |
| Unrelated | 0.249 | 1.28 (0.83–1.99) | 0.266 |
| Donor/recipient blood group match | |||
| Same | – | 1 | |
| Different | –0.294 | 0.75 (0.59–0.95) | 0.016 |
| Blood transfusion | |||
| No | – | 1 | |
| Donor specific | –0.027 | 0.97 (0.79–1.2) | 0.800 |
| Third party | –0.077 | 0.93 (0.36–2.4) | 0.873 |
| Ischaemia time, min | |||
| <30 | – | 1 | |
| 30–60 | 0.205 | 1.23 (0.97–1.5) | 0.089 |
| >60 | 0.327 | 1.39 (0.94–2.05) | 0.103 |
| Time to diuresis | |||
| Immediate | – | 1 | |
| Delayed | 0.140 | 1.15 (0.83–1.6) | 0.395 |
| Induction therapy | |||
| No | – | 1 | |
| Polyclonal | 0.688 | 1.99 (1.53–2.6) | <0.001 |
| Monoclonal | –0.448 | 0.64 (0.35–1.18) | 0.151 |
| Maintenance immunosuppression | |||
| Conventional | – | 1 | |
| CsA based | 0.116 | 1.12 (0.27–4.6) | 0.872 |
| Tacrolimus | –0.142 | 0.87 (0.21–3.5) | 0.843 |
| Steroids in first 3 months, g | |||
| <5 | – | 1 | |
| 5–10 | –0.149 | 0.86 (0.68–1.1) | 0.216 |
| >10 | –0.044 | 0.96 (0.69–1.3) | 0.786 |
| Rejection | |||
| No | – | 1 | |
| One acute rejection | 0.301 | 1.35 (1–1.8) | 0.049 |
| ⩾two acute rejections | 0.681 | 1.97 (1.45–2.7) | <0.001 |
| Chronic rejection | 0.878 | 2.41 (1.9–3) | <0.001 |
| PTGN | |||
| No | – | 1 | |
| Recurrent GN | –0.226 | 0.79 (0.56–1.13) | 0.207 |
| de novo GN | 1.205 | 3.33 (1.5–7.4) | 0.003 |
| Transplant GN | 0.530 | 1.69 (0.77–3.76) | 0.191 |
Discussion
The frequency of graft–antigen recognition and impact of alloimmune injury have been significantly reduced with the evolution of immunosuppression, which has improved post-transplantation long-term survival [7]. Hume et al. [8] reported that transplanted patients are not invulnerable from being at risk of GN, in all its forms, in the graft. The frequency of PTGN reached 40% and the cumulative probability increases with transplantation time [9]. A possible link between HCV infection and glomerulopathy has previously been reported [10]. In our present cohort, most of the donors among the PTGN group were parents, which supports the possible genetic predisposition reported previously [11]. GN has been found to be associated with mutations in several genes and the coding of several proteins that encode for podocytes function [11]. It has been reported that recurrent GN represents 70% of PTGN cases and IgA nephropathy was the most common histological form [12]. In our present series, recurrent FSGS was the predominated histopathological type, followed by MPGN and this was statistically significant (P < 0.001). The low incidence of reported IgA in our present series may be explained by the delayed introduction of immunofluorescence studies in our graft biopsies. Our present data are consistent with a Canadian study, which reported a higher frequency of recurrent FSGS compared with patients with other disorders [1]. The incidence of PTGN is higher in patients known to have chronic kidney disease than in the normal population [13], [14]. De novo GN is associated with frequent episodes of acute rejection, while chronic rejection overlapped with transplant glomerulopathy. Ibrahim et al. [15] reported the possibility of an acute rejection association with PTGN and that the frequency of PTGN is not affected by early steroid withdrawal.
Multivariate analysis for the risk of developing PTGN revealed two independent risk factors. Renal grafts from middle-aged donors carried a 1.8-fold higher risk of PTGN than grafts from other donor age groups; this may be explained by the fact that autoimmune diseases are more prevalent in the middle-aged population. Furthermore, the most common histopathological GN type in our present cohort was FSGS and it was documented that one of the major risk factors of recurrence of FSGS was renal ischaemic injury [16], combined with the genetic susceptibility transferred with the graft from the parent donor [11]. A sirolimus-based immunosuppression protocol was associated with a 4.6-fold higher risk of developing GN after transplantation. Our present data are consistent with reports documenting the immunosuppressive therapy role: cyclosporine was reported to cause renal injury including FSGS and sirolimus toxicity can lead to tubular injury and FSGS in patients with a genetic susceptibility [17]. Recurrent GN is associated with worse graft outcome than de novo GN or transplant glomerulopathy. The present data highlight the importance of PTGN as a cause of graft loss in renal transplant recipients and it is associated with a dramatic reduction in graft survival [5]. Graft prognosis depends on the severity and histological form of GN, as well as whether the GN is recurrent or de novo [18], [19]. There is no effective treatment for PTGN, intensive plasma exchange or Rituximab may be of benefit in some cases of FSGS but is of no benefit in many instances [20]. Efforts should be made to outline a standard approach to define risk factors of different forms of this serious disease affecting the survival of the graft that will lead to more specific therapy [21].
In conclusion, from our present results GN as a cause of renal failure represents a medical dilemma that may persist after transplantation. Early identification and understanding of the ongoing precipitating mechanisms may change our monitoring and immunosuppression strategies and improve long-term graft outcome.
Authorship
Ahmed Ibrahim Akl planned the research design, doing statistical analysis and wrote the manuscript. Hany Adel collected the clinical data and shared in writing the manuscript. Wahba Wafa Ehab and Ahmed A. Shokeir reviewed the final version of the manuscript.
Conflict of interest
Authors confirm no conflict of interest.
Support and financial disclosure
Nothing to declare.
Renal Transplantation
Footnotes
Peer review under responsibility of Arab Association of Urology.
Appendix A
Treatment strategies for different types of GN.
| Treatment | Clinically relevant∗ recurrent risk†, % | Risk of graft loss due to recurrence 5–10 years after transplantation†, % | Prevention/treatment strategies |
|---|---|---|---|
| IgAN | 13–46 | 2–16 | ACEI and/or ARB for patients with proteinuria ± renal impairment due to recurrent IgAN [22], [23] |
| FSGS | 20–50 | 13–20 | Avoid living donors for patients with history of rapid graft loss from recurrence [24] |
| Pre-emptive perioperative plasmapheresis (PP) for 2 weeks for patients with high risk of recurrence [25], [26] | |||
| Chronic PP with or without cyclophosphamide or cyclosporine for patients with relapse after initial course of PP [27], [28], [29] | |||
| Avoid omission of calcineurin inhibitors in sirolimus-based immunosuppressive regimen [30], [31] | |||
| Avoid induction therapy [32], [33] | |||
| MPGN | |||
| Type I | 20–25 | ≈15 | No effective preventive or treatment measures |
| Type II | 80–100 | 15–30 | Exclude secondary causes |
| Membranous nephropathy | 10–30 | 10–15 | No effective preventive or treatment measures |
| Exclude secondary causes | |||
| ANCA-associated glomerulonephritis | ≈17 | 6–8 | Defer transplant till disease inactive [34] |
| Cyclophosamide for recurrence [34], [35] | |||
| Combine therapy with PP, cyclophosphamide ± i.v. immunoglobulin for recurrence with high titre of ANCA and cellular crescents in renal biopsies [35], [37] | |||
| SLE | 2–9 | 2–4 | Defer transplant until disease inactive [38], [39] |
| Consider mycophenolate mofetil for recurrence [40], [41] | |||
| Anti-GBM | Rare | Rare | Defer transplant until disease inactive |
| Combine therapy with PP/immunoabsorption and cyclophosphamide for recurrence with high anti-GBM titre and cellular crescents in renal biopsies [36], [42] | |||
| ∗Clinically relevant refers to patients with clinical symptoms of proteinuria/haematuria/renal impairment. | |||
| †% of transplanted patients. IgAN, recurrent IgA nephropathy; SLE, systemic lupus erythematosus; ACEI, angiotensin-converting enzyme inhibitor; ANCA, anti-neutrophil cytoplasmic autoantibody; GBM, glomerular basement membrane. | |||
References
- 1.Chailimpamontree W., Dmitrienko S., Li G., Balshaw R., Magil A., Shapiro R.J. Probability, predictors, and prognosis of post-transplantation glomerulonephritis. J Am Soc Nephrol. 2009;20:843–851. doi: 10.1681/ASN.2008050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaston R. Current and evolving immunosuppressive regimens in kidney transplantation. Am J Kidney Dis. 2006;47:S3–21. doi: 10.1053/j.ajkd.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Briganti E.M., Russ G.R., McNeil J.J., Atkins R.C., Chadban S.J. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347:103–109. doi: 10.1056/NEJMoa013036. [DOI] [PubMed] [Google Scholar]
- 4.Choy B., Chan T., Lai K. Recurrent glomerulonephritis after kidney transplantation. Am J Transplant. 2006;6:2535–2542. doi: 10.1111/j.1600-6143.2006.01502.x. [DOI] [PubMed] [Google Scholar]
- 5.Hariharan S., Adams M., Brennan D., Davis C., First M., Johnson C. Recurrent and de novo glomerular disease after renal transplantation: a report from renal allograft disease registry (RADR) Transplantation. 1999;68:635–641. doi: 10.1097/00007890-199909150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Requião-Moura L.R., Moscoso-Solorzano G.T., Franco M.F., Ozaki K.S., Pacheco-Silva A., Kirsztajn G.M. Prognostic factors associated with poor graft outcomes in renal recipients with post-transplant glomerulonephritis. Clin Transplantation. 2007;21:363–370. doi: 10.1111/j.1399-0012.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- 7.Hariharan S., Johnson C., Bresnahan B., Taranto S., McIntosh M. StableinD: improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 8.Hume D.M., Bryant C.P. The development of recurrent glomerulonephritis. Transplantation Proc. 1972;4:673–677. [PubMed] [Google Scholar]
- 9.Hariharan S., Peddi V.R., Savin V.J., Johnson C.P., First M.R., Roza A.M. Recurrent and de novo renal diseases after renal transplantation: a report from the renal allograft disease registry. Am J Kid Dis. 1998;31:928–931. doi: 10.1053/ajkd.1998.v31.pm9631835. [DOI] [PubMed] [Google Scholar]
- 10.Sabry A.A., Sobh M.A., Irving W.L., Grabowska A., Wagner B.E., Fox S. A comprehensive study of the association between hepatitis C virus and glomerulopathy. Nephrol Dial Transplantation. 2002;17:239–245. doi: 10.1093/ndt/17.2.239. [DOI] [PubMed] [Google Scholar]
- 11.Gusmano R., Mazzucco G., Monga G., Ghiggeri G.M. Focal and segmental glomerulosclerosis (FSGS). Clinical, morphological and genetic features. J Nephrol. 2004;17:139–157. [PubMed] [Google Scholar]
- 12.Floege J. Recurrent glomerulonephritis following renal transplantation: an update. Nephrol Dial Transplantation. 2003;18:1260–1265. doi: 10.1093/ndt/gfg102. [DOI] [PubMed] [Google Scholar]
- 13.Couser W. Recurrent glomerulonephritis in the renal allograft: Australia and New Zealand dialysis and transplant registry: ANZ-update of selected areas. Exp Clin Transplantation. 2005;3:283–288. [PubMed] [Google Scholar]
- 14.Daskalakis N., Winn M. Focal and segmental glomerulosclerosis: varying biologic mechanisms underlie a final histopathologic end point. Semin Nephrol. 2006;26:89–94. doi: 10.1016/j.semnephrol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim H., Rogers T., Casingal V. Graft loss from recurrent glomerulonephritis is not increased with a rapid steroid discontinuation protocol. Transplantation. 2006;81:214–219. doi: 10.1097/01.tp.0000188656.44326.53. [DOI] [PubMed] [Google Scholar]
- 16.Cibrik D.M., Kaplan B., Campbell D.A., Meier-Kriesche H.U. Renal allograft survival in transplant recipients with focal segmental glomerulosclerosis. Am J Transplantation. 2003;3:64–67. doi: 10.1034/j.1600-6143.2003.30111.x. [DOI] [PubMed] [Google Scholar]
- 17.Letavernier E., Bruneval P., Mandet C., VanHuyen J., Peraldi M., Helal I. High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol. 2007;2:326–333. doi: 10.2215/CJN.03751106. [DOI] [PubMed] [Google Scholar]
- 18.Swaminathan S., Lager D., Qian X., Stegall M., Larson T., Griffin M. Collapsing and non-collapsing focal segmental glomerulosclerosis in kidney transplants. Nephrol Dial Transplantation. 2006;21:2607–2614. doi: 10.1093/ndt/gfl225. [DOI] [PubMed] [Google Scholar]
- 19.Pardon A., Audard V., Caillard S., Moulin B., Desvaux D., Bentaarit B. Risk factors and outcome of focal and segmental glomerulosclerosis recurrence in adult renal transplant recipients. Nephrol Dial Transplantation. 2006;21:1053–1059. doi: 10.1093/ndt/gfk005. [DOI] [PubMed] [Google Scholar]
- 20.Gossmann J., Scheuermann E.H., Porubsky S., Kachel H.G., Geiger H., Hauser I.A. Abrogation of nephrotic proteinuria by rituximab treatment in a renal transplant patient with relapsed focal segmental glomerulosclerosis. Transplant Int. 2007;20:558–562. doi: 10.1111/j.1432-2277.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 21.Matas A. Recurrent disease after kidney transplantation: it is time to unite to address this problem! Am J Transplantation. 2006;6:2527–2528. doi: 10.1111/j.1600-6143.2006.01571.x. [DOI] [PubMed] [Google Scholar]
- 22.Oka K., Imai E., Moriyama T., Akagi Y., Ando A., Hori M. A clinicopathological study of IgA nephropathy in renal transplant recipients: beneficial effect of angiotensin-converting enzyme inhibitor. Nephrol Dial Transplantation. 2000;15:689–695. doi: 10.1093/ndt/15.5.689. [DOI] [PubMed] [Google Scholar]
- 23.Calvino J., Lens X.M., Romero R., Sanchez-Guisande D. Long-term anti-proteinuric effect of Losartan in renal transplant recipients treated for hypertension. Nephrol Dial Transplantation. 2000;15:82–86. doi: 10.1093/ndt/15.1.82. [DOI] [PubMed] [Google Scholar]
- 24.Stephanian E., Matas A.J., Mauer S.M., Chavers B., Nevins T., Kashtan C. Recurrence of disease in patients retransplanted for focal segmental glomerulosclerosis. Transplantation. 1992;53:755–757. doi: 10.1097/00007890-199204000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Ohta T., Kawaguchi H., Hattori M., Komatsu Y., Akioka Y., Nagata M. Effect of pre- and postoperative plasmapheresis on posttransplant recurrence of focal segmental glomerulosclerosis in children. Transplantation. 2001;71:628–633. doi: 10.1097/00007890-200103150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Gohh R.Y., Yango A.F., Morrissey P.E., Monaco A.P., Gautam A., Sharma M. Pre-emptive plasmapheresis and recurrence of FSGS in high risk renal transplant recipients. Am J Transplantation. 2005;5:2907–2912. doi: 10.1111/j.1600-6143.2005.01112.x. [DOI] [PubMed] [Google Scholar]
- 27.Savin V.J., Sharma R., Sharma M., McCarthy E.T., Swan S.K., Ellis E. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 28.Dall’Amico R., Ghiggeri G., Carraro M., Artero M., Ghio L., Zamorani E. Prediction and treatment of recurrent focal segmental glomerulosclerosis after renal transplantation in children. Am J Kidney Dis. 1999;34:1048–1055. doi: 10.1016/S0272-6386(99)70010-7. [DOI] [PubMed] [Google Scholar]
- 29.Schachter A.D., Harmon W.E. Single-center analysis of early recurrence of nephrotic syndrome following renal transplantation in children. Pediatric Transplantation. 2001;5:406–409. doi: 10.1034/j.1399-3046.2001.t01-2-00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dittrich E., Schmaldienst S., Soleiman A., Hörl W.H., Pohanka E. Rapamycin-associated post-transplantation glomerulonephritis and its remission after reintroduction of calcineurin-inhibitor therapy. Transplant Int. 2004;17:215–220. doi: 10.1007/s00147-004-0700-0. [DOI] [PubMed] [Google Scholar]
- 31.Morelon E., Kreis H. Sirolimus therapy without calcineurin inhibitors: Necker Hospital 8-year experience. Transplantation Proc. 2003;35:52S–57S. doi: 10.1016/s0041-1345(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 32.Raafat R., Travis L.B., Kalia A., Diven S. Role of transplant induction therapy on recurrence rate of focal segmental glomerulosclerosis. Pediatric Nephrol. 2000;14:189–194. doi: 10.1007/s004670050038. [DOI] [PubMed] [Google Scholar]
- 33.Hubsch H., Montané B., Abitbol C., Chandar J., Shariatmadar S., Ciancio G. Recurrent focal glomerulosclerosis in pediatric renal allografts: the Miami experience. Pediatric Nephrol. 2005;20:210–216. doi: 10.1007/s00467-004-1706-7. [DOI] [PubMed] [Google Scholar]
- 34.Nachman P.H., Segelmark M., Westman K., Hogan S.L., Satterly K.K., Jennette J.C. Recurrent ANCA-associated small vessel vasculitis after transplantation: a pooled analysis. Kidney Int. 1999;56:1544–1550. doi: 10.1046/j.1523-1755.1999.00666.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosenstein E.D., Ribot S., Ventresca E., Kramer N. Recurrence of Wegener’s granulomatosis following renal transplantation. Br J Rheumatology. 1994;33:869–871. doi: 10.1093/rheumatology/33.9.869. [DOI] [PubMed] [Google Scholar]
- 36.Nyberg G., Akesson P., Norden G., Wieslander J. Systemic vasculitis in a kidney transplant population. Transplantation. 1997;63:1273–1277. doi: 10.1097/00007890-199705150-00014. [DOI] [PubMed] [Google Scholar]
- 37.Lobbedez T., Comoz F., Renaudineau E., Pujo M., Ryckelynck J.P., Hurault de Ligny B. Recurrence of ANCA-positive glomerulonephritis immediately after renal transplantation. Am J Kidney Dis. 2003;42:E2–E6. doi: 10.1016/s0272-6386(03)00917-x. [DOI] [PubMed] [Google Scholar]
- 38.Stone J.H., Millward C.L., Olson J.L., Amend W.J., Criswell L.A. Frequency of recurrent lupus nephritis among ninety-seven renal transplant patients during the cyclosporine era. Arthritis Rheumatology. 1998;41:678–686. doi: 10.1002/1529-0131(199804)41:4<678::AID-ART15>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Moroni G., Tantardini F., Gallelli B., Quaglini S., Banfi G., Poli F. The long-term prognosis of renal transplantation in patients with lupus nephritis. Am J Kidney Dis. 2005;45:903–911. doi: 10.1053/j.ajkd.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 40.Denton M.D., Galvanek E.G., Singh A., Sayegh M.H. Membranous lupus nephritis in a renal allograft: response to mycophenolate mofetil therapy. Am J Transplantation. 2001;1:288–292. doi: 10.1034/j.1600-6143.2001.001003288.x. [DOI] [PubMed] [Google Scholar]
- 41.Ahuja T.S., Boughton J., Weiss V., Memon A., Remmers A., Jr, Rajaraman S. Late recurrence of lupus nephritis in a renal transplant recipient: response to mycophenolate mofetil. Am J Med Sci. 2001;322:166–169. doi: 10.1097/00000441-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Khandelwal M., McCormick B.B., Lajoie G., Sweet J., Cole E., Cattran D.C. Recurrence of anti-GBM disease 8 years after renal transplantation. Nephrol Dial Transplantation. 2004;19:491–494. doi: 10.1093/ndt/gfg393. [DOI] [PubMed] [Google Scholar]