Abstract
General anesthesia is a relatively safe medical procedure, which for nearly 170 years has allowed life saving surgical interventions in animals and people. However, the molecular mechanism of general anesthesia continues to be a matter of importance and debate. A favored hypothesis proposes that general anesthesia results from direct multisite interactions with multiple and diverse ion channels in the brain. Neurotransmitter-gated ion channels and two-pore K+ channels are key players in the mechanism of anesthesia; however, new studies have also implicated voltage-gated ion channels. Recent biophysical and structural studies of Na+ and K+ channels strongly suggest that halogenated inhalational general anesthetics interact with gates and pore regions of these ion channels to modulate function. Here, we review these studies and provide a perspective to stimulate further advances.
Main Text
General anesthetics encompass a diverse array of compounds seemingly having little in common besides hydrophobicity. Thus, the identification of common molecular targets based on structure-activity relationships has been challenging, especially for the inhalational anesthetics. These volatile drugs are uncharged and small, lacking often even the ability to form hydrogen bonds (1). From a molecular recognition standpoint, these drugs are almost featureless and thus very promiscuous. Consequently, not all binding events might elicit physiological effects. For example, although it is clear that lipid bilayers bind inhalational anesthetics, the search for relevant physiological consequences of this interaction has proven difficult and wanting (2, 3). The realization that proteins also have hydrophobic recognition surfaces, typically cavities or pockets, initially shifted the attention to soluble enzymes. Bacterial and firefly luciferases are early examples of enzymes with an easily measured function that was altered by roughly appropriate concentrations of anesthetic compound (4, 5, 6). However, modulation of the catalytic activity of nonneuronal and, most importantly, nonmammalian proteins is not sufficient evidence to deem these receptors clinically relevant. Anesthetic targets ought to play a major role as regulators of excitability in the central nervous system of animals that exhibit typical reversible endpoints of anesthesia (e.g., unresponsiveness, immobility, etc.). Based on this criterion, ion channels emerge as the most reasonable candidates.
Remarkably, general anesthetics influence the activity of many ion channels with widely divergent properties (2, 7, 8, 9, 10). It is thus unlikely that only one class of ion channels is responsible for all endpoints of anesthesia (unitary protein hypothesis). Conversely, there is growing recognition that multiple molecular pathways to anesthesia exist and that different anesthetics can use one or more of these pathways to produce an anesthetic state (10). Anesthetics have been separated into groups based on these pathway preferences. For example, propofol, etomidate, barbiturates, neurosteroids, and many inhalational anesthetics potently influence the GABAA receptor and thus were termed GABAergic, whereas ketamine, nitrous oxide, and xenon are more active on NMDA receptors, and were termed glutamatergic (10). These monikers are based almost entirely on electrophysiological studies of various reductionist systems, and the choices of ion channel were in part dictated by old evidence suggesting that general anesthetics act on synaptic transmission rather than conduction (3, 11). Definitive support for this hypothesis remains elusive because in vivo studies using either genetic or pharmacological manipulations of these largely synaptic ligand-gated ion channels have been far less convincing than the electrophysiology, particularly for the inhalational anesthetics (12, 13).
What are we missing? For one, a big family of ion channels has been largely ignored, as early studies suggested that the voltage-gated ion channels are less sensitive than the ligand-gated and background K+ channels (2, 3). In other words, relatively high anesthetic concentrations modulate these ion channels; with EC50 values somewhat higher than those required for GABAAR modulation and in vivo general anesthesia. However, voltage-gated ion channels are certainly relevant as they are found in the brain and other excitable tissues, help set the membrane potential, determine initiation, propagation and repetitive firing of action potentials, and govern vesicular transmitter release (8, 14, 15, 16). Also, the in vitro sensitivity criterion has been challenged on the grounds that it assumes a linear relationship between channel activity, cellular activity, and ultimately organism function (17). Even the simplest of models lay this notion to rest (10, 17). Furthermore, recent studies have demonstrated robust and selective effects of inhalational anesthetics on voltage-gated ion channels, identified putative sites, and have gone further to demonstrate in vivo relevance (8, 9, 18, 19, 20, 21, 22, 23, 24).
Focusing on mechanisms of action, this perspective summarizes recent evidence supporting the emergence of the voltage-gated Kv and Nav channel families to full status as functionally relevant molecular targets of general anesthetics. Certainly, other voltage-gated ion channels are also emerging as anesthetic target candidates (Cav and HCN channels) (16, 25, 26, 27); however, significant structural and functional aspects of these ion channels overlap with Nav and Kv channels, and thus our focus will not be entirely exclusionary.
Electrophysiological and biophysical Insights
Modulation of Kv channels by halogenated inhalational anesthetics
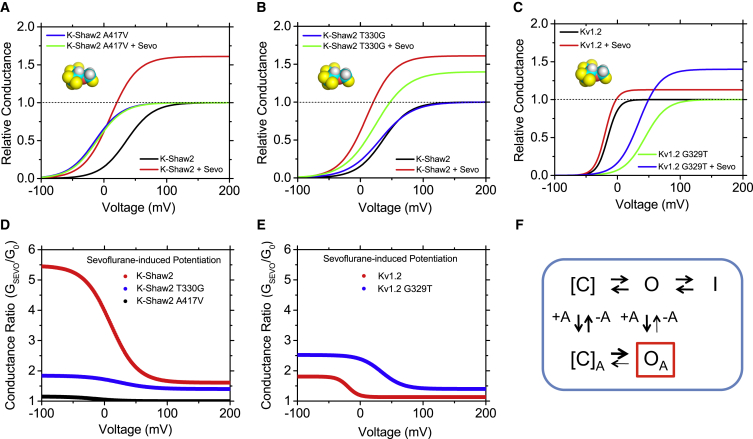
Pioneering studies of the Drosophila Shaker mutant and heterologously expressed K+ channels suggest that specific Kv channels could be significant targets of inhalational general anesthetics (28, 29, 30, 31, 32, 33). In particular, Li and Correa demonstrated that isoflurane potentiates the conductance of the Drosophila ShakerB Kv channel by increasing the open probability and the unitary conductance (29). Independently, we showed that n-alcohols (with an apparent cut-off at C6) and most general anesthetics, such as chloroform, halothane, isoflurane, and propofol inhibit K-Shaw2, another Drosophila Kv channel in the Shaker superfamily (20, 34, 35, 36). This inhibition is reversible, exhibits a saturable profile over a range covering relevant concentrations, involves amphiphilic interactions, and has fast on/off millisecond kinetics (37, 38). Despite their distinct chemical structures, but consistent with their physicochemical properties and the relatively nonspecific nature of the interactions, n-butanol and halothane display overlapping sites in the channel protein (38, 39). Overall, the data suggest that n-alcohols and halogenated inhalational anesthetics inhibit K-Shaw2 by preferentially interacting with the channel’s resting closed state and stabilizing it. In contrast, we later discovered that sevoflurane uniquely potentiates the K-Shaw2 conductance in a manner that is reminiscent of the effect of isoflurane on the conductance of the ShakerB Kv channel (20, 28, 29). This finding is particularly relevant because sevoflurane is a relatively new inhalational anesthetic commonly used in the clinic (40, 41). Sevoflurane-dependent potentiation of K-Shaw2 exhibits high and low affinity interactions with apparent equilibrium dissociation constants of the order of 60 μM and 4 mM, respectively (20). Positive modulation of the K-Shaw2 conductance by sevoflurane results from two major effects: shifting in the conductance-voltage (G-V) relation toward negative voltages, and increasing the maximum conductance (Fig. 1, A and D) (20).
Figure 1.
Positive allosteric modulation of Kv channels by sevoflurane. (A–C) Idealized G-V curves of Kv channels. (D and E) Idealized conductance ratio (conductance in the presence of 1 mM sevoflurane over control conductance) – voltage relations. These curves are based on previously published experimental data (20). The magnitude of the changes is better appreciated by examining the conductance ratio curves, which show significant sevoflurane-induced increases in conductance over a physiologically critical range of membrane potentials for K-Shaw2 and Kv1.2, Kv1.2-G329T. (F) Kinetic scheme that explains the positive allosteric modulation of Kv channels by sevoflurane (20) (see text). [C], O, and I indicate distinct gating states: preopen closed, open, and open nonconducting (possibly inactivated), respectively. The preopen closed might include an aggregate of resting/nonconducting states connected by voltage-dependent transitions in the activation pathway. +A and –A represent binding and unbinding of the anesthetic.
To explain these effects, kinetic modeling suggested that sevoflurane increases the equilibrium constant of the channel’s opening step and inhibits a nonconducting state outside the activation pathway (20) (Fig. 1 F). Additionally, sevoflurane similarly potentiates ShakerB, and the mammalian homologs Kv1.2 (Fig. 1, C and E), Kv1.3, and Kv1.5 (9, 20). The latter is particularly important because it suggests that Kv1 channels might be relevant sevoflurane targets in mammalian excitable tissues. Currently, we do not know why sevoflurane selectively potentiates some Kv channels in contrast to the inhibitory action of other halogenated inhalational anesthetics and propofol (20). Seemingly, relatively subtle differences in anesthetic structure and Kv channel structure/conformation determine the modulation. For instance, isoflurane, a halogenated ether closely related to sevoflurane, potentiates the activity of the ShakerB Kv channel but inhibits the K-Shaw2 channel. In excitable tissues, these differences might also be dictated by the diverse heteromeric architecture of native Kv channel complexes, which include accessory subunits (42, 43).
Given the apparently unique ability of sevoflurane to potentiate Kv channels, it is also not clear how such a modulation could be associated with anesthesia. Considering that there are many ways to achieve anesthesia endpoints, the modulation of specific molecular targets is not as critical as their placement within the neuronal circuitry. If anesthesia depends on tilting the excitation-inhibition balance toward inhibition, any manipulation that selectively dampens excitability in these circuits might produce anesthesia. Sevoflurane could achieve this by specifically potentiating Kv1 channels in critical neural circuits that oppose arousal. Alternatively, potentiation of Kv1 channels by sevoflurane might be responsible for undesired anesthetic effects not directly associated with hypnosis, such as dysphoria (44).
Halogenated inhalational anesthetics target electromechanical coupling and pore properties in Kv channels
Based on the differential actions of n-alcohols on Kv channels and comparison of the amino acid sequences, we previously hypothesized that general anesthetics target regions directly involved in the electromechanical transduction mechanism that governs voltage-dependent gating of Kv channels (36, 45, 46). Supporting this hypothesis, we found that swapping of the S4–S5 linker (13 amino acids) between K-Shaw2 (anesthetic-sensitive) and Kv3.4 (anesthetic-resistant) fully removes and confers inhibition by n-alcohols, respectively (36, 47). Moreover, there is a positive correlation between the apparent anesthetic binding energy and the α-helicity of the S4–S5 linker (37, 47, 48); and a single mutation in the critical PVPV motif of the K-Shaw2 S6 tail (PVPA) converts the inhibition by n-alcohols or halothane into potentiation (39, 49). This potentiation is greatly dependent on the context provided by the S4–S5 linker both in K-Shaw2 and other related chimeric Kv channels (47). Strengthening the critical role of these interactions, gating perturbations induced by n-butanol, and halothane yielded substantial interaction energies between the S4–S5 linker residue A326 and the S6-tail residues A417, Y419, and Y420 (2–>4 kcal/mol) (38). Therefore, regions critically responsible for electromechanical transduction in Kv channels (S4–S5 linker and the S6-tail) are major determinants of inhalational anesthetic action.
Recent studies continue to strengthen this conclusion. We discovered that a single residue at a critical pivot point between the S4–S5 linker and the S5 segment determines the specific potentiation of Kv1.2 and K-Shaw2 by sevoflurane (G329 and T330 at equivalent positions in Kv1.2 and K-Shaw2, respectively). Whereas G329T confers dramatic potentiation of the Kv1.2 conductance by sevoflurane (and other general anesthetics), T330G eliminates the robust sevoflurane-induced potentiation of the K-Shaw2 conductance. However, the latter mutation does not affect the sevoflurane-induced increase in maximum conductance mentioned previously (Fig. 1, A and B). This indicates that at least two separable mechanisms are involved in the positive modulation of the Kv channels by sevoflurane. One mechanism might involve sites allosterically coupled to the electromechanical transduction directly responsible for controlling voltage-dependent gating (i.e., cavities in the vicinity of contacts between the S4–S5 linker and the S6-tail). The other mechanism might involve distinct sites, which could modulate the channel’s pore region and thereby influence the stability of the open state and/or the unitary conductance.
A plausible mechanism of sevoflurane-dependent positive modulation of Kv channels
Although sevoflurane similarly potentiates K-Shaw2 and Kv1.2 channels (Fig. 1), there are quantitative differences. The sevoflurane-induced changes are larger in K-Shaw2, suggesting that differential efficacies might depend on intrinsic biophysical/structural properties of the Kv channels. Whereas the opening-reluctant K-Shaw2 exhibits relatively high-voltage activation and unfavorable opening (zFV1/2 = 4 kcal/mol), the opening-permissive Kv1.2 exhibits relatively low-voltage activation and more favorable opening (zFV1/2 = −5 kcal/mol). Therefore, there is a plausible nonadditive inverse link between the energetics of the channel opening and sevoflurane action. Accordingly, a Kv1.2 mutation (G329T) that makes the channel opening-reluctant (zFV1/2 = 6 kcal/mol) greatly magnifies the sevoflurane-induced positive modulation. A broader examination of the modulation of a larger set of wild-type and mutant Kv channels from different subfamilies by sevoflurane revealed a similar link (Q.L. and M.C., unpublished data). We propose that the sevoflurane-induced positive modulation of Kv channels is tightly linked to voltage-dependent gating. If the channel’s native/mutant conformation exhibits an unfavorable opening step (opening-reluctant), sevoflurane binding to cavities involving regions that govern electromechanical coupling would have significant leeway to promote opening (i.e., a significant shift of the G-V relation toward more negative voltages). By contrast, if the opening step is already more favorable (opening-permissive), the leeway for positive modulation by sevoflurane is limited and, therefore, the resulting shift of the G-V relation is smaller. Supporting this relationship, K-Shaw2 A417V exhibits a strongly leftward shifted G-V relation and lacks modulation by sevoflurane (Fig. 1, A and D). There are, however, exceptions to this relationship; the G-V relation for K-Shaw2 T330G is not shifted and yet the ability of sevoflurane to modulate this mutant is greatly diminished (Fig. 1, B and D). Overall, these observations reveal that voltage-dependent gating and positive modulation by sevoflurane are nonadditive (i.e., they are not independent), which strongly suggests that the two processes interact. We suggest that the actions of sevoflurane and voltage are not separable because voltage sensing and sevoflurane binding act on the conformational change that governs opening of the K+-selective pore (Fig. 2).
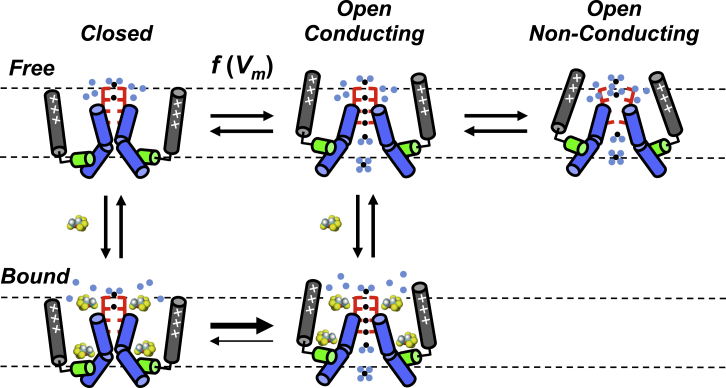
Figure 2.
Cartoon of a possible mechanism of sevoflurane action on a Kv channel. For clarity, only two opposing pore-forming subunits are shown. Gray, green, and blue rods represent voltage sensors, S4–S5 linker and pore-lining S6 segments, respectively. Red brackets represent the selectivity filter and ion binding sites. Light blue dots represent water molecules, and f(Vm) indicates a voltage-dependent equilibrium. Binding of sevoflurane (space-filled) to external (around selectivity filter) and internal sites (S4–S5 linker sites) stabilizes the open state by promoting opening and inhibiting the rapid equilibrium between the open/conducting and open/nonconducting conformations (note a possible distortion of the selectivity filter in this conformation).
The hypothesis discussed previously cannot explain the voltage-independent effect of sevoflurane (i.e., the increase in relative maximum conductance), which is resistant to a mutation (K-Shaw2 T330G) that nearly abolishes the voltage-dependent effect discussed previously. We propose that the increase in maximum conductance might result from increasing the unitary conductance and/or increasing the maximum open probability by inhibiting a nonconducting state outside the activation pathway. This inhibition could stabilize the conducting conformation of the channel’s selectivity filter (Fig. 2).
Modulation of Nav channels by halogenated inhalational anesthetics
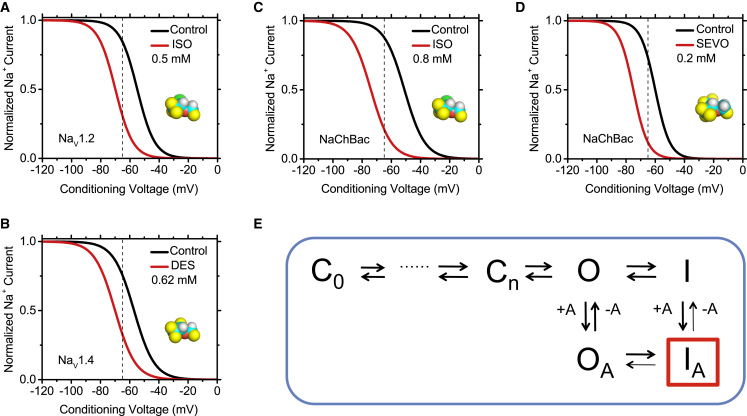
Inhalational general anesthetics primarily inhibit Nav channels through modulation of inactivation gating (8, 50, 51, 52, 53, 54, 55). Ouyang and Hemmings demonstrated that isoflurane inhibition of Nav 1.2, 1.4, and 1.5 results from a negative shift of the steady-state inactivation curve associated with fast inactivation (52) (Fig. 3). Additionally, Nav1.2 showed significant tonic block by isoflurane. Further work by Ouyang et al. revealed that various halogenated anesthetics differed in their ability to inhibit the Nav1.4 channel, possibly due to anesthetic-specific differences in the predominant mode of action (56). For example, desflurane induced a large hyperpolarizing shift of the steady-state inactivation curve, whereas halothane and isoflurane exhibited use-dependent block, slowed recovery from inactivation, and enhanced current decay.
Figure 3.
Halogenated ethers generally induce a relative stabilization of the Nav channel inactivated state. (A–D) Idealized steady-state inactivation curves for various Nav channels before and after exposure to the indicated concentrations of common halogenated inhalational anesthetics (ISO, isoflurane; DES, desflurane; SEVO, sevoflurane). These curves are based on data independently published by various authors (19, 50, 52, 54, 56, 57). The anesthetics induce a negative shift in the steady-state inactivation curve, consistent with a relative stabilization of the inactivated conformation. The dashed vertical line marks the typical resting membrane potential of mammalian neurons. Note a substantial shift in the available Na+ current at this membrane potential. (E) Kinetic scheme that explains the major effects of halogenated inhalational anesthetics on Nav channels. The anesthetics bind with higher affinity to the inactivated state and stabilize it (modulated receptor hypothesis).
Mechanisms of the inhibition of a bacterial Nav channel by halogenated inhalational anesthetics
Although multiple studies point to inactivation gating of Nav channels as a key target of inhalational anesthetic modulation, the large size and pseudotetrameric architecture of mammalian Nav channels has made it difficult to pinpoint mechanistically relevant interactions. The discovery and subsequent crystallization of bacterial Nav channels offered an avenue for more detailed mechanistic exploration of inhalational anesthetic action. Ouyang et al. demonstrated that, like mammalian Nav channels, NaChBac is inhibited by clinically relevant concentrations of isoflurane (0.2–0.8 mM) (57). NaChBac shares similarity with eukaryotic Nav and Cav channels (58). However, in contrast to the eukaryotic counterparts, NaChBac is tetrameric (resembling Kv channels), which facilitates structure-function studies. This might also affect anesthetic interactions. Nevertheless, the inhibition of NaChBac by isoflurane involves electrophysiological alterations similar to those observed in mammalian Nav channels, namely a negative shift of the steady-state inactivation curve and use-dependent block. Ouyang et al. proposed that enhanced inactivation is the primary mechanism of isoflurane-induced inhibition of NaChBac (Fig. 3).
We independently assessed NaChBac modulation by sevoflurane, which displays some unexpected features (19). At low and high concentrations (0.2 and 2 mM), sevoflurane shifts both the G-V relation and steady-state inactivation curve in the hyperpolarizing direction. Additionally, sevoflurane modestly accelerates recovery from inactivation at both low and high concentrations. This acceleration is opposite to the effect of other inhalational anesthetics on both NaChBac and mammalian Nav channels, which exhibit slowed recovery from inactivation consistent with stabilization of an inactivated state. Gating modulation by sevoflurane displayed no significant concentration dependence, suggesting high-affinity interactions possibly saturated at 0.2 mM. However, 2 mM sevoflurane additionally induces substantial acceleration of current decay, indicative of enhanced inactivation and/or slow open-channel block. Semiquantitative computational modeling based on a strictly sequential six-state kinetic scheme previously proposed for NaChBac (59) recapitulates sevoflurane modulation by combining enhanced inactivation gating indirectly resulting from more favorable voltage-dependent activation (resembling Kv channels), and slow open-channel block (19). The first and second assumptions are presumably associated with sevoflurane’s high- and low-affinity interactions, respectively.
Altogether, halogenated inhalational anesthetics act on Nav channels to promote steady-state inactivation coupled to voltage-dependent activation (Fig. 3). In most instances this modulation is associated with slowed recovery from inactivation indicating preferential binding to inactivated Nav channels and stabilization of the inactivated state, which also gives rise to use-dependent inhibition (an apparent block resulting from cumulative inactivation). In NaChBac, the unique but modestly accelerated recovery from inactivation induced by sevoflurane appears to be insufficient to counteract much more favorable steady-state inactivation. Other common effects are consistent with enhanced inactivation (accelerated current decay), but could also result from slow open channel block. In the light of these features, we could generally explain the action of halogenated inhalational anesthetics by assuming classical mechanisms of local anesthetic action (60). Combinations of modulated and guarded receptor hypotheses could account for the state-dependent phenomenology depending on the relatively specific interactions that may allow distinct halogenated inhalational anesthetics to differentially induce stabilization of the channel’s inactivated state (modulated receptor) and open channel pore block (guarded receptor). Whereas the first mechanism depends on preferential interaction with the channel’s inactivated state, the second involves gated accessibility to an anesthetic pore-blocking site (e.g., opening of an internal activation gate). The interaction with the inactivated state may not necessarily depend on the opening of an internal activation gate. Given that halogenated inhalational anesthetics share a general ability to stabilize the inactivated state of bacterial and mammalian Nav channels, a heuristic approach prompted us to adopt a modulated receptor hypothesis as the dominant mechanism of drug action (Fig. 3). By analogy to the Kv channel scenario (above), we propose that inactivation gating and gating perturbation by inhalational anesthetic binding in Nav channels are nonadditive processes acting on the same conformational change. The inactivation-independent pore blocking mechanisms (tonic and use-dependent) are not sufficiently defined, despite otherwise suggestive electrophysiological features (52). At any rate, the complexity of the phenomenology and the proposed interactions suggest multiple modes of actions involving multiple sites on prokaryotic and eukaryotic Nav channels.
Structural and computational insights
Putative anesthetic sites in KV channels: K-Shaw2, Kv1.2
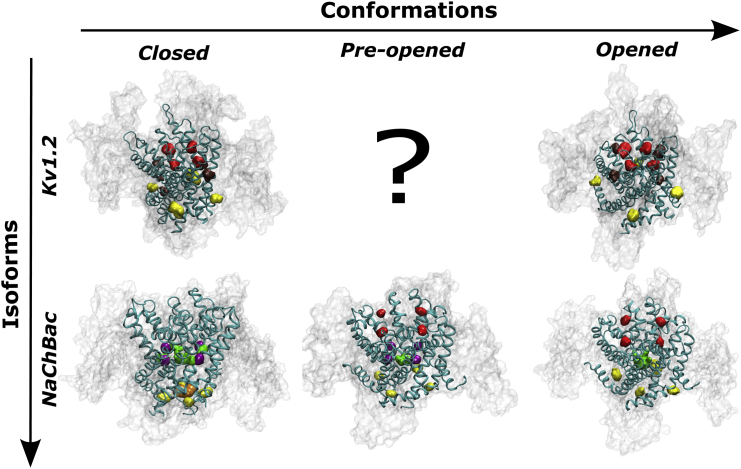
Based on the available x-ray crystal structures of activated/open Kv1.2 channels in the lipid bilayer environment, we have built experimentally consistent atomistic models of the resting-closed conformation (61). To resolve site-specific anesthetic affinities, molecular docking and molecular dynamics-based free energy calculations yielded distinct classes of putative binding sites in wild-type and mutant Kv channels (Fig. 4). Kv1.2 harbors two sevoflurane sites within the transmembrane domain of the channel at the internal S4–S5 linker and the S5–S6 interface (pore domain) of adjacent subunits, and a third site flanking the selectivity filter at the extracellular face of the channel. Similar putative binding sites were identified for halothane and sevoflurane in K-Shaw2 and Kv1.2-G329T (38). Essentially, the modeled Kv1.2 sevoflurane binding sites can be grouped into two subsets displaying relatively low and high affinities. The low-affinity subset includes the S4–S5 linker and pore domain sites, and the high-affinity subset includes pockets around the selectivity filter. The S4–S5 linker site that is closest to the key residue G329 displays ∼sevenfold greater affinity for the resting-closed conformation relative to the activated-open conformation. These patterns are consistent with the complexity of the functional effects previously discussed. In particular, the putative binding site subset implicating the S4–S5 linker may help explain specific aspects of the negative/positive modulation that is sensitive to mutations affecting this region. Further work is necessary to understand how additional anesthetic binding sites might contribute to functional modulation (e.g., the sevoflurane-induced increase in maximum conductance).
Figure 4.
Putative sevoflurane sites. Lateral views of Kv1.2 and NaChBac models in different functional conformations. From in silico docking of Kv1.2, three main binding sites are located at the extracellular region next to the selectivity filter (red), at the S5–S6 interface of adjacent subunits (brown), and at the S4–S5 linker (yellow). NaChBac clustering analysis of flooding simulations revealed four sites, extracellular site (red), linker site (yellow), activation gate site (orange), and cavity/fenestration site (green and purple).
Putative anesthetic sites in Nav channels: NaChBac
Although structural information about eukaryotic Nav channels is scant, careful phylogenetic analyses together with several independent x-ray crystallography studies have made accessible an entire class of prokaryotic Na+-selective voltage-gated ion channels (62, 63, 64). Their significance as proxies for the physiologically relevant mammalian counterparts has been ascertained on the basis of functional and structural studies (57, 58, 59, 63, 65). Molecular dynamics flooding simulations on membrane-bound structural models of NaChBac revealed putative binding of isoflurane and sevoflurane at four distinct locations (19, 66) (Fig. 4). One site is in the pore of the channel, suggesting that halogenated ethers may hinder ion conduction. Surprisingly, this site is accessible even when the pore and the aqueous compartment at the intracellular side are disconnected. The fenestrations present in the membrane-embedded region of the channel seem to act as the long-hypothesized hydrophobic drug access pathway (67). Adjacent to this binding site, but somewhat distinct from it, is a second set of sites located at the internal fenestration entrance; a third set of binding sites is found at the interface between the S4–S5 linker and the S6 helix bundle. In the open state, these sites are formed by mostly hydrophobic side chains of S6 residues, which create pockets with an ideal chemical character to bind halogenated inhalational anesthetics. Finally, a fourth set of binding sites is in a region surrounding the selectivity filter at the interface between adjacent subunits (a pocket created by residues belonging to the so-called pore-turret and pore-helix). This is the only set of binding sites that is directly accessible from the extracellular side. The potential relevance of the binding sites surrounding the selectivity filter is supported by the observation of a marked state-dependent affinity: binding is possible only in the most activated states (Fig. 4). Conversely, binding at the fenestrations occurs only in the closed deactivated states. Free energy calculations confirmed the putative physiological relevance of the sites by providing affinities in the submillimolar range. The lack of systematic mutational studies, however, limits the extent to which we can presently relate these putative sites to functional effects of the anesthetics (e.g., stabilization of the inactivated state versus pore block).
Do Kv and Nav channels share molecular mechanisms of sevoflurane action?
Sevoflurane is a relatively specific gating modulator that induces opposite net effects on Kv and NaChBac channels (Figs. 1 and 2), which have similar architectures and basic gating mechanisms (58). This selectivity is surprising because general anesthetics have been long regarded as nonspecific agents. The conformational specificity probably depends on relatively subtle structural differences in critical gating regions. Consequently, in a state-dependent manner, sevoflurane binding shifts the channel’s gating equilibrium in a particular direction to stabilize/destabilize either closed, open, or inactivated conformations. At a fundamental level sevoflurane probably interacts similarly with both Kv and Nav channels (below). Supporting this idea, the binding patterns for sevoflurane in Kv1.2, K-Shaw, and NaChBac are remarkably similar (Fig. 4). Specifically, aside from the NaChBac sites in the pore and fenestrations, all sites are detected in regions lying at the level of the membrane interface, suggesting that partially solvated protein pockets in these regions and the dipolar field of the lipid bilayer provide a chemical environment that matches the amphiphilic character of halogenated ethers. The specific locations of the putative binding sites in Kv and NaChBac channels are also relevant. For instance, binding might occur at the subunit interface behind the selectivity filter. In typical K+ channels, this interface helps regulate the stability of P/C-type inactivation (68). Another interesting binding region involves the S4–S5 linker, which plays a crucial role in the electromechanical mechanism that governs voltage-dependent activation gating (45, 46). At this site, interactions with the anesthetic could modulate the overall gating cycle. In contrast to the external and internal sites common to Kv and NaChBac channels, the cavity and fenestration sites are characteristic of NaChBac and possibly other Nav channels. These sites might be significant specific determinants of the state-dependent mechanism underlying anesthetic-induced stabilization of the inactivated state that is typical of Nav channels. At critical access locations, these sites might work independently or in conjunction with sites involving the S4–S5 linker to modulate gating and pore blockade. Whether or not we can directly extrapolate the information gained from studying NaChBac to mammalian systems depends on learning more about the three-dimensional structures of eukaryotic Nav channels at the atomic level. Moreover, studies of mammalian Kv and Nav channels at an organismal level would be necessary to ultimately confirm the relevant roles of these ion channels in general anesthesia.
Conclusions
The differential outcomes of the interactions between halogenated inhalational anesthetics and Kv and Nav channels probably depend on how the voltage-dependent conformational energy landscape of a particular ion channel affects anesthetic binding and vice versa. However, inferring the nature and location of real anesthetic binding sites from the modulatory behavior alone (top-down approach) is a lengthy process that can be carried out only for a few ligand chemotypes, channel-types, and conformations. Whether or not we can transfer these inferences to a larger set of ligands, ion channels, and conformations remains a challenge. Special attention will have to be devoted to channel/drug interactions in intermediate channel conformations as growing evidence from both theoretical and experimental studies points to the critical role of these hidden interactions. Critically, what is still missing is a bottom-up solution of the problem. This approach implies demonstrating actual ligand binding (from NMR studies and photolabeling) and predicting from first principles how this interaction could ultimately affect the conformational equilibriums of the ion channel. This, however, is not a trivial problem as ligand binding may be a necessary but not sufficient condition to achieve a modulatory effect. Shedding light on this topic will require an entire new series of high-resolution experimental and computational studies aiming at describing gating energetics of ion channels in the absence and presence of site-specific bound ligands. New advances in time-resolved x-ray/neutron interferometry and free-energy molecular dynamics calculations might help achieve this goal (19, 66, 69).
Author Contributions
M.C. made the figures, drafted the Kv channel sections, edited the final version, and finalized the manuscript; A.B. drafted the Nav channel section and edited the text; V.C. drafted the computational insights and edited the text; W.T. drafted the computational insights and edited the text; R.G.E. drafted the introduction and edited text. M.C. and R.G.E. wrote the conclusions.
Acknowledgments
This work was supported by a research grant from the National Institutes of Health, US Department of Health and Human Services (P01 GM055876).
Editor: Brian Salzberg.
References
- 1.Eckenhoff R.G., Johansson J.S. Molecular interactions between inhaled anesthetics and proteins. Pharmacol. Rev. 1997;49:343–367. [PubMed] [Google Scholar]
- 2.Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 3.Franks N.P., Lieb W.R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 4.Curry S., Lieb W.R., Franks N.P. Effects of general anesthetics on the bacterial luciferase enzyme from Vibrio harveyi: an anesthetic target site with differential sensitivity. Biochemistry. 1990;29:4641–4652. doi: 10.1021/bi00471a020. [DOI] [PubMed] [Google Scholar]
- 5.Moss G.W., Lieb W.R., Franks N.P. Anesthetic inhibition of firefly luciferase, a protein model for general anesthesia, does not exhibit pressure reversal. Biophys. J. 1991;60:1309–1314. doi: 10.1016/S0006-3495(91)82168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda I., Kamaya H. Kinetic and thermodynamic aspects of the mechanism of general anesthesia in a model system of firefly luminescence in vitro. Anesthesiology. 1973;38:425–436. doi: 10.1097/00000542-197305000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Franks N.P. Molecular targets underlying general anaesthesia. Br. J. Pharmacol. 2006;147(Suppl 1):S72–S81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herold K.F., Hemmings H.C., Jr. Sodium channels as targets for volatile anesthetics. Front. Pharmacol. 2012;3:50. doi: 10.3389/fphar.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lioudyno M.I., Birch A.M., Alkire M.T. Shaker-related potassium channels in the central medial nucleus of the thalamus are important molecular targets for arousal suppression by volatile general anesthetics. J. Neurosci. 2013;33:16310–16322. doi: 10.1523/JNEUROSCI.0344-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp Lugli A., Yost C.S., Kindler C.H. Anaesthetic mechanisms: update on the challenge of unravelling the mystery of anaesthesia. Eur. J. Anaesthesiol. 2009;26:807–820. doi: 10.1097/EJA.0b013e32832d6b0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards C.D. Actions of general anaesthetics on synaptic transmission in the CNS. Br. J. Anaesth. 1983;55:201–207. doi: 10.1093/bja/55.3.201. [DOI] [PubMed] [Google Scholar]
- 12.Jurd R., Arras M., Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 13.Werner D.F., Swihart A., Homanics G.E. Inhaled anesthetic responses of recombinant receptors and knockin mice harboring α2(S270H/L277A) GABA(A) receptor subunits that are resistant to isoflurane. J. Pharmacol. Exp. Ther. 2011;336:134–144. doi: 10.1124/jpet.110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hille B. Sinauer Associates; Sunderland, MA: 2001. Ionic Channels of Excitable Membranes. [Google Scholar]
- 15.Tsantoulas C., McMahon S.B. Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci. 2014;37:146–158. doi: 10.1016/j.tins.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orestes P., Todorovic S.M. Are neuronal voltage-gated calcium channels valid cellular targets for general anesthetics? Channels (Austin) 2010;4:518–522. doi: 10.4161/chan.4.6.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckenhoff R.G., Johansson J.S. On the relevance of “clinically relevant concentrations” of inhaled anesthetics in in vitro experiments. Anesthesiology. 1999;91:856–860. doi: 10.1097/00000542-199909000-00039. [DOI] [PubMed] [Google Scholar]
- 18.Alkire M.T., Asher C.D., Hahn E.L. Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology. 2009;110:766–773. doi: 10.1097/aln.0b013e31819c461c. [DOI] [PubMed] [Google Scholar]
- 19.Barber A.F., Carnevale V., Covarrubias M. Modulation of a voltage-gated Na+ channel by sevoflurane involves multiple sites and distinct mechanisms. Proc. Natl. Acad. Sci. USA. 2014;111:6726–6731. doi: 10.1073/pnas.1405768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber A.F., Liang Q., Covarrubias M. Novel activation of voltage-gated K(+) channels by sevoflurane. J. Biol. Chem. 2012;287:40425–40432. doi: 10.1074/jbc.M112.405787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmings H.C., Jr. Sodium channels and the synaptic mechanisms of inhaled anaesthetics. Br. J. Anaesth. 2009;103:61–69. doi: 10.1093/bja/aep144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eger E.I., 2nd, Raines D.E., Sonner J.M. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth. Analg. 2008;107:832–848. doi: 10.1213/ane.0b013e318182aedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouyang W., Wang G., Hemmings H.C., Jr. Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals. Mol. Pharmacol. 2003;64:373–381. doi: 10.1124/mol.64.2.373. [DOI] [PubMed] [Google Scholar]
- 24.Westphalen R.I., Desai K.M., Hemmings H.C., Jr. Presynaptic inhibition of the release of multiple major central nervous system neurotransmitter types by the inhaled anaesthetic isoflurane. Br. J. Anaesth. 2013;110:592–599. doi: 10.1093/bja/aes448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X., Shu S., Bayliss D.A. Subunit-specific effects of isoflurane on neuronal Ih in HCN1 knockout mice. J. Neurophysiol. 2009;101:129–140. doi: 10.1152/jn.01352.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cacheaux L.P., Topf N., Goldstein P.A. Impairment of hyperpolarization-activated, cyclic nucleotide-gated channel function by the intravenous general anesthetic propofol. J. Pharmacol. Exp. Ther. 2005;315:517–525. doi: 10.1124/jpet.105.091801. [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Sirois J.E., Bayliss D.A. HCN subunit-specific and cAMP-modulated effects of anesthetics on neuronal pacemaker currents. J. Neurosci. 2005;25:5803–5814. doi: 10.1523/JNEUROSCI.1153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correa A.M. Gating kinetics of Shaker K+ channels are differentially modified by general anesthetics. Am. J. Physiol. 1998;275:C1009–C1021. doi: 10.1152/ajpcell.1998.275.4.C1009. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Correa A.M. Single-channel basis for conductance increase induced by isoflurane in Shaker H4 IR K(+) channels. Am. J. Physiol. Cell Physiol. 2001;280:C1130–C1139. doi: 10.1152/ajpcell.2001.280.5.C1130. [DOI] [PubMed] [Google Scholar]
- 30.Li J., Correa A.M. Kinetic modulation of HERG potassium channels by the volatile anesthetic halothane. Anesthesiology. 2002;97:921–930. doi: 10.1097/00000542-200210000-00026. [DOI] [PubMed] [Google Scholar]
- 31.Friederich P., Benzenberg D., Urban B.W. Interaction of volatile anesthetics with human Kv channels in relation to clinical concentrations. Anesthesiology. 2001;95:954–958. doi: 10.1097/00000542-200110000-00026. [DOI] [PubMed] [Google Scholar]
- 32.Elliott J.R., Urban B.W. Integrative effects of general anaesthetics: why nerve axons should not be ignored. Eur. J. Anaesthesiol. 1995;12:41–50. [PubMed] [Google Scholar]
- 33.Tinklenberg J.A., Segal I.S., Maze M. Analysis of anesthetic action on the potassium channels of the Shaker mutant of Drosophila. Ann. N. Y. Acad. Sci. 1991;625:532–539. doi: 10.1111/j.1749-6632.1991.tb33884.x. [DOI] [PubMed] [Google Scholar]
- 34.Covarrubias M., Rubin E. Ethanol selectively blocks a noninactivating K+ current expressed in Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 1993;90:6957–6960. doi: 10.1073/pnas.90.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Covarrubias M., Vyas T.B., Wei A. Alcohols inhibit a cloned potassium channel at a discrete saturable site. Insights into the molecular basis of general anesthesia. J. Biol. Chem. 1995;270:19408–19416. doi: 10.1074/jbc.270.33.19408. [DOI] [PubMed] [Google Scholar]
- 36.Harris T., Shahidullah M., Covarrubias M. General anesthetic action at an internal protein site involving the S4-S5 cytoplasmic loop of a neuronal K+ channel. J. Biol. Chem. 2000;275:4928–4936. doi: 10.1074/jbc.275.7.4928. [DOI] [PubMed] [Google Scholar]
- 37.Shahidullah M., Harris T., Covarrubias M. Molecular features of an alcohol binding site in a neuronal potassium channel. Biochemistry. 2003;42:11243–11252. doi: 10.1021/bi034738f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barber A.F., Liang Q., Covarrubias M. Molecular mapping of general anesthetic sites in a voltage-gated ion channel. Biophys. J. 2011;101:1613–1622. doi: 10.1016/j.bpj.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattacharji A., Klett N., Covarrubias M. Inhalational anaesthetics and n-alcohols share a site of action in the neuronal Shaw2 Kv channel. Br. J. Pharmacol. 2010;159:1475–1485. doi: 10.1111/j.1476-5381.2010.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith I., Nathanson M., White P.F. Sevoflurane–a long-awaited volatile anaesthetic. Br. J. Anaesth. 1996;76:435–445. doi: 10.1093/bja/76.3.435. [DOI] [PubMed] [Google Scholar]
- 41.Reference deleted in proof.
- 42.Pongs O., Schwarz J.R. Ancillary subunits associated with voltage-dependent K+ channels. Physiol. Rev. 2010;90:755–796. doi: 10.1152/physrev.00020.2009. [DOI] [PubMed] [Google Scholar]
- 43.Trimmer J.S. Subcellular localization of K+ channels in mammalian brain neurons: remarkable precision in the midst of extraordinary complexity. Neuron. 2015;85:238–256. doi: 10.1016/j.neuron.2014.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells L.T., Rasch D.K. Emergence “delirium” after sevoflurane anesthesia: a paranoid delusion? Anesth. Analg. 1999;88:1308–1310. doi: 10.1097/00000539-199906000-00020. [DOI] [PubMed] [Google Scholar]
- 45.Blunck R., Batulan Z. Mechanism of electromechanical coupling in voltage-gated potassium channels. Front. Pharmacol. 2012;3:166. doi: 10.3389/fphar.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chowdhury S., Haehnel B.M., Chanda B. Interfacial gating triad is crucial for electromechanical transduction in voltage-activated potassium channels. J. Gen. Physiol. 2014;144:457–467. doi: 10.1085/jgp.201411185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharji A., Kaplan B., Covarrubias M. The concerted contribution of the S4-S5 linker and the S6 segment to the modulation of a Kv channel by 1-alkanols. Mol. Pharmacol. 2006;70:1542–1554. doi: 10.1124/mol.106.026187. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J., Qu X., Germann M.W. Insight into the modulation of Shaw2 Kv channels by general anesthetics: structural and functional studies of S4-S5 linker and S6 C-terminal peptides in micelles by NMR. Biochim. Biophys. Acta. 2013;1828:595–601. doi: 10.1016/j.bbamem.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris T., Graber A.R., Covarrubias M. Allosteric modulation of a neuronal K+ channel by 1-alkanols is linked to a key residue in the activation gate. Am. J. Physiol. Cell Physiol. 2003;285:C788–C796. doi: 10.1152/ajpcell.00113.2003. [DOI] [PubMed] [Google Scholar]
- 50.Herold K.F., Nau C., Hemmings H.C., Jr. Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8. Anesthesiology. 2009;111:591–599. doi: 10.1097/ALN.0b013e3181af64d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horishita T., Eger E.I., 2nd, Harris R.A. The effects of volatile aromatic anesthetics on voltage-gated Na+ channels expressed in Xenopus oocytes. Anesth. Analg. 2008;107:1579–1586. doi: 10.1213/ane.0b013e318184b966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.OuYang W., Hemmings H.C., Jr. Isoform-selective effects of isoflurane on voltage-gated Na+ channels. Anesthesiology. 2007;107:91–98. doi: 10.1097/01.anes.0000268390.28362.4a. [DOI] [PubMed] [Google Scholar]
- 53.Stadnicka A., Kwok W.M., Bosnjak Z.J. Effects of halothane and isoflurane on fast and slow inactivation of human heart hH1a sodium channels. Anesthesiology. 1999;90:1671–1683. doi: 10.1097/00000542-199906000-00024. [DOI] [PubMed] [Google Scholar]
- 54.Duch D.S., Rehberg B., Vysotskaya T.N. Volatile anesthetics significantly suppress central and peripheral mammalian sodium channels. Toxicol. Lett. 1998;100-101:255–263. doi: 10.1016/s0378-4274(98)00193-3. [DOI] [PubMed] [Google Scholar]
- 55.Rehberg B., Xiao Y.H., Duch D.S. Central nervous system sodium channels are significantly suppressed at clinical concentrations of volatile anesthetics. Anesthesiology. 1996;84:1223–1233. doi: 10.1097/00000542-199605000-00025. discussion 27A. [DOI] [PubMed] [Google Scholar]
- 56.Ouyang W., Herold K.F., Hemmings H.C., Jr. Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology. 2009;110:582–590. doi: 10.1097/ALN.0b013e318197941e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouyang W., Jih T.Y., Hemmings H.C., Jr. Isoflurane inhibits NaChBac, a prokaryotic voltage-gated sodium channel. J. Pharmacol. Exp. Ther. 2007;322:1076–1083. doi: 10.1124/jpet.107.122929. [DOI] [PubMed] [Google Scholar]
- 58.Charalambous K., Wallace B.A. NaChBac: the long lost sodium channel ancestor. Biochemistry. 2011;50:6742–6752. doi: 10.1021/bi200942y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuzmenkin A., Bezanilla F., Correa A.M. Gating of the bacterial sodium channel, NaChBac: voltage-dependent charge movement and gating currents. J. Gen. Physiol. 2004;124:349–356. doi: 10.1085/jgp.200409139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldschen-Ohm M.P., Chanda B. Probing gating mechanisms of sodium channels using pore blockers. Handbook Exp. Pharmacol. 2014;221:183–201. doi: 10.1007/978-3-642-41588-3_9. [DOI] [PubMed] [Google Scholar]
- 61.Stock L., Souza C., Treptow W. Structural basis for activation of voltage-gated cation channels. Biochemistry. 2013;52:1501–1513. doi: 10.1021/bi3013017. [DOI] [PubMed] [Google Scholar]
- 62.Payandeh J., Scheuer T., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bagnéris C., DeCaen P.G., Wallace B.A. Prokaryotic NavMs channel as a structural and functional model for eukaryotic sodium channel antagonism. Proc. Natl. Acad. Sci. USA. 2014;111:8428–8433. doi: 10.1073/pnas.1406855111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Payandeh J., Gamal El-Din T.M., Catterall W.A. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–139. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richardson J., Blunck R., Correa A.M. Distance measurements reveal a common topology of prokaryotic voltage-gated ion channels in the lipid bilayer. Proc. Natl. Acad. Sci. USA. 2006;103:15865–15870. doi: 10.1073/pnas.0607532103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raju S.G., Barber A.F., Carnevale V. Exploring volatile general anesthetic binding to a closed membrane-bound bacterial voltage-gated sodium channel via computation. PLOS Comput. Biol. 2013;9:e1003090. doi: 10.1371/journal.pcbi.1003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ostmeyer J., Chakrapani S., Roux B. Recovery from slow inactivation in K+ channels is controlled by water molecules. Nature. 2013;501:121–124. doi: 10.1038/nature12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tronin A.Y., Nordgren C.E., Blasie J.K. Direct evidence of conformational changes associated with voltage gating in a voltage sensor protein by time-resolved X-ray/neutron interferometry. Langmuir. 2014;30:4784–4796. doi: 10.1021/la500560w. [DOI] [PMC free article] [PubMed] [Google Scholar]