Abstract
Secretory meningioma, a histologic subtype of meningioma of World Health Organization grade 1, is clinically significant because it is frequently accompanied by peritumoral brain edema. The patient was a 53-year-old woman suffering from dysarthria and motor weakness of the right arm. Enhanced magnetic resonance images showed an enhancing mass measuring 2.5 cm in size located in the right parietal convexity. Intraoperative squash cytology showed moderately cellular smears composed mainly of clusters of ovoid cells with scattered whorl formations. The cells had round nuclei and a moderate amount of eosinophilic cytoplasm with ill-defined cell borders. Neither atypia nor mitosis was observed. Some scattered round shaped eosinophilic refractile hyaline globules, measuring from 5 to 25 µm, were observed, and a periglobular halo was occasionally observed. The diagnosis of secretory meningioma should be made as early as possible so that neurosurgeons can prevent postoperative aggravation of peritumoral edema. We emphasize that cytologic findings including eosinophilic, non-fibrillary cytoplasm with eosinophilic refractile hyaline globules are helpful in differentiating secretory meningioma from other subtypes of meningioma, primary and metastatic brain tumors.
Keywords: Meningioma, secretory; Cytology; Brain
INTRODUCTION
Secretory meningioma is an unusual type of meningioma, categorized as grade I according to recent World Health Organization (WHO) classification [1]. However, secretory meningiomas show a high rate of unproportionate peritumoral edema, compared to their tumor size, in 41-64% of cases; thus, it has clinical significance [2,3]. Exact pathophysiologic mechanism of development of peritumoral edema remains unclarified. However, several hypotheses have been proposed, including tumor volume, progesterone hormones, or production of vascular endothelial growth factor and interleukin-6 expression, as well as possible involvement of mast cell-derived histamine or serotonin [4]. As in microcystic menin-gioma, another subtype of meningioma showing peritumoral edema, change of hemodynamics inducing hypervascularity or the tumor occluding large veins or dural sinuses evoking venous stasis with subsequent extravasated fluid into the interstitial area of the brain may be related to peritumoral brain edema [5]. Due to the potential for increasing peritumoral edema-related mass effect-related neurological symptoms or even loss of consciousness, early diagnosis of secretory meningioma enables intensive postoperative management. However, few intraoperative cytologic reports of secretory meningioma have been described [6,7].
We recently experienced a case of secretory meningioma which showed typical intraoperative crush cytologic smears leading to a correct diagnosis. We emphasize the immense diagnostic utility of intraoperative cytologic findings of this uncommonly encountered secretory meningioma along with literature review.
CASE REPORT
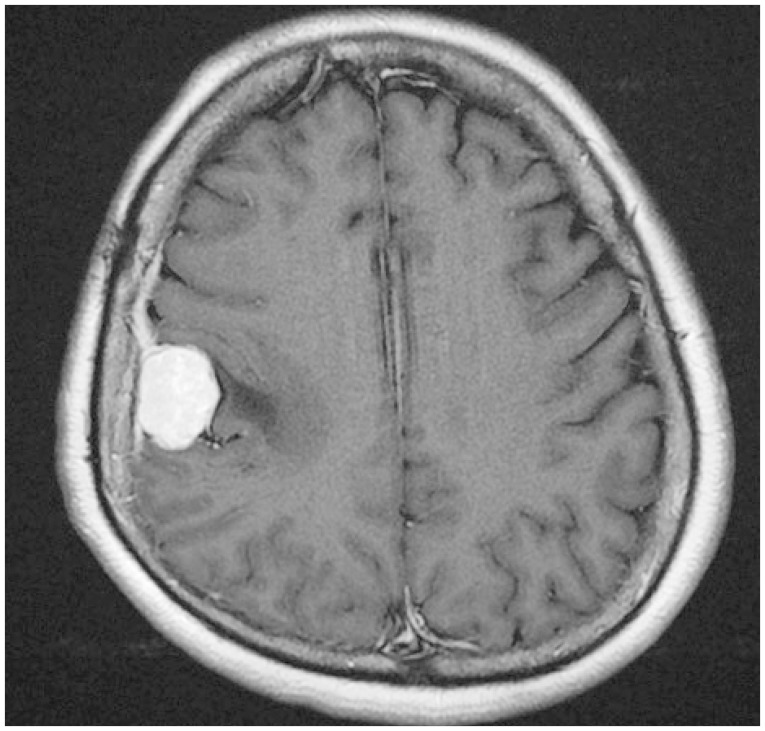
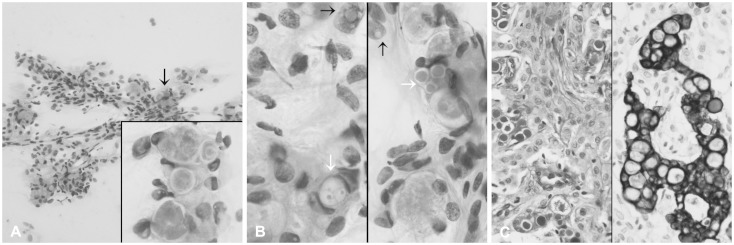
A 53-year-old Korean woman presented with a three-month history of dysarthria and motor weakness of the right arm. Enhanced brain MR images showed a mass measuring approximately 2.5 cm in size located in the right parietal convexity with strong enhancement (Fig. 1). Gross total resection of the mass and intraoperative crush cytology were performed. The squash smears were moderately cellular; tightly cohesive sheets or clusters of round to ovoid cells had ill-defined cell borders with thick collagenous eosinophilic cytoplasm (Fig. 2A). The tumor cells had occasional intranuclear inclusions or nuclear grooves and psammomatous calcification, with the tight clusters occasionally forming whorls. Numerous round shaped eosinophilic hyaline globules were found mainly in the intracytoplasmic area as well as extracellularly scattered within the clusters. The size ranged from approximately 5 to 25 µm (Fig. 2B). No high grade parameters such as mitoses, small-cell changes, or necrosis were found. Examination of the resected specimen showed that the mass was composed of meningothelial whorls of bland-looking round to ovoid tumor cells with intervening collagen deposits and some psammoma bodies. Eosinophilic granular globules were loosely scattered or collected in groups within the intracytoplasmic spaces as well as scattered within the extracellular area. The morphology and size of eosinophilic globules varied; small dot-like globules surrounded by a clear halo to homogeneous and brightly eosinophilic large spherical forms, ranging from 5 to 25 µm in diameter. They showed strong staining with periodic acid-Schiff (PAS) (Fig. 2C, left), and diastase-resistant. Secretory meningioma was diagnosed. Immunohistochemically, the tumor cells were positive for monoclonal carcinoembryonic antigen (CEA, II-7; prediluted, Dako, Glostrup, Denmark), pancytokeratin (AE1/AE3; 1:50 dilution, Dako) (Fig. 2C, right), epithelial membrane antigen (E29; prediluted, Dako), and vimentin (V9; prediluted, Dako), and negative for matrix metalloproteinase-2 (MMP-2; 1:250 dilution, Fuji Chemical Industries, Toyama, Japan) or MMP-9 (1:250 dilution, Fuji Chemical Industries). Ki-67 (MIB-1; 1:100 dilution, Dako) proliferation index was 2%.
Fig. 1. T1-weighted MR image shows an enhancing mass located in the right parietal convexity with peritumoral edema.
Fig. 2. Intraoperative crush and squash cytology of the resected mass. A: Cellular smear shows sheets of cohesive polygonal tumor cells forming occasional whorls (hematoxylin and eosin stain, ×200). Arrow and inset indicate eosinophilic globules (hematoxylin and eosin stain, ×1,000). B: Intracytoplasmic inclusions of variable size (white arrows) have a centrally located eosinophilic homogeneous core and a well-defined, thin, clear rim. Note frequent nuclear pseudoinclusions (black arrows, hematoxylin and eosin stain, ×1,000). C: Left: a resected mass is composed of large lobules of syncytially arranged ovoid shaped cells with periodic acid-Schiff-positive eosinophilic granular bodies in aggregated or singly scattered form. Note a periglobular thin halo-like rim (periodic acid-Schiff stain, ×400). Right: cells containing cytoplasmic secretory inclusions are occasionally positive for pancytokeratin (pancytokeratin immunostain, ×400).
Electron microscopic examination was performed using a deparaffinized tissue block; deparaffinized, rinsed in 0.1 phosphate buffer, post fixed in solutions of 2% glutaraldehyde and 1% osmium tetroxide in the same buffer, dehydrated in graded alcohols and embedded in Epon 812 (Oken Shoji Ltd., Tokyo, Japan). The thick sections (1 µm) were stained with toluidine blue and Azure B solution to confirm that the block contain-ed the desired cells. Thin sections were post-stained with uranyl acetate and lead citrate and examined using a transmission scanning electron microscope (H-7100, Hitachi High-Technologies, Tokyo, Japan) at an accelerating voltage of 75 kv. Ultrastructurally, scattered ovoid to spindle shaped tumor cells had round nuclei with occasional indentation and cytoplasmic invagination. The tumor cells contained a moderate amount of cytoplasm filled with intermediate filaments and well-formed desmosomes.
Postoperative serum titer for CEA was 1.12 ng/mL (reference level: 0-5 ng/mL). Preoperative titer of CEA was not measured. The patient showed no recurrence during the 12-month follow-up period.
Institutional Review Board approval was obtained for this case report.
DISCUSSION
The diagnosis of secretory meningioma should be made as early as possible so that neurosurgeons can prevent postoperative aggravation of peritumoral edema [3]. In particular, for neurosurgeons encountering preoperative images of disproportion between peritumoral edema and tumor size, postoperative immediate management including prompt intraoperative frozen diagnosis of this subtype is required.
Cohesive syncytial cell clusters with poorly-defined cell borders are the most characteristic and most commonly encountered cytologic features of meningiomas [1]. Observation of cytologic features such as prominent macronucleoli, brisk mitoses (>4/10 high power field), high N/C ratio, spontaneous necrosis, or sheet-like growth under crush cytology indicates suspicion of atypical or anaplastic meningioma [1]. The vesicular nuclei and eosinophilic globules, i.e., so called pseudopsammoma bodies, are salient cytologic details of secretory meningioma (WHO grade 1) [6]. However, the cytologic features of meningiomas may not reflect the histologic subtype, except for psammomatous type filled with variant, which can be easily recognized in touch preps/imprints; therefore, cytologic description of variable histologic subtypes of meningioma is limited [8]. According to the previous cytologic review by Hinton et al. [6] and Kim et al. [7], intraoperative crush smears show clusters of cohesive epithelioid cells containing variable numbers of inclusions. These hyalinized or granular globular inclusions range from 3 to 40 µm in size [9]. Differentiation from other tumors mimicking secretory globules is important. Russell bodies seen in lymphoplasmacyte-rich meningioma or chordoid meningioma should be distinguished from eosinophilic globules seen in secretory meningioma [10]. Russell bodies also have eosinophilic, large, homogeneous refractile immunoglobulin-containing inclusions found in plasmacytic cells resulting from excessive synthesis of immunoglobulin; these Russell bodies correspond to the distended endoplasmic reticulums [11]. The average diameter of Russell bodies is 4-5 µm, smaller than that of pseudopsammoma bodies seen in secretory meningioma. The distinguishing points are small dot-like eosinophilic globules surrounded by a clear halo, which are not found in Russell bodies, and tendencies of aggregates of several eosinophilic cytoplasmic globules are commonly found in Russell bodies. Pseudopsammoma bodies are secretory materials located in intracellular lumina lined by microvilli or scattered secretory materials outside within the extracellular spaces [12]. Lymphoplasmacytic infiltrates may also be observed with secretory meningioma, but uncommonly show a mucinous background, which is a characteristic cytologic finding of chordoid meningioma [10]. Histologically, these pseudopsammoma bodies show staining with both PAS and epithelial and secretory markers such as pancytokeratin, CEA, IgA, IgG, or IgM [9]. Eosinophilic granular bodies may be seen in gliomas such as pilocytic astrocytoma. However, pilocytic astrocytoma is composed of spindle cells having eosinophilic fibrillary cytoplasmic processes which can be distinguished from the thick non-fibrillary collagenous cell processes in secretory meningioma [13]. The eosinophilic granular bodies of pilocytic astrocytoma usually appear as singly scattered, and rarely aggregated. Granules in pilocytic astrocytoma tend to be coarse and fragile in nature [14], and a thin periglobular halo frequently found in secretory meningioma has been rarely found in eosinophilic granular bodies of pilocytic astrocytoma [7]. Other diseases that should be distinguished from secretory meningioma include inclusions or cytoplasmic vacuolization seen in metastatic brain tumor from breast ductal carcinoma, hepatocellular carcinoma, or gastric adenocarcinoma [15,16]. Although uncommon, meningioma metastasis from extracranial adenocarcinoma having a secretory component may show eosinophilic globule-like materials [14,17].
In conclusion, intraoperative cytology has diagnostic utility in central nervous system tumors. Cytologic findings such as granular to non-fibrillary cytoplasm and eosinophilic hyaline globules as well as periglobular halo and tendency for scattering are diagnostically helpful in differentiating secretory meningioma from other subtypes of meningioma or intracranial mimicking entities.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Meningiomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. Pathology and Genetics, World Health Organization Classification of Tumours of the Central Nervous System. 4th ed. Lyon: IARC press; 2007. pp. 164–172. [Google Scholar]
- 2.Osawa T, Tosaka M, Nagaishi M, Yoshimoto Y. Factors affecting peritumoral brain edema in meningioma: special histological subtypes with prominently extensive edema. J Neurooncol. 2013;111:49–57. doi: 10.1007/s11060-012-0989-y. [DOI] [PubMed] [Google Scholar]
- 3.Regelsberger J, Hagel C, Emami P, Ries T, Heese O, Westphal M. Secretory meningiomas: a benign subgroup causing life-threatening complications. Neuro Oncol. 2009;11:819–824. doi: 10.1215/15228517-2008-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitzer M, Nägele T, Geist-Barth B, et al. Role of hydrodynamic processes in the pathogenesis of peritumoral brain edema in meningiomas. J Neurosurg. 2000;93:594–604. doi: 10.3171/jns.2000.93.4.0594. [DOI] [PubMed] [Google Scholar]
- 5.Seok JY, Kim NR, Cho HY, Chung DH, Yee GT, Kim EY. Crush cytology of microcystic meningioma with extensive sclerosis. Korean J Pathol. 2014;48:77–80. doi: 10.4132/KoreanJPathol.2014.48.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinton DR, Kovacs K, Chandrasoma PT. Cytologic features of secretory meningioma. Acta Cytol. 1999;43:121–125. doi: 10.1159/000330964. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Lee KG, Kim TS. Cytologic features of secretory meningioma in squash preparation: a case report. Korean J Cytopathol. 2004;15:52–55. [Google Scholar]
- 8.Siddiqui MT, Mahon BM, Cochran E, Gattuso P. Cytologic features of meningiomas on crush preparations: a review. Diagn Cytopathol. 2008;36:202–206. doi: 10.1002/dc.20780. [DOI] [PubMed] [Google Scholar]
- 9.Taraszewska A, Matyja E. Secretory meningiomas: immunohistochemical pattern of lectin and ultrastructure of pseudopsammoma bodies. Folia Neuropathol. 2014;52:141–150. doi: 10.5114/fn.2014.43785. [DOI] [PubMed] [Google Scholar]
- 10.Takei H, Bhattacharjee MB, Adesina AM. Chordoid glioma of the third ventricle: report of a case with cytologic features and utility during intraoperative consultation. Acta Cytol. 2006;50:691–696. doi: 10.1159/000326044. [DOI] [PubMed] [Google Scholar]
- 11.Ribourtout B, Zandecki M. Plasma cell morphology in multiple myeloma and related disorders. Morphologie. 2015;99:38–62. doi: 10.1016/j.morpho.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Jaskólski D, Papierz T, Liberski PP, Sikorska B. Ultrastructure of meningiomas: autophagy is involved in the pathogenesis of "intranuclear vacuoles". Folia Neuropathol. 2012;50:187–193. [PubMed] [Google Scholar]
- 13.Browne TJ, Goumnerova LC, De Girolami U, Cibas ES. Cytologic features of pilocytic astrocytoma in cerebrospinal fluid specimens. Acta Cytol. 2004;48:3–8. doi: 10.1159/000326275. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Kim TS. Squash smear findings of eosinophilic granular bodies in pilocytic astrocytoma. Acta Cytol. 2005;49:112–114. [PubMed] [Google Scholar]
- 15.Monabati A, Kumar PV, Kamkarpour A. Intraoperative cytodiagnosis of metastatic brain tumors confused clinically with brain abscess. A report of three cases. Acta Cytol. 2000;44:437–441. doi: 10.1159/000328494. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt HP. Metastases of malignant neoplasms to intracranial tumours: the "tumour-in-a-tumour" phenomenon. Virchows Arch A Pathol Anat Histopathol. 1984;405:155–160. doi: 10.1007/BF00694933. [DOI] [PubMed] [Google Scholar]
- 17.Hockley AD. Metastatic carcinoma in a spinal meningioma. J Neurol Neurosurg Psychiatry. 1975;38:695–697. doi: 10.1136/jnnp.38.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]