Abstract
Background: Dietary supplement use is widespread in the United States. Although it has been suggested in both in vitro and small in vivo human studies that chromium has potentially beneficial effects in type 2 diabetes (T2D), chromium supplementation in diabetes has not been investigated at the population level.
Objective: The objective of this study was to examine the use and potential benefits of chromium supplementation in T2D by examining NHANES data.
Methods: An individual was defined as having diabetes if he or she had a glycated hemoglobin (HbA1c) value of ≥6.5%, or reported having been diagnosed with diabetes. Data on all consumed dietary supplements from the NHANES database were analyzed, with the OR of having diabetes as the main outcome of interest based on chromium supplement use.
Results: The NHANES for the years 1999–2010 included information on 62,160 individuals. After filtering the database for the required covariates (gender, ethnicity, socioeconomic status, body mass index, diabetes diagnosis, supplement usage, and laboratory HbA1c values), and when restricted to adults, the study cohort included 28,539 people. A total of 58.3% of people reported consuming a dietary supplement in the previous 30 d, 28.8% reported consuming a dietary supplement that contained chromium, and 0.7% consumed supplements that had “chromium” in the title. Compared with nonusers, the odds of having T2D (HbA1c ≥6.5%) were lower in persons who consumed chromium-containing supplements within the previous 30 d than in those who did not (OR: 0.73; 95% CI: 0.62, 0.86; P = 0.001). Supplement use alone (without chromium) did not influence the odds of having T2D (OR: 0.89; 95% CI: 0.77, 1.03; P = 0.11).
Conclusions: Over one-half the adult US population consumes nutritional supplements, and over one-quarter consumes supplemental chromium. The odds of having T2D were lower in those who, in the previous 30 d, had consumed supplements containing chromium. Given the magnitude of exposure, studies on safety and efficacy are warranted.
Keywords: chromium, diabetes, glucose intolerance, insulin resistance, dietary supplements, safety, NHANES
Introduction
In 2012, the CDC estimated that 29.1 million Americans had diabetes, accounting for 12.3% of the adult population (1). Modifiable social and behavioral factors, such as adopting different dietary patterns and consuming nutritional supplements aimed at preventing or controlling type 2 diabetes (T2D)7, have been a field of interest to address this public health burden (2). Although there are multiple classes of pharmacologic agents approved for diabetes treatment, many persons use nutritional supplements to help manage their diabetes, as well as a myriad of other conditions. In 2007, Americans spent nearly $34 billion of their personal money on complementary and alternative medicines, including dietary supplements (3). α-Lipoic acid, banaba, bitter lemon, blonde psyllium, cinnamon, dandelion, fenugreek, ginseng, gymnema sylvestre, milk thistle, nopal from the prickly pear, vanadium, and others are among the many dietary supplements with purported benefit in T2D, despite the low level of evidence to support effectiveness or safety (4). Nutritional substances, when taken to excess or for extended periods of time, may have adverse effects.
Many people appear to use chromium supplements for glycemia despite the fact that randomized clinical trials have generally not demonstrated differences in fasting glucose or oral glucose tolerance between chromium picolinate– and placebo-treated groups (5–9), and meta-analysis of published investigations likewise does not support clinical use (10–13). Chromium was previously classified as an essential trace element, important in carbohydrate, lipid, and protein metabolism, because when it is deficient, it might lead to glucose intolerance and insulin resistance (6, 14, 15). Historically, the nutritional importance of chromium was initially described in 1957 when rodents provided a diet lacking chromium became unable to adequately metabolize glucose, but when chromium was supplemented in the form of brewer’s yeast, glucose metabolism was restored. The chromium-dependent molecule responsible for the restoration of glucose metabolism was initially named glucose tolerance factor (16), although, if existent, identification of glucose tolerance factor and mode of action remains elusive (17). The essentiality of chromium for humans was subsequently described in a patient who received total parenteral nutrition and developed glucose intolerance, weight loss, and peripheral neuropathy, a condition that was unresponsive to insulin, but that was resolved with the administration of chromium (18). Additional reports of patients receiving total parenteral nutrition who developed T2D, which was resolved with chromium replacement, supported the essential status (19, 20). However, the essential function of chromium remains incompletely substantiated and the European Food Safety Authority expert scientific panel and others currently do not support continued essential classification (17, 21).
There are no large or long-term studies, to our knowledge, evaluating the efficacy or safety of chromium in treating or preventing T2D in humans. To this end, we examined the NHANES database, created and administered by the CDC (22, 23), to describe the proportion of Americans who are consuming dietary supplements containing chromium, and how this affects T2D prevalence.
Methods
To investigate the relation between dietary chromium supplement use and T2D, we examined the publicly available NHANES database for the years 1999 through 2010 (23). The NHANES database contains extensive individual-level information on demographic and socioeconomic information, family history, dietary habits, chronic diseases, infectious diseases, mental health, biochemical laboratory data, and more (22).
Diabetes status was defined in 2 ways. In the first method, T2D was defined on the basis of HbA1c values only (T2D-M1), with an HbA1c value <6.5% (47.5 mmol/mol) coded as “0” or nondiabetic and a value ≥6.5% coded as “1” or diabetic, as recommended by the US Department of Health and Human Services and the NIH (24), because many individuals with T2D may be undiagnosed. In the second method, T2D was defined on the basis of both HbA1c values and doctor diagnosis (T2D-M2). In the T2D-M2 method, if individuals reported that a doctor had ever told them they have diabetes or they had an HbA1c value ≥6.5%, they were defined as having diabetes, whereas to be categorized as nondiabetic, a person needed to have never been diagnosed as diabetic by a doctor and have an HbA1c value <6.5%. With the different coding strategies, there are necessarily a higher proportion of people classified as diabetic in the second method than in the first. We did not run statistical analyses to test whether there were differences between groups (T2D-M1 and T2D-M2) because, by definition, some overlap occurs between groups. Restricted and broader definitions of diabetes were used in sensitivity analysis to increase robustness of results, ensuring general findings were consistent regardless of the disease definitions, and not artifacts of potential misclassification. Summary statistics of demographics and other variables are presented as mean values and 95% CIs or proportions (%) of the sample (n).
To quantify the number of people using dietary chromium supplements on at least a semiregular basis, we collected full dietary supplement information from the NHANES database, and extracted information from all dietary supplements that contained chromium (NHANES supplement use data are provided as the number of supplements taken in the past 30 d at the time of NHANES interview). In the NHANES database, information is provided for the name of each dietary supplement consumed by an individual in the previous 30 d, along with frequency of use, the ingredients for each supplement, and the quantity of each ingredient in the supplement. Individual ingredients in any supplement could span in quantity from a primary ingredient (e.g., iron in an iron supplement) to minute amounts. With this data, we calculated the quantity of chromium consumed in any of its varieties from all consumed dietary supplements over the 30 d period. We did not estimate nonsupplement dietary chromium consumption. A wide variety of chromium supplement consumption values was created with the use of this method, ranging from individuals who consumed a single dietary supplement with a small amount of chromium only once in the 30 d period, to individuals who consumed multiple dietary supplements with high chromium quantities multiple times in the 30 d period. Two subsets of dietary supplements were then created: those whose ingredients list included chromium and those supplements that contained the word “chromium” in its title (by definition, a subset of the former group). This was in order to separate out individuals actively seeking chromium supplementation (those taking supplements with “chromium” in the title) and those simply consuming the chromium from a multivitamin or a supplement whose main purpose may not be chromium supplementation (with chromium appearing only in the ingredients list). Information on participant age, gender, BMI, ethnicity, and socioeconomic status (SES) was also collected. SES was coded as the ratio of family income to poverty, with values below 1.00 representing household income below poverty. All analyses were restricted to individuals ≥18 y of age.
Logistic regression analyses were performed with independent variables in the model categorized in the following manner, with an asterisk denoting the baseline referent category for analyses: Gender (male = 0*, and female = 1); age (18–29, 30–49*, 50–69, and ≥70); ethnicity (non-Hispanic white*, Mexican American, other Hispanic, non-Hispanic black, and other); BMI (in kg/m2; <18.5, 18.5–24.9*, 25–29.9, and ≥30); and SES (categorized by quartiles, with lowest 25% as baseline category).
We investigated the association between diabetes prevalence and chromium supplementation as a dichotomous variable (did or did not take chromium), and explored several dose-response models. Logistic regression analysis was performed with the chromium variable dichotomized at 2000 μg of consumed chromium from supplements in 30 d. In this way, we considered people who took no chromium, only the small amounts of chromium typically found in a daily multivitamin, and larger amounts, which could potentially be viewed as more purposeful usage. To take into consideration the possibility that people who consume any type of dietary supplement may be less likely than others to have T2D to begin with, we further categorized consumption habits in the following manner: did not take any supplement, took at least one supplement but not one containing chromium, and took at least one supplement containing chromium. By doing this, we disentangled the confounding effects of overall supplement use on diabetes. We also evaluated diabetes prevalence based on chromium supplement use spanning 30 d, categorized as: 0 μg, >0–1999 μg, 2000–5999 μg, 6000–11,999 μg, and ≥12,000 μg Cr/30 d, as a reflection of the total range of chromium intake.
The survey package -svy- in Stata 13 was used, and data were weighted according to the NHANES recommended guidelines for database analysis (25). All logistic regression coefficients are presented as ORs (95% CIs), and reported P values were 2-tailed and based on an α = 0.05. Regression coefficients were identified as having a significant relation with the outcome variable, T2D status, if their P value was ≤0.05. Interactions between the main independent variable of interest (chromium supplementation) and other independent variables were investigated, but none of significance was identified.
Results
The NHANES database for the years 1999–2010 included information on 62,160 individuals. Of the entire population, 55.2% of people reported consuming at least one dietary supplement in the previous 30 d, and 26.8% of all people took a supplement that contained chromium, whereas 0.6% took a supplement that had “chromium” in its name. After filtering the database for individuals who had the full complement of data in the required variables (gender, ethnicity, BMI, SES, diabetes diagnosis, chromium supplement usage, and laboratory HbA1c values), the sample population included 35,998 individuals in total, because some data, such as laboratory HbA1c values, are collected for only a representative subsample of the yearly sample, and 28,539 when restricted to adults aged ≥18 y, representing the study cohort. With the use of the T2D-M2 definition of diabetes, the sample size is slightly smaller, with 28,211 observations, because less data was available on whether or not a doctor had ever diagnosed a participant with diabetes. Descriptive statistics of the sample population are presented in Table 1 and 2. The study cohort was 51.7% female, with a mean age of 45.3 y and mean HbA1c of 5.5%.
TABLE 1.
Descriptive statistics of the NHANES full subsample population and the population of individuals who consumed at least one supplement of any kind1
| Variable | Total | Male | Female |
| Entire subpopulation (n = 28,539) | |||
| Proportion of participants | — | 48.3 (47.8, 48.9) | 51. 7 (51.1, 52.2) |
| Age,1 y | 45.3 (44.9, 45.8) | 44.6 (44.1, 45.5) | 46.0 (45.5, 46.5) |
| HbA1c1 | 5.49 (5.47, 5.51) | 5.52 (5.50, 5.55) | 5.46 (5.44, 5.48) |
| T2D-M12 | 6.4 (6.0, 6.9) | 7.0 (6.4, 7.5) | 5.8 (5.3, 6.3) |
| T2D-M22 | 8.9 (8.4, 9.4) | 9.3 (8.7, 9.9) | 8.4 (7.8, 9.1) |
| Any supplement taken2 | 58.3 (57.2, 59.3) | 53.0 (51.6, 54.3) | 63.3 (62.1, 64.6) |
| Chromium taken2 | 28.8 (27.8, 29.8) | 28.7 (27.4, 29.9) | 28.9 (27.8, 30.0) |
| Chromium taken by name2,3 | 0.7 (0.5, 0.8) | 0.6 (0.5, 0.9) | 0.7 (0.6, 0.9) |
| At least 1 supplement taken (n = 15,103) | |||
| Chromium taken2 | 49.4 (47.9, 50.8) | 54.1 (52.3, 56.0) | 45.7 (44.0, 47.3) |
| Chromium taken by name2,3 | 1.2 (0.9, 1.4) | 1.2 (0.9, 1.6) | 1.1 (0.9, 1.5) |
Values for age are means (95% CIs); all other values are percentages (95% CIs). HbA1c, glycated hemoglobin; T2D-M1, type 2 diabetes defined on the basis of HbA1c values only; T2D-M2, type 2 diabetes defined by both HbA1c values and doctor diagnosis.
Presented as a proportion of the full subpopulation with 95% CIs.
Represents the population that consumed a supplement that had the word “chromium” in its name.
TABLE 2.
Proportion of population consuming dietary supplements across T2D-M1 and T2D-M2 diabetes definitions1
| Supplement use | T2D-M1 diabetes (n = 2878) | T2D-M2 diabetes (n = 3850) |
| Any supplement taken | ||
| No | 44.2 (41.4, 47.0) | 41.9 (39.4, 44.5) |
| Yes | 55.8 (53.0, 58.6) | 58.1 (55.5, 60.7) |
| Supplement with chromium taken | ||
| No | 74.4 (71.5, 76.7) | 72.4 (70.2, 74.6) |
| Yes | 25.6 (23.3, 28.5) | 27.6 (25.4, 29.8) |
| Supplement with chromium taken, if at least 1 supplement taken | ||
| No | 53.9 (49.4, 58.3) | 52.8 (49.0, 56.5) |
| Yes | 46.1 (41.7, 50.6) | 47.2 (43.5, 51.0) |
| Supplement taken with chromium in name | ||
| No | 0.6 (0.5, 0.7) | 0.5 (0.4, 0.7) |
| Yes | 1.9 (1.1, 2.8) | 2.0 (1.3, 2.7) |
Values are % (95% CI). HbA1c, glycated hemoglobin; T2D-M1, type 2 diabetes defined on the basis of HbA1c values only; T2D-M2, type 2 diabetes defined by both HbA1c values and doctor diagnosis.
Of those in the study cohort, 58.3% reported consuming a dietary supplement in the previous 30 d, 28.8% reported consuming a dietary supplement that contained at least some chromium, and 0.7% consumed supplements that had “chromium” in the title, similar to the total sample. A total of 49.4% of persons who reported taking a dietary supplement used a supplement that contained chromium (Table 1). Because of the small number of individuals intentionally taking chromium supplements in our sample, additional analysis was not performed on this subset with the use of named chromium supplements. A total of 25.9% of individuals with diabetes based on HbA1c values ≥6.5% (T2D-M1) reported taking a supplement with chromium included in its ingredients, and 1.9% reported taking a supplement with chromium by name. Similarly, 25.6% of people with diabetes based on the T2D-M2 definition took supplements with chromium in the ingredients list, and 2.0% reported the use of a supplement with chromium in its name.
Diabetes prevalence and chromium supplement use in the study population.
Diabetes prevalence was 6.4% based on HbA1c alone, or 8.9% by self-report and/or HbA1c (as defined by T2D-M1 and T2D-M2, respectively) (Table 1). For those who did not take any supplements, the proportion of the sample population with diabetes was 6.7% and 8.9% for T2D-M1 and T2D-M2, respectively (data not shown). In persons who took any type of dietary supplement, 6.1% and 8.8% had diabetes as defined by T2D-M1 and T2D-M2, respectively, with no difference between dietary supplement users and nonusers (T2D-M1: P = 0.64; T2D-M2: P = 0.56). The proportion of people with diabetes among those who took a supplement containing chromium was 5.7% and 8.5% for T2D-M1 and T2D-M2, respectively, again with no difference between users of a chromium-containing supplement and non-users of supplements (T2D-M1: P = 0.55; T2D-M2: P = 0.37,). Furthermore, for those individuals who had diabetes, 55.8% and 58.1% consumed some type of dietary supplement based on T2D-M1 and T2D-M2 definitions, respectively, whereas 25.6% and 27.6% consumed a supplement containing chromium (Table 2).
The proportion of people reporting the highest dosage range (≥12,000 μg Cr/30 d) was larger in those with diabetes (both T2D-M1 and T2D-M2) than without in all populations, suggesting a tendency for people with diabetes to consume larger amounts of chromium. This dosage category captures 0.8% of the population (95% CI for mean proportion of individuals in the ≥12,000 μg Cr/30 d dosage category: 0.6, 0.9). For the entire subpopulation, when the highest dosage category was combined with the next-lowest dosage category (6000–11,999 μg Cr/30 d), a larger proportion of persons with diabetes than without diabetes were in this category, representing ∼2.8% of the population (95% CI for the proportion of individuals in the ≥6000 μg Cr/30 d dosage category: 2.6, 3.1) (Table 3). Likewise, for the subpopulation of individuals who consumed at least one supplement of any kind, a greater proportion of people described as having diabetes consumed ≥6000 μg Cr/30 d, representing 4.9% of the population (95% CI for the proportion of individuals using at least one supplement of any kind who consumed ≥6000 μg Cr/30 d: 4.4, 5.3).
TABLE 3.
Proportion of people with diabetes across differing chromium exposure levels for the full subpopulation and the subpopulation that consumed at least one supplement of any kind1
| T2D-M1 |
T2D-M2 |
||||
| Chromium dosage, μg/30 d | % of total | No | Yes | No | Yes |
| Full subpopulation (n = 28,539) | |||||
| 0 | 71.2 | 71 | 74 | 71.1 | 73 |
| 0.1–1999 | 12.6 | 12.7 | 11.2 | 12.7 | 11.3 |
| 2000–5999 | 13.4 | 13.5 | 11.8 | 13.4 | 12.8 |
| 6000–11,999 | 2.1 | 2.1 | 1.8 | 2.1 | 1.8 |
| >12,000 | 0.8 | 0.7 | 1.2 | 0.7 | 1.1 |
| ≥6000 | 2.8 | 2.8 | 3 | 2.9 | 2.9 |
| At least one supplement taken (n = 15,103) | |||||
| 0 | 50.6 | 50.4 | 53.9 | 50.5 | 52.6 |
| 0.1–1999 | 21.6 | 21.7 | 19.9 | 21.7 | 19.8 |
| 2000–5999 | 22.9 | 23 | 20.9 | 23 | 22.5 |
| 6000–11,999 | 3.5 | 3.6 | 3.2 | 3.5 | 3.1 |
| >12,000 | 1.3 | 1.3 | 2.1 | 1.3 | 2 |
| ≥6000 | 4.9 | 4.9 | 5.3 | 4.8 | 5.1 |
Values are percentages. HbA1c, glycated hemoglobin; T2D-M1, type 2 diabetes defined on the basis of HbA1c values only; T2D-M2, type 2 diabetes defined by both HbA1c values and doctor diagnosis.
Chromium supplement use and diabetes.
While controlling for gender, age, ethnicity, BMI, SES, and the use of any type of dietary supplement besides chromium, persons using chromium supplements had lower odds of having diabetes than did nonusers (either no supplement use or use supplements without chromium). (Table 4). Graphic representation of the associations between both T2D-M1 and T2D-M2 and differing levels of chromium supplementation can be found in Figure 1. Compared with the population that did not take any supplements, the odds of having an HbA1c value ≥6.5% (T1D-M1) were nearly 27% lower for those who reported taking a supplement that contained chromium in the previous 30 d (OR: 0.73; 95% CI: 0.62, 0.86; P < 0.001) (Analysis 1A, Table 4). In contrast, there were no significant differences in the odds of having diabetes (T2D-M1) when comparing persons who consumed any type of dietary supplement not containing chromium with those not consuming any supplement (OR: 0.89; CI: 0.77, 1.03; P = 0.11). Similarly, chromium supplement use was associated with lower odds of having diabetes based on HbA1c values and/or self-reporting (T2D-M2 diabetes definition) compared with no supplement use (OR: 0.81; 95% CI: 0.71, 0.91; P = 0.001), but no difference was observed when comparing individuals who consumed supplements not containing chromium with nonusers of supplements (OR: 0.95; 95% CI: 0.82, 1.08; P = 0.42) (Analysis 1B, Table 4).
TABLE 4.
Multivariable logistic regression analyses of the effect of chromium supplementation on diabetes in the full subpopulation1
| Predictor variable | OR | SE | (95% CI) | P > t |
| Analysis 1A, T2D-M1 (n = 28,539) | ||||
| Supplement, yes; chromium, yes | 0.73 | 0.06 | (0.62, 0.86) | <0.001 |
| Supplement, yes; chromium, no | 0.89 | 0.06 | (0.77, 1.03) | 0.11 |
| Female | 0.71 | 0.04 | (0.64, 0.79) | <0.001 |
| Age category | ||||
| 18–29 y | 0.24 | 0.04 | (0.17, 0.32) | <0.001 |
| 50–70 y | 4.15 | 0.38 | (3.46, 4.98) | <0.001 |
| ≥70 y | 5.56 | 0.45 | (4.73, 6.54) | <0.001 |
| Ethnicity | ||||
| Mexican American | 1.99 | 0.17 | (1.68, 2.36) | <0.001 |
| Other Hispanic | 1.78 | 0.29 | (1.28, 2.46) | <0.001 |
| Non-Hispanic black | 1.95 | 0.15 | (1.68, 2.26) | <0.001 |
| Other | 2.58 | 0.36 | (1.95, 3.40) | <0.001 |
| BMI category | ||||
| <18.5 kg/m2 | 0.14 | 0.07 | (0.06, 0.36) | <0.001 |
| 25 to <30 kg/m2 | 1.73 | 0.17 | (1.43, 2.10) | <0.001 |
| ≥30 kg/m2 | 4.799 | 0.46 | (3.96, 5.79) | <0.001 |
| SES category | ||||
| 25 to <50% | 1.07 | 0.10 | (0.90, 1.28) | 0.43 |
| 50 to <75% | 0.81 | 0.06 | (0.70, 0.93) | 0.004 |
| 75–100% | 0.60 | 0.05 | (0.52, 0.72) | <0.001 |
| Analysis 1B, T2D-M2 (n = 28,211) | ||||
| Supplement, yes; chromium, yes | 0.81 | 0.05 | (0.71, 0.91) | 0.001 |
| Supplement, yes; chromium, no | 0.95 | 0.07 | (0.82, 1.08) | 0.42 |
| Analysis 1 C, T2D-M1 (n = 28,539) | ||||
| Supplement, yes; chromium, ≥2000 μg/30 d | 0.72 | 0.07 | (0.60, 0.87) | 0.001 |
| Supplement, yes; chromium, <2000 μg/30 d | 0.75 | 0.08 | (0.60, 0.93) | 0.01 |
| Supplement, yes; chromium, no | 0.89 | 0.06 | (0.77, 1.03) | 0.11 |
| Analysis 1D, T2D-M2 (n = 28,211) | ||||
| Supplement, yes; chromium, ≥2000 μg/30 d | 0.83 | 0.06 | (0.72, 0.96) | 0.01 |
| Supplement, yes; chromium, <2000 μg/30 d | 0.78 | 0.07 | (0.66, 0.92) | 0.004 |
| Supplement, yes; chromium, no | 0.95 | 0.07 | (0.82, 1.09) | 0.42 |
HbA1c, glycated hemoglobin; SES, socioeconomic status; T2D-M1, type 2 diabetes defined on the basis of HbA1c values only; T2D-M2, type 2 diabetes defined by both HbA1c values and doctor diagnosis.
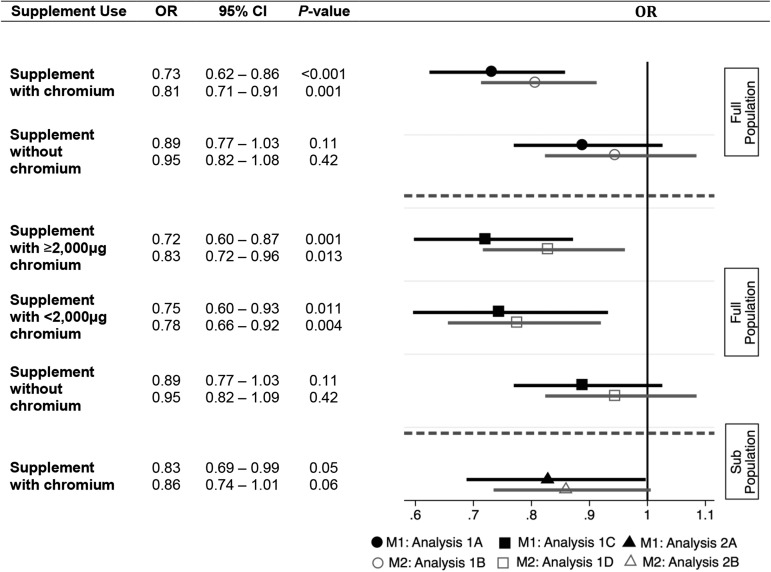
FIGURE 1.
The effect of varying levels of dietary chromium supplement use on the odds of having diabetes as defined by both HbA1c values and physician diagnosis. Associations between categorical variables for analyses with the use of the full population (analyses 1A, 1B, 1C, and 1D; n = 28,539) are in comparison with persons who consumed no dietary supplement of any kind. Associations between categorical variables for analyses with the use of the subpopulation (analyses 2A and 2B; n = 15,103) of people who consumed at least one dietary supplement of any kind are in comparison with people who consumed dietary supplements containing no chromium. M1 and M2 are in reference to T2D-M1 and T2D-M2, respectively. HbA1c, glycated hemoglobin; T2D-M1, type 2 diabetes defined on the basis of HbA1c values only; T2D-M2, type 2 diabetes defined by both HbA1c values and doctor diagnosis.
Persons consuming ≥2,000 μg Cr/30 d had lower odds of having diabetes (T2D-M1) than did those who consumed no dietary supplements (OR: 0.72; 95% CI: 0.60, 0.87; P = 0.001). Likewise, when comparing those consuming >0 μg but <2000 μg Cr/30 d to those who consumed no supplements of any kind, the odds of having diabetes (T2D-M1) were lower for those consuming supplements (OR: 0.75; 95% CI: 0.60, 0.93; P = 0.01). In contrast, the odds of having diabetes (T2D-M1) did not differ between persons who consumed a supplement not containing chromium and those who took no supplements at all (OR: 0.89; 95% CI: 0.77, 1.03; P = 0.11) (Analysis 1C, Table 4). Persons who consumed ≥2000 μg Cr/30 d had lower odds of having diabetes (T2D-M2) than did people who consumed no dietary supplements (OR: 0.83; 95% CI: 0.72, 0.96; P = 0.01); moreover, those who consumed >0 μg but <2000 μg Cr/30 d also had lower odds of having T2D-M2 than did those who took no supplements (OR: 0.78; 95% CI: 0.66, 0.92; P = 0.004) (Analysis 1D, Table 4). Finally, no difference in the odds of having diabetes (T2D-M2) was observed when comparing the use of nonchromium-containing supplements with no supplement use (OR: 0.95; 95% CI: 0.82, 1.09; P = 0.42).
Comparing odds of having diabetes in the subpopulation using at least one supplement: Supplements with varying amounts of chromium compared with supplements without chromium.
Because persons using supplements may be inherently healthier than nonusers, we repeated analysis while considering only those people who consumed at least one supplement of any kind in the previous 30 d. In this population of 15,103 persons, lower odds of having diabetes (T2D-M1) were once again observed for those who had consumed chromium in a supplement compared with persons using any supplements without chromium (OR: 0.83; 95% CI: 0.69, 0.99; P = 0.046) (Analysis 2A, Table 5); the same relation for the T2D-M2 definition of diabetes was negatively trending (OR 0.86, 95% CI: 0.74, 1.01; P = 0.06) (Analysis 2B, Table 5). These associations can also be found in Figure 1.
TABLE 5.
Multivariable logistic regression analyses of the effect of chromium supplementation on diabetes in the subpopulation of individuals who consumed at least one dietary supplement of any kind1
| Predictor variable | OR | SE | (95% CI) | P > t |
| Analysis 2A, T2D-M1 (n = 15,103) | ||||
| Chromium, yes | 0.83 | 0.08 | (0.69, 0.99) | 0.046 |
| Analysis 2B, T2D-M2 (n = 14,893) | ||||
| Chromium, yes | 0.86 | 0.07 | (0.74, 1.02) | 0.06 |
| Analysis 2C, T2D-M1 (n = 15,103) | ||||
| Chromium, ≥2000 μg/30 d | 0.82 | 0.09 | (0.66, 1.02) | 0.08 |
| Chromium, <2000 μg/30 d | 0.84 | 0.10 | (0.67, 1.06) | 0.14 |
| Analysis 2D, T2D-M2 (n = 14,893) | ||||
| Chromium, ≥2000 μg/30 d | 0.89 | 0.09 | (0.73, 1.07) | 0.21 |
| Chromium, <2000 μg/30 d | 0.82 | 0.07 | (0.69, 0.99) | 0.03 |
HbA1c, glycated hemoglobin; T2D-M1, type 2 diabetes defined on the basis of HbA1c values only; T2D-M2, type 2 diabetes defined by both HbA1c values and doctor diagnosis.
When considering supplementation of ≥2,000 μg Cr/30 d in the limited subset of individuals who consumed at least one dietary supplement of any kind, a similar, albeit nonsignificant negative trend was found between diabetes (T2D-M1) and chromium supplement use for those using a higher dose of chromium compared with persons who took at least one supplement of any kind that did not include chromium (OR: 0.82; 95% CI: 0.66, 1.02; P = 0.08). No significant difference was observed between individuals who consumed >0 μg but <2000 μg Cr/30 d and the same population of supplement users (OR: 0.84; 95% CI: 0.67, 1.06; P = 0.14) (Analysis 2C, Table 5). For the T2D-M2 definition, no difference was observed between those who consumed ≥2000 μg Cr/30 d and supplement users who consumed no chromium (OR: 0.89; 95% CI: 0.73, 1.07; P = 0.21). Finally, persons who consumed >0 μg but < 2000 μg Cr/30 d had lower odds of having diabetes (T2D-M2) than did people who consumed no chromium from supplements (OR: 0.82; 95% CI: 0.69, 0.99; P = 0.03) (Analysis 2D, Table 5).
Consideration of confounding factors.
In the whole study cohort, women had lower odds of having diabetes than did men, and compared with the referent age category (30–49 y), younger people had much lower odds of having diabetes, whereas older individuals showed progressively higher odds. All ethnicities had higher odds of having diabetes than did non-Hispanic whites. With the normal BMI value of 18.5–24.9 as a referent, underweight individuals had significantly lower odds of having diabetes, whereas overweight and obese people had respectively higher odds. Although individuals in the 25% to <50% SES category showed no significant deviation from the referent category of 0% to <25%, an increasing income-to-poverty ratio was associated with significantly reduced odds of having diabetes. Detailed results of the logistic regression analyses are presented in Table 4. Because of the marked similarity in the logistic regression coefficients for gender, age, ethnicity, BMI, and SES across analyses, these data are presented only for Analysis 1A. There was no significant effect from these covariates on the relation between chromium supplement use and diabetes status by either definition. Likewise, in the subpopulation of individuals who consumed at least one supplement of any kind, trends for all other explanatory variables were similar to those in the whole sample population.
Discussion
Given the large number of US adults with T2D and also reporting consuming dietary supplements, we were interested in determining what the potential impact of chromium supplementation may be on T2D at the population level. Although numerous studies have been conducted to evaluate the potential role of chromium on glucose metabolism, they have largely been conducted in vitro, in preclinical models, or in human cohorts of small sample sizes. Our present analysis shows that nutritional supplement use is widespread, with over 58% of the US population, as captured by the NHANES survey, reporting nutritional supplement use. The prevalence of diabetes among them is between 6% and 9%, which is concordant to the recently reported prevalence of diabetes (26). Furthermore, over one-quarter of the adult population (28.8%) reported consuming a dietary supplement that contains at least some chromium, and 0.7% consumed supplements that had “chromium” in the title, representing a substantial number of persons, given the ∼250 million adults ≥18 y of age in the United States. Although we did not go into deeper analysis of intentional chromium use (defined in this study by the consumption of a dietary supplement that has “chromium” in its title) because of a small sample size within NHANES, we found that intentional chromium use was reported by 0.6% of nondiabetics in our study and by 1.9–2.0% of those with diabetes, depending on the criteria for identifying those with disease (T2D-M1 and T2D-M2), translating into ∼5.7 million US adults with diabetes.
When considering the entire NHANES population as a whole, the proportion of individuals with diabetes was similar between those who consumed no supplements, those who consumed a supplement of any kind, and those who consumed a supplement containing chromium. However, when taking into account socioeconomic, demographic, and BMI information, persons who took dietary supplements containing chromium had significantly lower odds of having T2D than 1) those who did not take any type of dietary supplement, and 2) those who took any type of supplement that did not contain chromium. We did not find that persons taking supplements without chromium had lower odds of having diabetes than did those with no supplement use. To reduce the possibility that using chromium supplements might show lower odds of having diabetes because supplement users may be more likely to be healthy than nonusers, we compared people who consumed dietary supplements containing chromium with those who consumed dietary supplements without chromium. Again, we found that people using supplements with chromium had lower odds of having diabetes than did those using supplements without chromium. However, no dose-response relation between supplementation with >2000 μg Cr/30 d and diabetes was found. This may be due to low statistical power in the higher levels of chromium supplementation obtained from the NHANES database, making comparison of the relations between diabetes and supplement use of ≥2,000 μg Cr/30 d difficult to disentangle. Alternatively, there may be a biological threshold underlying this finding, in which an adequate level of chromium consumption may be enough to improve the odds of not having diabetes, but for which further consumption offers no additional benefits.
Neither the Institute of Medicine nor the Agency for Toxic Substances and Diseases Registry have established a safe upper intake level for chromium, and the NIH Office of Dietary Supplements notes that the efficacy and safety of chromium supplements need more investigation. Given the large number of US adults currently taking chromium supplements, an equally important question is the safety profile and thresholds of chromium in a nondeficient population. Mice fed chromium picolinate in doses up to 50,000 ppm for 2 y did not exhibit deleterious effects (27). Safety in rodents does not ensure safety in humans. Small human trials have evaluated the efficacy of chromium at doses of 200–1000 μg/d, with potential improvement in insulin sensitivity and glucose, no alterations in blood chemistries and hematologic variables, and no serious adverse effects in the small numbers of participants studied over the relatively short duration of administration, which generally supports use (6, 12, 28–30). The US National Academy of Sciences initially established an estimated safe and adequate daily intake for chromium of 50–200 μg (31). The recommended DRIs for chromium were established in 2001, with insufficient data to establish RDA, but providing Adequate Intake of 25–35 μg/d based on average intakes of chromium from food (32, 33).
Chromium may play a role in carbohydrate, lipid, and protein metabolism (6, 14, 15), but it is often marketed by dietary supplement companies for improving insulin sensitivity. We found more people with diabetes using chromium supplements, consistent with perceived (albeit poorly substantiated) indicated use. Chromium deficiency had been suggested as causing abnormal glucose metabolism, which can be corrected with replacement (34), and it has been suggested as improving insulin action (35). Although it has been shown that individuals who were parenterally deprived of trivalent chromium can develop insulin resistance and diabetes, there is no evidence that those with T2D develop clinically relevant chromium deficiencies, or that chromium supplementation in those who are replete provides additional benefit (36). It remains controversial whether or not chromium should be recommended for glycemic control in people with diabetes (2, 14), and chromium supplementation is not recommended by the American Diabetes Association (37). Multiple studies suggest that chromium supplementation might improve carbohydrate and lipid metabolism, yet the results have been inconsistent (2, 11, 13, 36, 38), potentially as a result of lack of adequate control groups (39, 40) or lack of statistical comparison between the chromium and control groups (41, 42), variations in the subject’s insulin resistance or diabetic status (36, 43), nutritional status, and formulation, and dosage of chromium (44). Multiple controlled studies find no benefit in fasting glucose or oral glucose tolerance with chromium picolinate dosed between 400–1000 μg/d over 3–8 mo in persons without diabetes (5–9, 11). However, although glycemia improved in some studies of patients with T2D (30), meta-analyses showed no improvement in fasting glucose with chromium supplementation in those with diabetes (45). Notably, to our knowledge, there are no studies performed that investigate the long term health effects of chromium supplementation on glycemia (45).
Chromium can be found in our environment in different oxidation states as metallic, trivalent, and hexavalent forms. Trivalent chromium is the potentially biologically active form found in foods and nutritional supplements, which are available as chromium chloride, chromium nicotinate, chromium picolinate, high-chromium yeast, and chromium citrate, with different profiles of absorption and bioavailability. Chromium in general has poor bioavailability (46), with absorption ranging from 0.4% to 2.8%, although the bioavailability of some chromium salts may be higher. Once absorbed, chromium is distributed throughout the body, and can be found in the liver, kidney, spleen, and bone (14, 32). Chromium is transported by transferrin and the oligopeptide low–molecular weight chromium-binding substance, or chromodulin, which has been suggested as binding chromic ions in response to an insulin-mediated chromic ion influx, and, in turn, as binding to the insulin-stimulated receptor to further activate its tyrosine kinase activity (47). Alternatively, chromium has been postulated as improving insulin sensitivity by reducing hepatic and muscle intracellular lipid accumulation and/or activation of glucose transporter 4 trafficking (48, 49). Coadministered anions (for example picolinate, nicotinate, or propionate) might also have biological properties.
In vitro studies suggest a mutagenic potential of trivalent chromium, with genotoxic properties at extremely high doses; however, these effects have not been confirmed in vivo (28). There are case reports of serious adverse events including kidney failure (50, 51) and rhabdomyolysis (52), but causality is unclear and events were presumably due to high doses of chromium. Hexavalent chromium results from industrial pollution and is considered toxic.
We acknowledge that a limitation of the NHANES database as a cross-sectional study tool is that individuals who were undiagnosed with diabetes, previously had T2D, or may have mild diabetes, and who, on the date of blood collection, had an HbA1c value <6.5%, are not identified as having diabetes. Although this limitation may create some misclassification bias, we estimate that the effect is small because of the robustness and analytic stability of HbA1c values, which have been shown to accurately reflect glycemia in the preceding weeks to months (53). We also acknowledge that we used a single determination of HbA1c for classification, and that the American Diabetes Association recommends confirmation by repeat testing (54). However, multiple secondary analysis of ORs for diabetes were consistent with rates published by the CDC, validating the classifications and manipulations of the data set. For example, we also found that diabetes is more prevalent in ethnic minorities, older age groups, and those with a higher BMI, as has been documented previously (26). Additionally, energy intake, energy expenditure, and physical activity are important to consider in T2D risk, but are considered unreliable in the NHANES database (55), so we forego these considerations, because there are no reliable alternatives for this population.
In summary, we showed chromium supplementation in adults to be associated with significantly lower odds of an individual having diabetes. Although causality cannot be determined, this study provides strong evidence that a large-scale study to determine the causal effect of chromium on diabetes is warranted. A very important matter that requires attention is that a substantial proportion of the US population is taking over-the-counter supplements that include chromium, and at a population level, many specifically are using chromium by name in their supplements. Clinical safety and efficacy data for chromium supplementation for improved insulin sensitivity and glycemic lowering is lacking. To support widespread use for diabetes treatment or prevention, and given the prevalence of current use, clinical trials adequately powered and aimed at evaluating safety and efficacy may be warranted.
Acknowledgments
JSB and ABG designed the research; DJM conducted the research; DJM analyzed the data and performed the statistical analysis; DJM, AMG, and ABG wrote the paper; and DJM had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: HbA1c, glycated hemoglobin; SES, socioeconomic status; T2D, type 2 diabetes; T2D-M1, type 2 diabetes defined on the basis of HbA1c values only; T2D-M2, type 2 diabetes defined by both HbA1c values and doctor diagnosis.
References
- 1.Center for Disease Control. 2011 National diabetes fact sheet-publications-diabetes DDT. [Internet]. [cited 2014 Apr 25]. Available from: http://www.cdc.gov/diabetes/pubs/factsheet11.htm.
- 2.Psaltopoulou T, Ilias I, Alevizaki M. The role of diet and lifestyle in primary, secondary, and tertiary diabetes prevention: a review of meta-analyses. Rev Diabet Stud 2010;7:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Diabetes Information Clearinghouse (NDIC). Diabetes 0verview [Internet]. [cited 2014 Apr 28]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/overview/#types.
- 4.Campbell AP. Diabetes and dietary supplements. Clin Diabetes 2010;28:35–9. [Google Scholar]
- 5.Boyd SG, Boone BE, Smith AR, Conners J, Dohm GL. Combined dietary chromium picolinate supplementation and an exercise program leads to a reduction of serum cholesterol and insulin in college-aged subjects. J Nutr Biochem 1998;9:471–5. [Google Scholar]
- 6.Cefalu WT. Effect of chromium tripicolinate on insulin sensitivity in vivo. J Trace Elem Exp Med 1999;12:71–83. [Google Scholar]
- 7.Frauchiger MT, Wenk C, Colombani PC. Effects of acute chromium supplementation on postprandial metabolism in healthy young men. J Am Coll Nutr 2004;23:351–7. [DOI] [PubMed] [Google Scholar]
- 8.Joseph LJ, Farrell PA, Davey SL, Evans WJ, Campbell WW. Effect of resistance training with or without chromium picolinate supplementation on glucose metabolism in older men and women. Metabolism 1999;48:546–53. [DOI] [PubMed] [Google Scholar]
- 9.Volpe SL, Huang HW, Larpadisorn K, Lesser II. Effect of chromium supplementation and exercise on body composition, resting metabolic rate and selected biochemical parameters in moderately obese women following an exercise program. J Am Coll Nutr 2001;20:293–306. [DOI] [PubMed] [Google Scholar]
- 10.Bailey CH. Improved meta-analytic methods show no effect of chromium supplements on fasting glucose. Biol Trace Elem Res 2014;157:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Althuis MD, Jordan NE, Ludington EA, Wittes JT. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am J Clin Nutr 2002;76:148–55. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RA, Cheng N, Bryden NA, Polansky MM, Chi J, Feng J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes 1997;46:1786–91. [DOI] [PubMed] [Google Scholar]
- 13.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care 2007;30:2154–63. [DOI] [PubMed] [Google Scholar]
- 14.Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care 2004;27:2741–51. [DOI] [PubMed] [Google Scholar]
- 15.Jain SK, Patel P, Rogier K. Trivalent chromium inhibits protein glycosylation and lipid peroxidation in high glucose-treated erythrocytes. Antioxid Redox Signal 2006;8:238–41. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz K, Mertz W. Chromium(III) and the glucose tolerance factor. Arch Biochem Biophys 1959;85:292–5. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JB. Chromium: celebrating 50 years as an essential element? Dalton Trans 2010;39:3787–94. [DOI] [PubMed] [Google Scholar]
- 18.Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, Bruce-Robertson A. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am J Clin Nutr 1977;30:531–8. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RA. Chromium and parenteral nutrition. Nutrition 1995; 11(1, Suppl)83–6. [PubMed] [Google Scholar]
- 20.Brown RO, Forloines-Lynn S, Cross RE, Heizer WD. Chromium deficiency after long-term total parenteral nutrition. Dig Dis Sci 1986;31:661–4. [DOI] [PubMed] [Google Scholar]
- 21.EFSA NDA Panel (EFSA Panel on Dietetic Products NaA). Scientific Opinion on Dietary Reference Values for chromium. EFSA Journal 2014;12:3845. [Google Scholar]
- 22.National Health and Nutrition Examination Survey (NHANES)—About the National Health and Nutrition Examination Survey [Internet]. [cited 2014 Apr 28]. Available from: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- 23.National Health and Nutrition Examination Survey (NHANES)—Questionnaires, Datasets, and Related Documentation. [Internet]. [cited 2014 Apr 28]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 24.National Diabetes Information Clearinghouse (NDIC). The A1C Test and Diabetes [Internet]. [cited 2014 Apr 25]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/A1CTest/.
- 25.National Health and Nutrition Examination Survey (NHANES)—Analytical Guidelines. [Internet]. [cited cited 2014 Apr 28]. Available from: http://www.cdc.gov/nchs/nhanes/analytic_guidelines.htm.
- 26.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med 2014;160:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stout MD, Nyska A, Collins BJ, Witt KL, Kissling GE, Malarkey DE, Hooth MJ. Chronic toxicity and carcinogenicity studies of chromium picolinate monohydrate administered in feed to F344/N rats and B6C3F1 mice for 2 years. Food Chem Toxicol 2009;47:729–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berner TO, Murphy MM, Slesinski R. Determining the safety of chromium tripicolinate for addition to foods as a nutrient supplement. Food Chem Toxicol 2004;42:1029–42. [DOI] [PubMed] [Google Scholar]
- 29.Campbell WW, Beard JL, Joseph LJ, Davey SL, Evans WJ. Chromium picolinate supplementation and resistive training by older men: effects on iron-status and hematologic indexes. Am J Clin Nutr 1997;66:944–9. [DOI] [PubMed] [Google Scholar]
- 30.Martin J, Wang ZQ, Zhang XH, Wachtel D, Volaufova J, Matthews DE, Cefalu WT. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care 2006;29:1826–32. [DOI] [PubMed] [Google Scholar]
- 31.Recommended Dietary Allowances. 10th Edition: The National Academies Press; 1989; ISBN Paperback: 978–0-309–04633–6. [cited 2015 Apr 28]. Available from: http://www.nap.edu/catalog/1349/recommended-dietary-allowances-10th-edition. [PubMed]
- 32.Chromium. [Internet]. [cited 2014 Apr 30]. Available from: http://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/#h7.
- 33.Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc: The National Academies Press; 2001. [PubMed]
- 34.Anderson RA. Chromium as an essential nutrient for humans. Regul Toxicol Pharmacol 1997;26:S35–41. [DOI] [PubMed] [Google Scholar]
- 35.Kimura K. Role of essential trace elements in the disturbance of carbohydrate metabolism. Nippon Rinsho 1996;54:79–84. [PubMed] [Google Scholar]
- 36.Cefalu WT, Rood J, Pinsonat P, Qin J, Sereda O, Levitan L, Anderson RA, Zhang XH, Martin JM, Martin CK, et al. Characterization of the metabolic and physiologic response to chromium supplementation in subjects with type 2 diabetes mellitus. Metabolism 2010;59:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Diabetes Association. Foundations of Care: Education, Nutrition, Physical Activity, Smoking Cessation, Psychosocial Care, and Immunization. Diabetes Care 2015;38 Suppl:S20–30. [DOI] [PubMed] [Google Scholar]
- 38.Broadhurst CL, Domenico P. Clinical studies on chromium picolinate supplementation in diabetes mellitus–a review. Diabetes Technol Ther 2006;8:677–87. [DOI] [PubMed] [Google Scholar]
- 39.Grant KE, Chandler RM, Castle AL, Ivy JL. Chromium and exercise training: effect on obese women. Med Sci Sports Exerc 1997;29:992–8. [DOI] [PubMed] [Google Scholar]
- 40.Kato I, Vogelman JH, Dilman V, Karkoszka J, Frenkel K, Durr NP, Orentreich N, Toniolo P. Effect of supplementation with chromium picolinate on antibody titers to 5-hydroxymethyl uracil. Eur J Epidemiol 1998;14:621–6. [DOI] [PubMed] [Google Scholar]
- 41.Gunton JE, Cheung NW, Hitchman R, Hams G, O’Sullivan C, Foster-Powell K, McElduff A. Chromium supplementation does not improve glucose tolerance, insulin sensitivity, or lipid profile: a randomized, placebo-controlled, double-blind trial of supplementation in subjects with impaired glucose tolerance. Diabetes Care 2005;28:712–3. [DOI] [PubMed] [Google Scholar]
- 42.Pasman WJ, Westerterp-Plantenga MS, Saris WH. The effectiveness of long-term supplementation of carbohydrate, chromium, fibre and caffeine on weight maintenance. International journal of obesity and related metabolic disorders. Int J Obes Relat Metab Disord 1997;21:1143–51. [DOI] [PubMed] [Google Scholar]
- 43.Wang ZQ, Qin J, Martin J, Zhang XH, Sereda O, Anderson RA, Pinsonate P, Cefalu WT. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism 2007;56:1652–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Racek J, Trefil L, Rajdl D, Mudrova V, Hunter D, Senft V. Influence of chromium-enriched yeast on blood glucose and insulin variables, blood lipids, and markers of oxidative stress in subjects with type 2 diabetes mellitus. Biol Trace Elem Res 2006;109:215–30. [DOI] [PubMed] [Google Scholar]
- 45.Landman GW, Bilo HJ, Houweling ST, Kleefstra N. Chromium does not belong in the diabetes treatment arsenal: Current evidence and future perspectives. World J Diabetes 2014;5:160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laschinsky N, Kottwitz K, Freund B, Dresow B, Fischer R, Nielsen P. Bioavailability of chromium(III)-supplements in rats and humans. Biometals 2012;25:1051–60. [DOI] [PubMed] [Google Scholar]
- 47.Vincent JB. The biochemistry of chromium. J Nutr 2000;130:715–8. [DOI] [PubMed] [Google Scholar]
- 48.Horvath EM, Tackett L, McCarthy AM, Raman P, Brozinick JT, Elmendorf JS. Antidiabetogenic effects of chromium mitigate hyperinsulinemia-induced cellular insulin resistance via correction of plasma membrane cholesterol imbalance. Mol Endocrinol 2008;22:937–50. Retraction in: Mol Endocrinol 2010;24:1308. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Sreejayan N, Dong F, Kandadi MR, Yang X, Ren J. Chromium alleviates glucose intolerance, insulin resistance, and hepatic ER stress in obese mice. Obesity (Silver Spring) 2008;16:1331–7. [DOI] [PubMed] [Google Scholar]
- 50.Cerulli J, Grabe DW, Gauthier I, Malone M, McGoldrick MD. Chromium picolinate toxicity. Ann Pharmacother 1998;32:428–31. [DOI] [PubMed] [Google Scholar]
- 51.Wasser WG, Feldman NS, D’Agati VD. Chronic renal failure after ingestion of over-the-counter chromium picolinate. Ann Intern Med 1997;126:410. [DOI] [PubMed] [Google Scholar]
- 52.Martin WR, Fuller RE. Suspected chromium picolinate-induced rhabdomyolysis. Pharmacotherapy 1998;18:860–2. [PubMed] [Google Scholar]
- 53.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care 2014;37 Suppl 1:S14–80. [DOI] [PubMed] [Google Scholar]
- 55.Archer E, Hand GA, Blair SN. Validity of US nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data, 1971–2010. PLoS One 2013;8:e76632. [DOI] [PMC free article] [PubMed] [Google Scholar]