Abstract
INTRODUCTION
Diabetes mellitus (DM) is the most common cause of amputations in Malaysia. This study aimed to identify the predictive factors for major lower limb amputation among patients with type 2 DM (T2DM) who were admitted to a hospital, in order to reduce its likelihood.
METHODS
This cross-sectional study involved 218 patients with T2DM who were admitted to Hospital Tengku Ampuan Afzan, Kuantan, Malaysia, for diabetic foot problems from June 2011 to July 2012. A form was developed to document the patients’ profiles, comorbidities, complications, investigations, treatment and clinical outcomes. The predictors for major lower limb amputations were determined using univariate and stepwise logistic regression analysis.
RESULTS
A total of 31 patients underwent major lower limb amputations (25 transtibial, 6 transfemoral). The following factors were found to be associated with the incidence of major lower limb amputations: T2DM duration ≥ 10 years, diabetic neuropathy, diabetic nephropathy, presentation with gangrene, diabetic foot conditions of Wagner grade 4 or 5, and necrotising fasciitis. Patients who underwent major amputations had significantly lower haemoglobin and albumin levels, and higher total white blood cell counts, erythrocyte sedimentation rates, and C-reactive protein, urea and creatinine levels. However, only T2DM duration ≥ 10 years, positive bacterial culture and albumin levels were significant on stepwise logistic regression analysis.
CONCLUSION
T2DM duration ≥ 10 years, positive bacterial culture and low albumin levels were found to be significant predictive factors for major lower limb amputation among patients with T2DM admitted for diabetic foot problems.
Keywords: amputation, diabetic foot, diabetes mellitus, lower limb, type II
INTRODUCTION
According to Malaysia’s third National Health and Morbidity Survey conducted in 2006, an estimated 3.4 million Malaysians would have diabetes mellitus (DM) in 2010.(1) Results of the two latest National Health and Morbidity Surveys showed a dramatic increase in the prevalence of DM, from 8.3% in 1996 to 14.9% in 2006 for Malaysian adults aged 30 years and above (an increase of 80% over a period of 10 years). An estimated one-third (36%) of the diabetic population remained undiagnosed. The same surveys showed that the most common diabetic complication was lower limb amputations (4.3%), followed by strokes (3.4%) and renal failure requiring dialysis or kidney transplants (1.6%).(1)
In a survey conducted at a specialist primary care clinic in Malaysia, the prevalence of diabetic peripheral neuropathy was reported to be about 50%.(2) It has also been reported that around 75% of the amputations performed in Malaysia were related to DM.(3,4) Although about 80% of amputees had their amputated limb replaced with a prosthetic one, prostheses are still perceived to be suboptimal options.(4) Patients with diabetic foot problems were also found to have a poorer health-related quality of life, especially in terms of physicality.(5) Given the abundant problems related to amputations and prostheses, it would be beneficial to reduce the amputation rate due to DM. The present study sought to determine predictive factors for major lower limb amputations in patients admitted for diabetic foot problems, in order to help reduce the amputation rate in Malaysia.
METHODS
This was a cross-sectional study involving 218 patients who were admitted to Hospital Tengku Ampuan Afzan, Kuantan, Malaysia, for diabetic foot problems from June 2011 to July 2012. Only patients with type 2 DM (T2DM) were included in the study. A case record form was developed for the documentation of data prior to the commencement of this study. The following predictive factors were analysed: age, gender, marital status, duration of T2DM, smoking habits, alcohol consumption, hypertension, ischaemic heart disease, ankle-brachial systolic index, neuropathy, peripheral vascular disease, hyperlipidaemia, glycated haemoglobin level, renal profile, total white blood cell count, C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR).
Neuropathy was examined according to three components, namely sensory, motor and autonomic neuropathy. The patient’s feet were examined and the colour, temperature, presence of callosities and/or deformities, brittleness of toe nails, loss of hair and dryness of the skin surrounding the foot and ankle were noted. Sensory neuropathy was assessed using the 10-g Semmes-Weinstein monofilament. The vibratory sensation of the patient’s feet was examined using a 128-Hz tuning fork that was applied to the medial malleolus. Peripheral vascular assessment was done using a handheld Doppler ultrasound; an ankle-brachial systolic index of less than 0.8 was used to indicate the presence of peripheral vascular disease.
Other information that was documented included the type of diabetic foot problems (i.e. abscess, osteomyelitis, gangrene, ulcers, cellulitis, necrotising fasciitis and/or charcot osteoarthropathy). Foot conditions were graded according to the Wagner classification (grade 0: high-risk foot; grade 1: superficial ulcer; grade 2: deep ulcer, penetrating tendon, bone or joint; grade 3: deep ulcer with abscess or osteomyelitis; grade 4: localised toe, forefoot or heel gangrene; grade 5: hindfoot gangrene). Patients who underwent below- or above-knee (i.e. transtibial or transfemoral, respectively) amputations were considered to have had a major lower limb amputation. Minor lower limb amputation was defined as an amputation distal to the ankle joint. The decision to amputate was made by the orthopaedic surgeons.
The best cutoff point for the duration of T2DM was determined using a receiver operating characteristic (ROC) curve. A duration of ten years and above was chosen as it yielded about 80% sensitivity and specificity for having a major lower limb amputation. Demographics as well as clinical and laboratory variables of the minor and major amputation groups were compared. Unless stated otherwise, numerical data was described using mean ± standard deviation, while categorical data was described using percentages. The statistical differences of the variables for minor and major amputations were tested using independent sample t-tests for numerical variables and chi-square tests for categorical variables. Fisher’s exact test was used where applicable. Important variables were modelled with logistic regression to determine significant predictors. The model’s fit was determined using the Hosmer-Lemeshow test. A p-value < 0.05 indicated statistical significance. Statistical analysis was performed using SPSS version 12.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
Of the 218 patients included in the present study, 147 were male and 71 were female. Their mean age was 60.97 (range 36–98) years. The majority of the patients were Malay (63.3%); the rest were Indian (23.9%) or Chinese (12.8%). Slightly more than half of the patients (n = 144, 66.1%) lived in suburban areas. Most of the patients (n = 197, 90.4%) had an income of less than MYR 2,000 per month and the majority (n = 200, 91.7%) had fairly good family support, with only 18 (8.3%) living alone. A large number of the patients (n = 189, 86.7%) had secondary school education; only 29 (13.3%) had obtained tertiary education. About half (n = 131, 60.1%) of the patients had hyperlipidaemia and 183 (83.9%) had hypertension; 79 (36.2%) patients were obese and 86 (39.4%) were smokers.
Nearly 60% of the patients (n = 130) were on oral hypoglycaemic agents and 88 (40.4%) patients were on insulin therapy. In terms of follow-up location, 143 (65.6%) patients were followed up at government health clinics, 57 (26.1%) at tertiary government hospitals and 18 (8.3%) at general practitioner clinics. Retinopathy was observed in 62 (28.4%) patients, neuropathy in 94 (43.1%) patients, nephropathy in 65 (29.8%) patients, and peripheral vascular disease in 80 (36.7%) patients. 15 (6.9%) patients had a history of cerebrovascular accidents and 55 (25.2%) had underlying coronary heart disease. The most common presentation was abscesses (n = 68, 31.2%), followed by osteomyelitis (n = 56, 25.7%), cellulitis (n = 45, 20.6%), gangrene (n = 30, 13.8%), necrotising fasciitis (n = 19, 8.7%), charcot osteoarthropathy (n = 2, 0.9%) and ulcers (n = 1, 0.5%). When the foot conditions were categorised according to the Wagner classification (n = 203), 27 (13.3%) were grade 1, 19 (9.4%) were grade 2, 114 (56.2%) were grade 3, 19 (9.4%) were grade 4 and 24 (11.8%) were grade 5. Among the 218 patients, 31 (14.2%) underwent major lower limb amputation – 6 (2.8%) were transfemoral amputations, while 25 (11.5%) were transtibial amputations.
We found that age, gender, ethnicity, monthly income, dependency on family, smoking status, obesity, hyperlipidaemia, hypertension and history of ischaemic heart disease were not associated with major lower limb amputation (Table I). The factors associated with major lower limb amputations included T2DM duration of ≥ 10 years, higher or tertiary education level, diabetic neuropathy, presentation with gangrene, diabetic foot condition of Wagner grade 4 or 5, and necrotising fasciitis (Table II). Coronary heart disease, abscesses, osteomyelitis, ulcers and cellulitis were not significant factors for major lower limb amputation. Gangrene was a significant factor because 12 (40%) patients with gangrene underwent major lower limb amputation compared to 19 (10.1%) patients without gangrene.
Table I.
Characteristics of the patients with type 2 diabetes mellitus (n = 218), according to whether they underwent major or minor lower limb amputation.
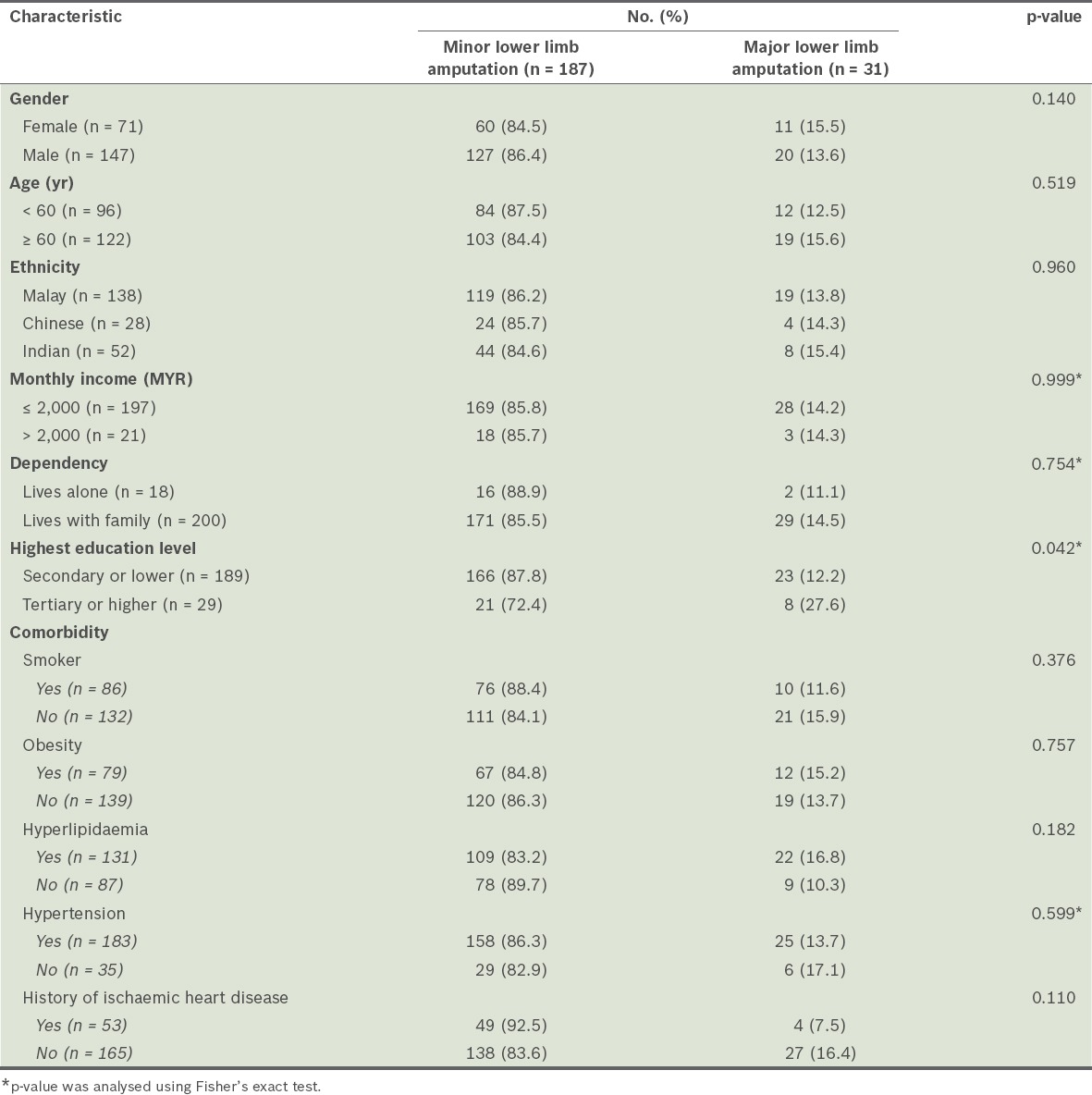
Table II.
Characteristics of type 2 diabetes mellitus (T2DM) in the patients (n = 218), according to whether they underwent major or minor lower limb amputation.
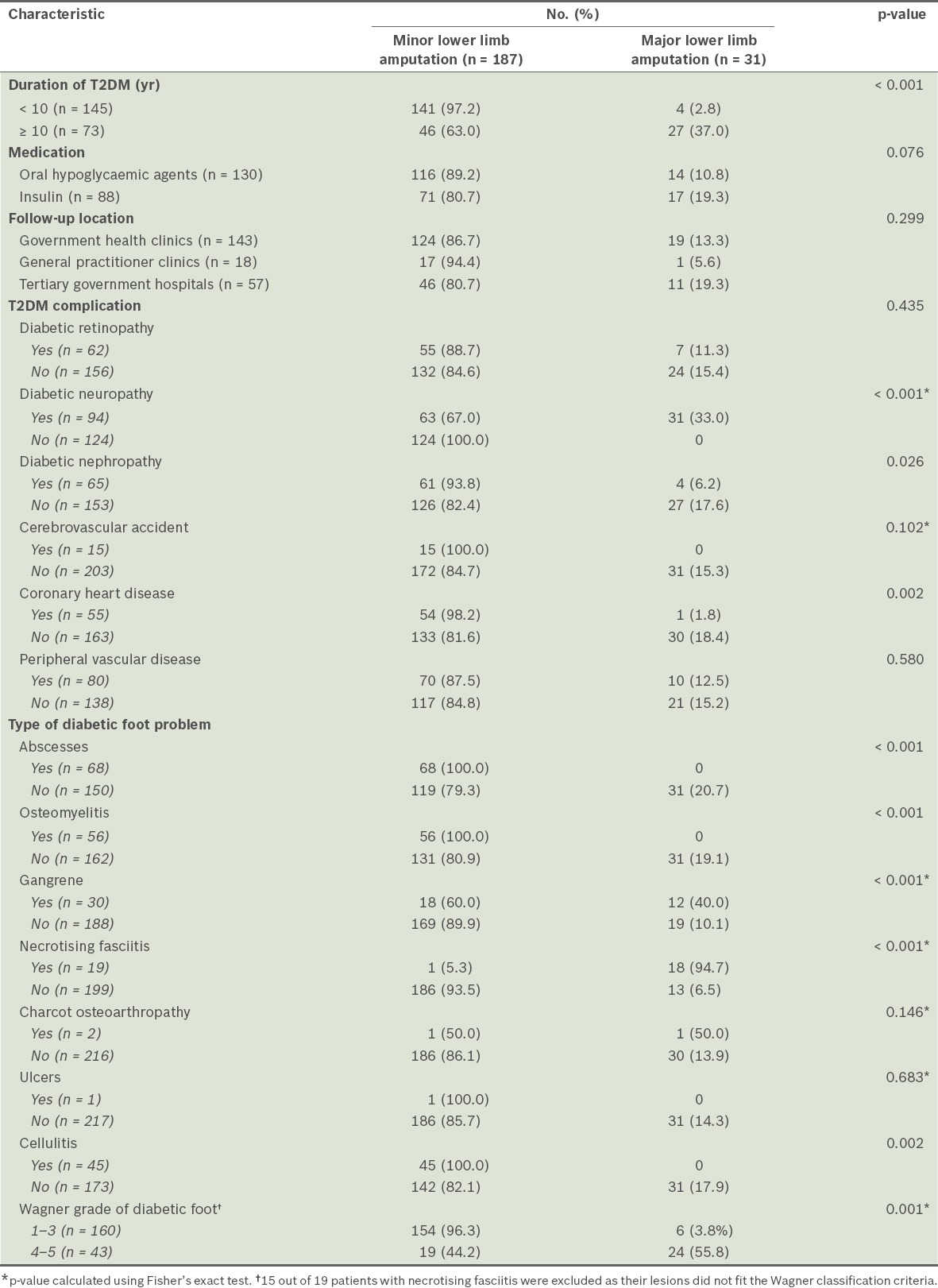
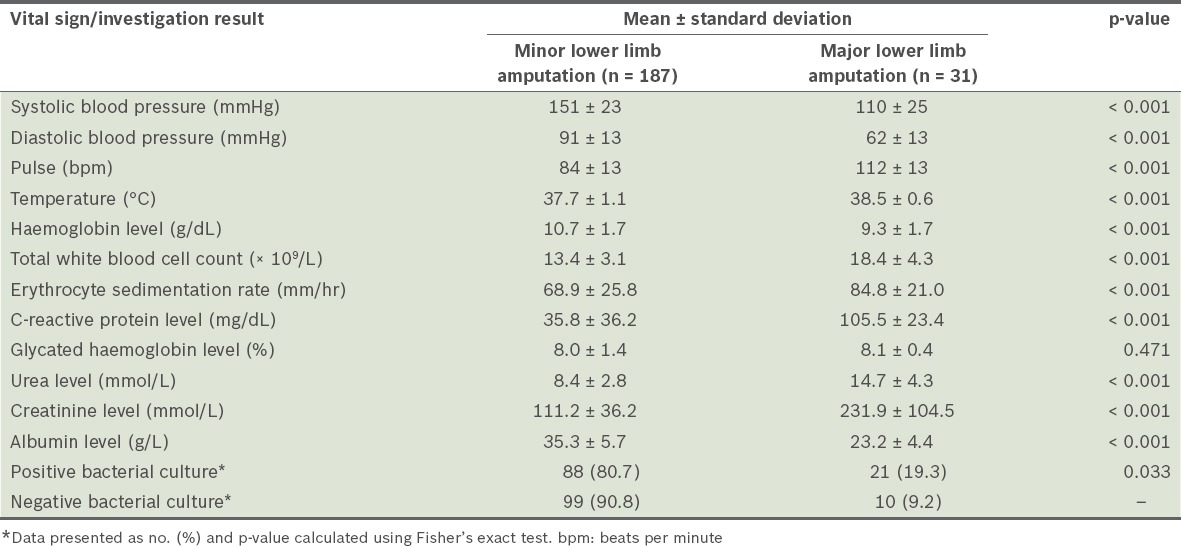
Compared to those who underwent minor lower limb amputations, the patients who underwent major lower limb amputations were found to have significantly lower haemoglobin and albumin levels, higher white blood cell count, ESR, and CRP, urea and creatinine levels. A significantly higher number of patients who underwent major amputations also had a positive bacterial culture result, compared to those who underwent minor amputations (Table III).
Table III.
Vital signs and investigation results of the patients during admission (n = 218).
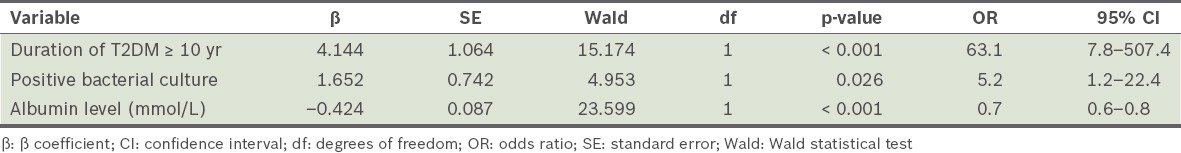
To determine the significant predictors for major lower limb amputation, we included those variables that we considered to be clinically important. The variables were duration of T2DM (< 10 years vs. ≥ 10 years), Wagner classification (grade 1–3 vs. grade 4–5), positive bacterial culture (regardless of type of bacterial specimen cultured) and plasma albumin level. A stepwise logistic regression was performed and the final model consisted of: duration of T2DM, positive bacterial culture and albumin level (Hosmer-Lemeshow test: χ2 = 1.868, degrees of freedom = 8, p = 0.985, 95% correct prediction). Based on a 95% confidence interval level, the independent significant predictive factors for major lower limb amputations among our patients were duration of T2DM of ≥ 10 years, positive bacterial culture and low albumin levels (Table IV).
Table IV.
Results of the stepwise logistic regression analysis.
DISCUSSION
The rate of major lower limb amputations for diabetic foot problems in the present study (14.2%) is lower than the rates reported by other studies. Nather et al reported an amputation rate of 27.2%,(6) while Leung et al reported an amputation rate of 30.3%.(7) An earlier study, conducted by Harwant et al at Hospital Kuala Lumpur, Malaysia, found an amputation rate of 20%.(8) These differences in the rates of major lower limb amputation could be due to poor acceptance of amputations as a means of diabetic foot management among our study population. In a cross-sectional study conducted by Wan Hazmy et al, only 63% of the 30 amputees interviewed retrospectively said they would have agreed to undergo amputation; 37% said that they would have refused amputation even if it was indicated.(4)
Among the 31 patients who had major lower limb amputations in the present study, 19 (61.3%) were Malay, 4 (12.9%) were Chinese and 8 (25.8%) were Indian. This distribution matches the racial distribution of patients admitted to our hospital. According to the third National Health and Morbidity Survey,(1) the prevalence of DM in Malaysia was highest among Indians (19.9%), followed by Malays (11.9%) and Chinese (11.4%). The prevalence of lower limb amputations was similar across the three ethnic groups [Malay (4.3%), Chinese (4.5%) and Indian (4.6%)].(1) Ethnicity was not found to be associated with major lower limb amputations in the present study. This finding is similar to that of previous studies conducted by Nather et al(6) and Aziz et al(9) in Singapore, which also included a multiracial population. However, another study that was also conducted in Singapore found that patients of Malay ethnicity were more likely to have lower extremity amputations as compared to patients of Chinese or Indian ethnicities.(10)
In the present study, a duration of T2DM of ≥ 10 years was found to be a significant independent predictive factor for major lower limb amputation. This finding was also observed by Lehto et al, and Selby and Zhang.(11,12) However, Leung et al observed that the duration of DM was not found to be a significant factor for major lower limb amputation.(7) The other aforementioned studies did not analyse the duration of DM as a possible factor for major lower limb amputation, considering only age as a possible factor. Leung et al noted that the patients who had major lower limb amputations were significantly older, with a mean age of 74.41 years compared to 67.25 years for those who did not have major limb amputations.(7) Nather et al and Aziz et al found that being above 60 years of age was a significant independent predictive factor for major lower limb amputation.(6,9) The study by Yang et al observed that being of an older age group (> 65 years) is a significant factor for lower extremity amputation in diabetic patients with renal disease.(10) In the present study, age was not found to be a significant predictive factor for major lower limb amputation. We also noted that only 101 (46.3%) patients were living with their spouses and 18 (8.3%) patients were living alone. Living alone was not found to be a significant factor for major amputation.
While neuropathy was found to be associated with major lower limb amputation in the present study, it was not a significant independent predictive factor. Nather et al and Leung et al reported similar findings.(6,7) Research conducted by Aziz et al and Gürlek et al did not show that neuropathy was a predictive factor for major lower limb amputation.(9,13) However, other studies by Lehto et al, and Selby and Zhang showed that it was a predictive factor.(11,12) One possible reason neuropathy was not shown to be significant in the multivariate model could be its high association with the duration of DM. It is also possible that neuropathy is dependent on infection and ischaemia.(6,7)
Although necrotising fasciitis was found to be associated with major lower limb amputation, following stepwise logistic regression, only a positive bacterial culture result was found to be a significant independent predictive factor for major lower limb amputation. Abscess, osteomyelitis, ulcers and cellulitis were not significant factors for major lower limb amputation, suggesting that they could be treated with minor limb amputation. Nather et al noted that infections (i.e. osteomyelitis and abscesses) were a significant independent predictive factor for major lower limb amputation,(6) while Aziz et al and Pittet et al found that wet gangrene was a significant independent factor for major lower limb amputation.(9,14) Total white blood cell count, and ESR and CRP levels are known markers of infection. Aziz et al observed that a total white blood cell count of more than 15.0 × 109/L was a predictive factor for major lower limb amputation, while ESR and CRP level were only associated factors.(9) In the present study, total white blood cell count, ESR and CRP levels were dependent factors for major lower limb amputation. While major lower limb amputation was not significantly associated with peripheral vascular disease (ankle-brachial systemic index < 0.8), it was significantly associated with gangrene at presentation and diabetic foot conditions of Wagner grades 4 or 5. Nather et al and Leung et al have observed that gangrene was more important than the ankle-brachial systolic index reading in predicting the need for major lower limb amputation.(6,7)
Albumin level was found to be an independent predictive factor for major lower limb amputation in the present study, similar to the findings of the studies conducted by Leung et al and Akinci et al.(7,15) In the present study, nephropathy and high levels of urea and creatinine were also found to be associated with major lower limb amputation. A low albumin level indicates poor nutritional and renal function. Speckman et al noted that DM and low albumin levels were risk factors for amputation among patients with end-stage renal failure.(16) Low albumin levels were also found to be associated with poor wound healing after Syme amputations, leading to major lower limb amputation in patients with DM.(17)
In conclusion, the present study showed that a duration of T2DM ≥ 10 years, positive bacterial culture and low serum albumin levels were predictors for major lower limb amputation in patients admitted with diabetic foot problems. Hence, we recommend early aggressive treatment for infections in patients with a long history of T2DM who are admitted for diabetic foot problems, without neglecting their clinical management and nutritional support.
REFERENCES
- 1.Letchuman GR, Wan Nazaimoon WM, Wan Mohamad WB, et al. Prevalence of diabetes in the Malaysian National Health Morbidity Survey III 2006. Med J Malaysia. 2010;65:180–6. [PubMed] [Google Scholar]
- 2.Mimi O, Teng CL, Chia YC. The prevalence of diabetic peripheral neuropathy in an outpatient setting. Med J Malaysia. 2003;58:533–8. [PubMed] [Google Scholar]
- 3.Yusof MI, Sulaiman AR, Muslim DA. Diabetic foot complications: a two-year review of limb amputation in a Kelantanese population. Singapore Med J. 2007;48:729–32. [PubMed] [Google Scholar]
- 4.Wan Hazmy CH, Chia WY, Fong TS, Ganendra P. Functional outcome after major lower extremity amputation: a survey on lower extremity amputees. Med J Malaysia. 2006;61(Suppl A):3–9. [PubMed] [Google Scholar]
- 5.Mazlina M, Shamsul AS, Jeffery FA. Health-related quality of life in patients with diabetic foot problems in Malaysia. Med J Malaysia. 2011;66:234–8. [PubMed] [Google Scholar]
- 6.Nather A, Bee CS, Huak CY, et al. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications. 2008;22:77–82. doi: 10.1016/j.jdiacomp.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Leung HB, Ho YC, Carnett J, Lam PK, Wong WC. Diabetic foot ulcers in the Hong Kong Chinese population: retrospective study. Hong Kong Med J. 2001;7:350–5. [PubMed] [Google Scholar]
- 8.Harwant S, Doshi HK, Moissinac K, Abdullah BT. Factors related to adverse outcome in inpatients with diabetic foot. Med J Malaysia. 2000;55:236–41. [PubMed] [Google Scholar]
- 9.Aziz Z, Lin WK, Nather A, Huak CY. Predictive factors for lower extremity amputations in diabetic foot infections. Diabet Foot Ankle. 2011:2. doi: 10.3402/dfa.v2i0.7463. [Epub 2011 Sep 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Østbye T, Tan SB, et al. Risk factors for lower extremity amputation among patients with diabetes in Singapore. J Diabetes Complications. 2011;25:382–6. doi: 10.1016/j.jdiacomp.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Risk factors predicting lower extremity amputations in patients with NIDDM. Diabetes Care. 1996;19:607–12. doi: 10.2337/diacare.19.6.607. [DOI] [PubMed] [Google Scholar]
- 12.Selby JV, Zhang D. Risk factors for lower extremity amputation in persons with diabetes. Diabetes Care. 1995;18:509–16. doi: 10.2337/diacare.18.4.509. [DOI] [PubMed] [Google Scholar]
- 13.Gürlek A, Bayraktar M, Savaş C, Gedik O. Amputation rate in 147 Turkish patients with diabetic foot: the Hacettepe University Hospital experience. Exp Clin Endocrinol Diabetes. 1998;106:404–9. doi: 10.1055/s-0029-1212006. [DOI] [PubMed] [Google Scholar]
- 14.Pittet D, Wyssa B, Herter-Clavel C, et al. Outcome of diabetic foot infections treated conservatively: a retrospective cohort study with long-term follow-up. Arch Intern Med. 1999;159:851–6. doi: 10.1001/archinte.159.8.851. [DOI] [PubMed] [Google Scholar]
- 15.Akinci B, Yener S, Yesil S, et al. Acute phase reactants predict the risk of amputation in diabetic foot infection. J Am Podiatr Med Assoc. 2011;101:1–6. doi: 10.7547/1010001. [DOI] [PubMed] [Google Scholar]
- 16.Speckman RA, Frankenfield DL, Roman SH, et al. Diabetes is the strongest risk factor for lower-extremity amputation in the new hemodialysis patients. Diabetes Care. 2004;27:2198–203. doi: 10.2337/diacare.27.9.2198. [DOI] [PubMed] [Google Scholar]
- 17.Pinzur MS, Stuck RM, Sage R, Hunt N, Rabinovich Z. Syme ankle disarticulation in patients with diabetes. J Bone Joint Surg Am. 2003;85:1667–72. doi: 10.2106/00004623-200309000-00003. [DOI] [PubMed] [Google Scholar]


