Abstract
Background: Data have shown that healthy children and adolescents have an inadequate intake of zinc, an essential nutrient for growth. It is unclear whether zinc supplementation can enhance bone health during this rapid period of growth and development.
Objective: The primary aim of this study was to determine the effect of zinc supplementation on biochemical markers of bone turnover and growth in girls entering the early stages of puberty. The secondary aim was to test moderation by race, body mass index (BMI) classification, and plasma zinc status at baseline.
Methods: One hundred forty seven girls aged 9–11 y (46% black) were randomly assigned to a daily oral zinc tablet (9 mg elemental zinc; n = 75) or an identical placebo (n = 72) for 4 wk. Fasting plasma zinc, procollagen type 1 amino-terminal propeptide (P1NP; a bone formation marker), carboxy-terminal telopeptide region of type 1 collagen (ICTP; a bone resorption marker), and insulin-like growth factor I (IGF-I) were assessed at baseline and post-test. Additional markers of bone formation (osteocalcin) and resorption (urinary pyridinoline and deoxypyridinoline) were also measured.
Results: Four weeks of zinc supplementation increased plasma zinc concentrations compared with placebo [mean change, 1.8 μmol/L (95% CI: 1.0, 2.6) compared with 0.2 μmol/L (95% CI: −0.3, 0.7); P < 0.01]. Zinc supplementation also increased serum P1NP concentrations compared with placebo [mean change, 23.8 μmol/L (95% CI: −14.9, 62.5) compared with −31.0 μmol/L (95% CI: −66.4, 4.2); P = 0.04). There was no effect from zinc supplementation on osteocalcin, ICTP, pyridinoline, deoxypyridinoline, or IGF-I. There was no moderation by race, BMI classification, or plasma zinc status at baseline.
Conclusions: Our data suggest that 4 wk of zinc supplementation increases bone formation in premenarcheal girls. Further studies are needed to determine whether supplemental zinc can improve childhood bone strength. This trial was registered at clinicaltrials.gov as NCT01892098.
Keywords: zinc, bone, bone turnover, growth, children
Introduction
Normal growth and maturation in children and adolescents depends on an Adequate Intake of many essential nutrients. Zinc in particular is involved in numerous aspects of cellular metabolism that ultimately influence growth and maturation (1), which is why the RDA for zinc is highest during adolescence. Indeed, classic characteristics of zinc deficiency include growth retardation and delayed sexual maturation. Zinc supplementation trials have shown improved height, weight, and other growth indexes in children with nutrient deficiencies, including in those with idiopathic short stature (2), sickle cell anemia (3), and cystic fibrosis (4). Though zinc supplementation studies in apparently healthy children as a strategy to optimize growth outcomes are limited, they may be warranted. Data from population-based surveys suggest that although most children meet the current recommended daily intake of zinc, inadequate intake based on US reference values has been observed in those entering the early stages of puberty (5, 6). Moreover, circulating zinc concentrations decline in response to rapid growth and hormonal changes that occur during this period (1), and because there is currently no reliable reference standard, the extent to which this may influence normal development is unclear.
It is possible that zinc supplementation may promote optimal growth and development specifically by augmenting childhood bone mineralization and strength. Zinc is the most abundant trace mineral in the human skeleton, and may be most beneficial in this period of rapid bone accretion to achieve optimal peak bone mass. In vitro, zinc has been shown to stimulate osteoblasts and inhibit osteoclasts ultimately to drive bone formation (7–9). In vivo, poor zinc status, and not overt zinc deficiency, is associated with suboptimal bone development, whereas a low intake of dietary zinc (e.g., 2–4 μg/g diet) has been shown to attenuate bone maturation toward the end of the pubertal growth spurt in primates (10, 11). Whether similar findings may be observed in healthy children is unknown. A recent cross-sectional study in healthy premenarcheal girls found that dietary zinc intake was positively associated with bone strength indexes (12), suggesting that higher zinc intake may be a viable strategy to optimize childhood bone health with potential long-term benefits.
To our knowledge, only one study has examined the effects of zinc supplementation on biochemical markers of bone turnover in healthy female children (13). In this investigation, researchers did not observe a significant effect on bone formation as assessed by serum osteocalcin, or bone resorption as assessed by urinary deoxypyridinoline (13). Although these findings suggest that zinc supplementation is not effective in improving bone health in otherwise healthy children, investigators did not assess the bone formation marker procollagen type 1 amino-terminal propeptide (P1NP)8, which has been recognized as the primary reference standard to assess among biochemical markers of bone formation (14). Assessment of this biochemical marker could provide additional information and advance our understanding of zinc’s effect on bone formation.
The primary aim of this study was to determine the effect of 4 wk of zinc supplementation on biochemical markers of bone turnover and growth in otherwise healthy girls entering the early stages of puberty. Primary outcomes were changes in serum P1NP (bone formation marker) and carboxy-terminal telopeptide region of type 1 collagen (ICTP), a bone resorption marker, and plasma insulin-like growth factor I (IGF-I) at 4 wk. Secondary outcomes were serum osteocalcin (a bone formation marker) and urinary pyridinoline (a bone resorption marker) and deoxypyridinoline (a bone resorption marker). To test for supplement compliance and to exclude potential adverse effects on copper status, changes in plasma zinc and ceruloplasmin activity in serum, respectively, were assessed. We also determined whether race, body weight, and plasma zinc status at baseline modify the bone turnover response to zinc supplementation.
Methods
Study participants and design
Healthy white and black girls (n = 147) participated in this 4 wk randomized, double-blind, placebo-controlled, parallel-group zinc supplementation trial. The 4 wk study period was selected because research has shown changes in biochemical markers of bone turnover as early as 1–2 wk after dietary intervention (15–17). Moreover, children exhibit a higher rate of bone remodeling during growth than the adults largely examined in these studies. Participants were recruited beginning in the summer of 2009 from the Athens–Clarke County area in northeast Georgia through newspaper advertisements, radio announcements, and community flyers. Trained study personnel administered a telephone screen to the parent/guardian of prospective participants to determine eligibility. During the screen, parents/guardians were informed that the purpose of the study was to investigate the effects of zinc on bone health, and they were made aware of adverse events that may occur with the treatment dose. Children were included if they were girls of non-Hispanic white or non-Hispanic black/African American race and aged 9–11 y. These criteria were used to eliminate further variability by race and potential differences by sex and pubertal maturation. Children were excluded if they were taking medications or had any medical condition that could affect growth, pubertal maturation, nutritional status, or metabolism, and if they had experienced menses. All testing procedures were conducted at the Bone and Body Composition Laboratory at The University of Georgia and were completed by spring 2010. The Institutional Review Board for Human Subjects at The University of Georgia approved all study procedures, and informed assent and consent were obtained from each participant and her parent/guardian, respectively.
Supplemental zinc and placebo tablets
Participant identification numbers were used to randomly assign children by following simple randomization procedures to either zinc (n = 75) or control (n = 72) groups. A laboratory technician labeled tablet bottles with the appropriate corresponding treatment code. All investigators, research personnel, and participants remained blinded to these codes until statistical analyses were complete.
Enrolled participants received a 4 wk supply of zinc tablets (9 mg/d elemental zinc) as zinc sulfate or identical placebo tablets (i.e., in color, size, and odor) at the baseline visit, provided by Vesta Pharmaceuticals. Participants were instructed to take 1 study tablet/d and to return empty tablet bottles along with any unused products at 4 wk. Compliance was checked by pill count and by measurements of plasma zinc concentrations. Compliance was calculated with the following formula: compliance (percentage) = (number of tablets actually taken/number of tablets that should have been taken) × 100. Safety was assessed at each visit by monitoring adverse events and measurement of ceruloplasmin activity.
Anthropometry and pubertal stage
Height and weight were measured to calculate BMI-for-age percentiles used for body weight classification as normal weight (<85th percentile), overweight (85th–94.99th percentile), or obese (≥95th percentile) (18). Pubertal maturation stage was determined with the use of a gender-specific questionnaire described by Tanner (19). There is agreement for breast development between self- and physician-evaluation of sexual maturity (20).
Biochemical analyses
Blood and urine samples were collected in the morning after an overnight fast at the baseline and 4 wk visits. Samples were used to determine concentrations of zinc and IGF-I, ceruloplasmin activity, bone formation markers (P1NP and osteocalcin), and bone resorption markers (ICTP, pyridinoline, and deoxypyridinoline). Plasma zinc, measured to confirm study adherence, was determined by atomic absorption spectrophotometry with a Perkin Elmer Analyst 400, and verified based on standards from the US National Institute of Standards and Technology. Normal values for plasma zinc were defined as between 10.7 and 16.8 μmol/L (21). Ceruloplasmin activity was assessed to determine the effect of zinc supplementation on copper status (22). Ceruloplasmin activity concentrations <62 IU/L were indicative of an adverse effect on copper status.
Plasma IGF-I was assayed in duplicate with the use of an ELISA. The intra- and interassay CVs were 7.5–8.3% and 3.5–4.3%, respectively (23). Serum P1NP, osteocalcin, and ICTP were assayed in duplicate with the use of RIA. The intra- and interassay CVs were 2.8% and 5.6% for P1NP, 10% and 5% for osteocalcin, and 3.4% and 5.9% for ICTP, respectively. Urinary pyridinoline and deoxypyridinoline were measured with the use of HPLC, and corrected for dilution by creatinine concentration, as previously described (15, 24).
Dietary intake
Dietary intake data were collected to help determine adherence to the study protocol. Participants were encouraged to maintain their usual dietary habits to ensure that changes in bone-related outcomes were attributed to the study regimen. All participants received the same instructions regarding diet to reduce the possibility of experimental bias between groups. Dietary intake was determined with the use of 3 d diet records completed at baseline and 4 wk, and records were analyzed with the use of the Food Processor SQL, version 9.7.3 (ESHA Research).
Statistical analyses
Sample size calculation.
Power calculations were computed based on primary outcomes P1NP, ICTP, and IGF-I. The computations were based upon an F test for main effect of treatment (zinc supplement compared with placebo). Required are assumptions concerning the difference in 4 wk changes in the response (i.e., differences in the mean gain scores) across treatment groups, and the variance of the gain score, from which effect size is computed. Using data previously collected in our laboratory, we pooled estimates in SD of change for P1NP (SD = 110 μg/L), ICTP (SD = 5 μg/L), and IGF-I (SD = 94 μg/L), and we estimated effect sizes (i.e., Cohen’s d) for these outcomes (P1NP, d = 0.55; ICTP, d = 0.50; and IGF-I, d = 0.54). We determined that 56–64 subjects/group would provide at least 80–82% power (α = 0.05) to detect a difference in mean change of outcome variable between treatment groups. With a given sample size of 64 subjects/group (α-level set at 0.05), the proposed study had a power of 0.80. Assuming a 15% sample size loss from either attrition or insufficient quality of measurements, a starting sample size of 75 subjects/group (n = 150) preserved the power of the study design.
Analyses.
Group differences at baseline were determined by independent samples t tests. Differences in proportions at baseline were tested by chi-square tests. Repeated-measures mixed models were used in an intention-to-treat analysis of each outcome measure with the use of all available data. Base models for each outcome measure included the fixed effects of group (zinc and control) and measurement time (baseline and 4 wk) and their interaction. To test moderation by race, body weight status, and plasma zinc status at baseline, main effects and 2- and 3-factor interactions of race × group × time, weight status × group × time, and plasma zinc status × group × time were examined for each outcome measure in separate models. Four-factor interactions were not examined because of small sample sizes within the combination of the variables. Participant nested within group was considered a random effect. Because there were only 2 measurement times, an unstructured covariance structure was assumed. If none of the 3-factor interactions were statistically significant, the model was reduced to examine the 2-factor interaction of group × time. Final models contained at a minimum the main effects and 2-factor interaction between group and time and any statistically significant 3-factor interactions. A Bonferroni adjustment to the overall α level was used to perform post hoc pairwise differences in changes of each measure (post-test − pretest) within group for models with only the 2-factor interaction with group and time, either within race and group, within weight status and group, or within plasma zinc status and group for models containing 3-factor interactions. All statistical analyses were performed with the use of SPSS, version 21, and statistical significance was set at P < 0.05.
Results
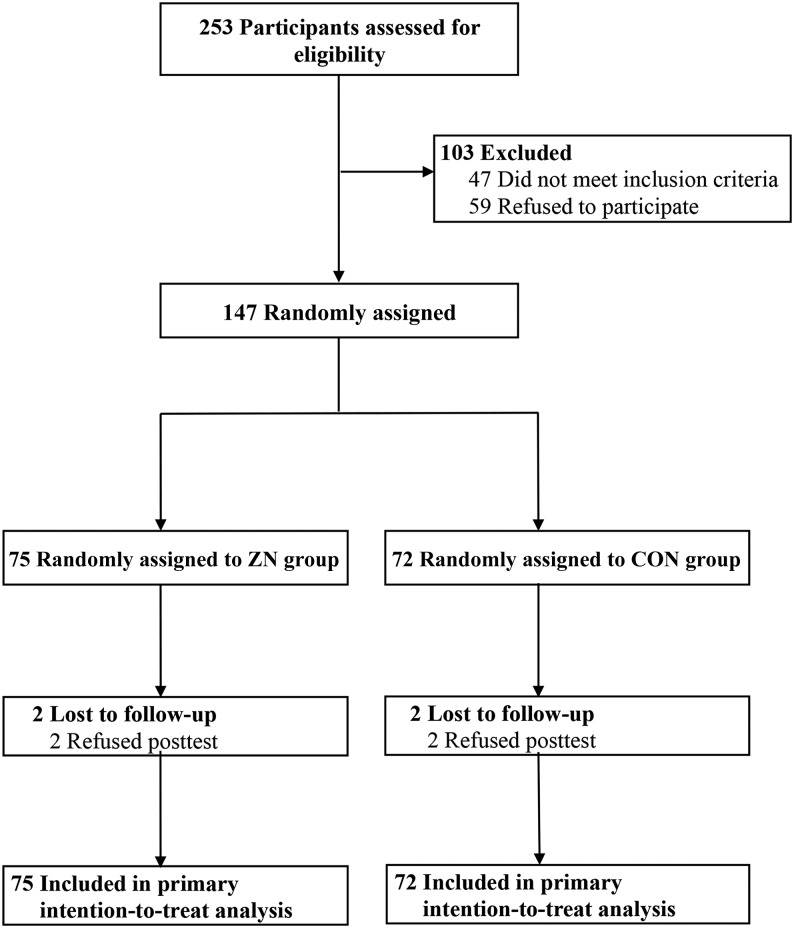
Participant flow is presented in Figure 1. We randomly assigned 147 white and black girls [age (mean ± SD) 10.5 ± 0.7 y, 46% black, 22% overweight, and 28% obese] to a daily oral zinc tablet containing 9 mg of elemental zinc (zinc group; n = 75) or an identical placebo tablet (control group; n = 72) for 4 wk. Fifty percent of the total sample was classified as in the normal weight range, and 22% and 28% were overweight and obese, respectively. Mean plasma zinc concentration in the total sample was 12.0 ± 3.2 μmol/L. Ninety seven percent of the sample (n = 143) was retained at post-test. Four participants (2 from the zinc group and from the control group) dropped out because of refusal to post-test. None of the participants reported signs of zinc toxicity (e.g., nausea).
FIGURE 1.
Flow diagram of study participants. CON, control; ZN, zinc.
The baseline characteristics for the zinc and control groups are shown in Table 1, which considers RDAs for calcium, vitamin D, and zinc among girls aged 9–13 y (25, 26). Age, racial distribution, pubertal maturation stage, anthropometric measurements, dietary intake, and biochemical measurements were similar between treatment arms. Compliance with the study protocol, as indicated by pill counts, was also similar between the zinc and control groups (82% compared with 81%, respectively; P = 0.71).
TABLE 1.
Baseline characteristics of girls aged 9–11 y randomly assigned to ZN or CON groups1
| ZN group (n = 75) | CON group (n = 72) | P2 | |
| Age, y | 10.6 ± 0.7 | 10.5 ± 0.7 | 0.62 |
| Race, white/black3 | 40/35 | 40/32 | 0.87 |
| Pubertal maturation stage (1–5) | 2.3 ± 0.5 | 2.3 ± 0.5 | 0.66 |
| Height, cm | 148 ± 6.8 | 149 ± 6.6 | 0.68 |
| Weight, kg | 46.8 ± 11.6 | 47.3 ± 10.9 | 0.79 |
| BMI percentile | 74.4 ± 26.3 | 75.8 ± 26.0 | 0.76 |
| BMI percentile category3 | 0.72 | ||
| Not overweight | 53 | 47 | |
| Overweight | 21 | 22 | |
| Obese | 26 | 31 | |
| Dietary intake4 | |||
| Energy, kcal/d | 1849 ± 718 | 1757 ± 661 | 0.37 |
| Calcium, mg/d | 699 ± 381 | 661 ± 356 | 0.54 |
| Vitamin D, μg/d | 2.9 ± 2.5 | 2.9 ± 2.8 | 0.96 |
| Zinc, mg/d | 5.0 ± 2.7 | 4.7 ± 2.3 | 0.48 |
| Biochemical measurements | |||
| Plasma zinc, μmol/L | 12.0 ± 3.2 | 12.1 ± 3.3 | 0.86 |
| Plasma zinc status3 | 0.43 | ||
| Normal range (10.7–16.8 μmol/L) | 52 | 49 | |
| Low range (<10.7 μmol/L) | 43 | 40 | |
| Ceruloplasmin activity, IU/L | 92.1 ± 25.1 | 87.3 ± 20.2 | 0.21 |
| Plasma IGF-I, μg/L | 415 ± 233 | 400 ± 258 | 0.72 |
| Bone formation markers | |||
| Serum P1NP, μg/L | 704 ± 188 | 709 ± 243 | 0.90 |
| Serum osteocalcin, μg/L | 32.0 ± 9.2 | 33.3 ± 9.1 | 0.40 |
| Bone resorption markers | |||
| Serum ICTP, μg/L | 24.2 ± 6.6 | 24.7 ± 6.5 | 0.73 |
| Urinary PYD/C, nmol/mmol | 102 ± 39.1 | 98.6 ± 40.1 | 0.64 |
| Urinary DPD/C, nmol/mmol | 29.6 ± 9.7 | 29.6 ± 11.0 | 0.99 |
Values are means ± SDs or %. CON, control; DPD/C, deoxypyridinoline/creatinine; ICTP, carboxy-terminal telopeptide of type 1 collagen; IGF-I, insulin-like growth factor I; P1NP, procollagen type 1 amino-terminal propeptide; PYD/C, pyridinoline/creatinine; ZN, zinc.
Tests of significance between groups were based on independent-samples t test unless otherwise indicated.
Tests of significance between groups were based on chi-square test.
Values of biochemical markers of bone turnover and growth by group at baseline and at 4 wk and changes over the testing period are shown in Table 2. The change in plasma zinc concentration was greater in the zinc group than in the control group (15% compared with 1.7%, P < 0.01), indicating overall compliance with the intervention. The change in ceruloplasmin activity was not different between groups (P = 0.65), demonstrating no negative effects on copper status. The change in serum P1NP, the primary outcome marker of bone formation, was greater in the zinc group than in the control group (23.8 compared with −31.0 μg/L, P = 0.04). The change in serum osteocalcin, the secondary outcome marker of bone formation, was not different between treatment arms (P = 0.40). The changes in plasma IGF-I and the bone resorption markers serum ICTP and urinary pyridinoline and deoxypyridinoline also were not different between groups (all P > 0.05). In addition, there were no significant interactions of group × time with race, body weight status, or plasma zinc status (all P > 0.05).
TABLE 2.
Biochemical markers at baseline and 4 wk in girls aged 9–11 y randomly assigned to ZN or CON groups1
| ZN group (n = 75) |
CON group (n = 72) |
||||||
| Baseline | 4 wk | Change | Baseline | 4 wk | Change | P-interaction2(time x group effect) | |
| Plasma zinc, μmol/L | 12.0 (11.3, 12.8) | 13.8 (12.8, 14.7) | 1.8 (1.0, 2.6) | 12.1 (11.3, 12.9) | 12.3 (11.3, 13.3) | 0.2 (−0.3, 0.7) | <0.01 |
| Ceruloplasmin activity, IU/L | 92.1 (86.9, 97.4) | 94.8 (88.6, 101) | 2.7 (−2.8, 8.2) | 87.3 (81.9, 92.7) | 91.7 (85.3, 98.1) | 4.4 (−1.0, 9.9) | 0.65 |
| Plasma IGF-I, μg/L | 415 (359, 471) | 458 (389, 526) | 42.8 (−8.1, 93.4) | 400 (343, 457) | 422 (352, 493) | 22.1 (−11.8, 55.8) | 0.51 |
| Serum P1NP, μg/L | 704 (648, 759) | 728 (669, 786) | 23.8 (−14.9, 62.5) | 709 (652, 766) | 678 (617, 739) | −31.0 (−66.4, 4.2) | 0.039 |
| Serum osteocalcin, μg/L | 32.0 (29.9, 34.1) | 32.5 (30.2, 34.8) | 0.5 (−0.8, 1.7) | 33.3 (31.2, 35.5) | 34.5 (32.2, 36.9) | 1.2 (−0.1, 2.4) | 0.40 |
| Serum ICTP, μg/L | 24.2 (22.3, 26.1) | 24.5 (22.4, 26.7) | 0.3 (−1.3, 2.0) | 24.7 (22.7, 26.7) | 24.8 (22.5, 27.0) | 0.1 (−1.7, 1.8) | 0.83 |
| Urinary PYD/C, nmol/mmol | 102 (92.6, 111) | 102 (93.4, 110) | 0.1 (−6.7, 6.5) | 98.6 (89.3, 108) | 101 (92.7, 110) | 2.7 (−5.7, 11.1) | 0.62 |
| Urinary DPD/C, nmol/mmol | 29.6 (27.3, 32.0) | 30.4 (27.9, 32.9) | 0.8 (−1.6, 3.0) | 29.6 (27.2, 32.0) | 30.5 (27.8, 33.0) | 0.9 (−1.5, 3.3) | 0.98 |
Values are means (95% CIs). CON, control; DPD/C, deoxypyridinoline/creatinine; ICTP, carboxy-terminal telopeptide of type 1 collagen; IGF-I, insulin-like growth factor I; P1NP, procollagen type 1 amino-terminal propeptide; PYD/C, pyridinoline/creatinine; ZN, zinc.
Tests of significance for group differences in change from baseline to 4 wk were conducted by using intention-to-treat mixed-model repeated-measures ANOVA.
Discussion
To our knowledge, this is the first randomized controlled trial to show that zinc supplementation may enhance bone development in healthy premenarcheal girls. We found that 9 mg/d of elemental zinc over 4 wk stimulated bone formation, as indicated by serum P1NP concentrations. Moreover, the zinc group exhibited a 15% increase in circulating plasma zinc and no decrease in ceruloplasmin activity, suggesting that this amount of zinc supplementation did not affect copper status.
The importance of adequate zinc for normal bone growth and development has been established in the large number of zinc supplementation trials conducted in children at risk of nutrient deficiencies. Indeed, studies in children with short stature found that ∼10 mg/d of elemental zinc supplementation improved growth (27), midupper arm circumference (28), and biochemical markers of bone formation (2). Although our study was conducted in apparently healthy premenarcheal girls, mean dietary zinc intake was below the 7 mg/d estimated average requirement and the 8 mg/d RDA (∼5 mg/d). Given that 41% of participants had plasma zinc concentrations <10.7 μmol/L, combined with reported intake below the RDA, it is possible that participants were marginally zinc deficient and susceptible to suboptimal bone development. Under these conditions, our findings would reflect those of a zinc repletion study. However, this seems unlikely, given that participants in the present study had a mean height of ∼149 cm, and CDC growth charts show that a value of 139 cm corresponds with a height-for-age z score that is within the normal range for 10-y-old children. Zinc concentrations in our participants were also higher than those observed in a study of children of short stature with known zinc deficiency (12.0 compared with 2.2 μmol/L) (29). In the present study, we showed that zinc supplementation with a dose similar to previous studies (27, 28) in addition to current dietary intake is practical and could be beneficial for bone formation in girls during growth when circulating zinc concentrations reach their nadir (30). These findings have important health implications, because zinc supplementation may optimize peak bone mass in childhood and adolescence, with long-term benefits in adulthood.
Few studies have examined the effects of zinc supplementation in apparently healthy children on biochemical markers of bone turnover. Clark et al. (13) found that 15 mg/d of elemental zinc in pubertal girls (mean age 12 y; n = 47) had no effect on serum osteocalcin and urinary deoxypyridinoline over 6 wk. A more recent study also found that 10 mg/d of elemental zinc in children (aged 8–9 y; n = 40) had no effect on serum osteocalcin (31). A possible limitation of these studies was that they did not measure serum P1NP. Although osteocalcin has long been considered an ideal marker of bone formation, it can also be released into circulation during bone resorption (32, 33). The serum concentration may therefore reflect components of both bone formation and resorption. P1NP, however, has several functional advantages over other biochemical markers of bone formation because of its low interindividual variability and relative stability in serum at room temperature (14, 34). P1NP was also recommended as the primary reference standard to assess among biochemical markers of bone formation by the Bone Marker Standards Working Group (14).
Similar to previous findings, we did not observe an effect from zinc supplementation on serum osteocalcin. We did, however, find that 9 mg/d of elemental zinc increased serum P1NP. Although it is unclear why we did not observe an effect on both biochemical markers, it is possible that P1NP and osteocalcin assays assess distinct molecular processes of bone formation that respond differently to 4 wk of zinc supplementation. Indeed, P1NP reflects newly synthesized type 1 collagen (35), whereas osteocalcin, considered to be a late marker of osteoblast activity, reflects matrix mineralization (36). Therefore, it is likely that a 4 wk trial may have been adequate for serum P1NP to capture zinc-stimulated proliferation of preosteoblasts, but not for serum osteocalcin to reflect zinc-stimulated matrix mineralization. Also in accordance with previous findings, we did not observe an effect from zinc supplementation on bone resorption. It is therefore possible that the effects of zinc supplementation are specific to the process of bone formation.
It has been postulated that zinc’s influence on increased bone formation may be via IGF-I, a growth factor produced primarily by the liver and, to a lesser extent, by nonhepatic tissues including bone. Once IGF-I is secreted by osteoblasts in response to growth hormone and parathyroid hormone (37), it recruits preosteoblasts to the bone-remodeling surface and stimulates the synthesis of bone collagen and matrix (38). Indeed, the relation between zinc intake and IGF-I has been observed in children with short stature (27, 29) and failure-to-thrive infants (39). However, there was no effect from zinc supplementation on plasma IGF-I concentrations in the present study, which is consistent with earlier findings in healthy children (13, 31). Given the purported mechanism, it is unclear why zinc supplementation increased circulating P1NP concentrations, but not IGF-I concentrations. It is possible that circulating IGF-I concentrations reflect hepatic IGF-I production and not the local bone contribution. In addition, our sample of healthy premenarcheal girls with normal plasma IGF-I concentrations (40) may not have had a marked response to zinc supplementation compared with those with lower IGF-I concentrations (27, 29). More research is warranted to explore additional mechanisms driving the zinc–bone association in healthy children.
A major strength of this study was that it was a randomized, double-blind, placebo-controlled trial. In addition, we included P1NP as a biochemical marker of bone formation in addition to osteocalcin, and we tested relations in a racially diverse sample of healthy children. We acknowledge some potential limitations. It is possible that 4 wk was inadequate to detect differences between groups in the other biochemical markers, although this is unlikely, given findings from other studies conducted over shorter testing periods (41–43). Our study was also limited to girls, and findings may not be generalizable because of differences in bone turnover by sex (44).
In conclusion, zinc supplementation was shown to stimulate bone formation in healthy premenarcheal girls over 4 wk. A longer-term clinical trial is warranted to determine whether zinc supplementation is a viable strategy to augment childhood bone structure and strength.
Acknowledgments
NKP, EML, AG, SAS, and RDL designed the research; NKP, EML, PJB, AG, SAS, K-HD, and CMI conducted the research; NKP performed the statistical analysis; PKB, NKP, and VC wrote the paper; and RDL had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ICTP, carboxy-terminal telopeptide of type 1 collagen; IGF-I, insulin-like growth factor I; P1NP, procollagen type 1 amino-terminal propeptide.
References
- 1.Story M, Stang J. Nutrition needs of adolescents. [Internet]. [cited 2015 Apr 16.] Available from: http://www.epi.umn.edu/let/pubs/adol_book.shtm.
- 2.Imamoğlu S, Bereket A, Turan S, Taga Y, Haklar G. Effect of zinc supplementation on growth hormone secretion, IGF-I, IGFBP-3, somatomedin generation, alkaline phosphatase, osteocalcin and growth in prepubertal children with idiopathic short stature. J Pediatr Endocrinol Metab 2005;18:69–74. [DOI] [PubMed] [Google Scholar]
- 3.Zemel BS, Kawchak DA, Fung EB, Ohene-Frempong K, Stallings VA. Effect of zinc supplementation on growth and body composition in children with sickle cell disease. Am J Clin Nutr 2002;75:300–7. [DOI] [PubMed] [Google Scholar]
- 4.Van Biervliet S, Vande Velde S, Van Biervliet JP, Robberecht E. The effect of zinc supplements in cystic fibrosis patients. Ann Nutr Metab 2008;52:152–6. [DOI] [PubMed] [Google Scholar]
- 5.Schenkel TC, Stockman NK, Brown JN, Duncan AM. Evaluation of energy, nutrient and dietary fiber intakes of adolescent males. J Am Coll Nutr 2007;26:264–71. [DOI] [PubMed] [Google Scholar]
- 6.Jennings A, Davies GJ, Costarelli V, Dettmar PW. Micronutrient intakes of pre-adolescent children living in London. Int J Food Sci Nutr 2010;61:68–77. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi M. Role of nutritional zinc in the prevention of osteoporosis. Mol Cell Biochem 2010;338:241–54. [DOI] [PubMed] [Google Scholar]
- 8.Togari A, Arakawa S, Arai M, Matsumoto S. Alteration of in vitro bone metabolism and tooth formation by zinc. Gen Pharmacol 1993;24:1133–40. [DOI] [PubMed] [Google Scholar]
- 9.Moonga BS, Dempster DW. Zinc is a potent inhibitor of osteoclastic bone resorption in vitro. J Bone Miner Res 1995;10:453–7. [DOI] [PubMed] [Google Scholar]
- 10.Golub MS, Keen CL, Gershwin ME, Styne DM, Takeuchi PT, Ontell F, Walter RM, Hendrickx AG. Adolescent growth and maturation in zinc-deprived rhesus monkeys. [see comment] Am J Clin Nutr 1996;64:274–82. [DOI] [PubMed] [Google Scholar]
- 11.Leek JC, Keen CL, Vogler JB, Golub MS, Hurley LS, Hendrickx AG, Gershwin ME. Long-term marginal zinc deprivation in rhesus monkeys. IV. Effects on skeletal growth and mineralization. Am J Clin Nutr 1988;47:889–95. [DOI] [PubMed] [Google Scholar]
- 12.Laudermilk MJ, Manore MM, Thomson CA, Houtkooper LB, Farr JN, Going SB. Vitamin C and zinc intakes are related to bone macroarchitectural structure and strength in prepubescent girls. Calcif Tissue Int 2012;91:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark PJ, Eastell R, Barker ME. Zinc supplementation and bone growth in pubertal girls. Lancet 1999;354:485. [DOI] [PubMed] [Google Scholar]
- 14.Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 2011;22:391–420. [DOI] [PubMed] [Google Scholar]
- 15.Shapses SA, Robins SP, Schwartz EI, Chowdhury H. Short-term changes in calcium but not protein intake alter the rate of bone resorption in healthy subjects as assessed by urinary pyridinium cross-link excretion. J Nutr 1995;125:2814–21. [DOI] [PubMed] [Google Scholar]
- 16.Kerstetter JE, Mitnick ME, Gundberg CM, Caseria DM, Ellison AF, Carpenter TO, Insogna KL. Changes in bone turnover in young women consuming different levels of dietary protein. J Clin Endocrinol Metab 1999;84:1052–5. [DOI] [PubMed] [Google Scholar]
- 17.Wastney ME, Martin BR, Peacock M, Smith D, Jiang XY, Jackman LA, Weaver CM. Changes in calcium kinetics in adolescent girls induced by high calcium intake. J Clin Endocrinol Metab 2000;85:4470–5. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data 2000;314:1–27. [PubMed] [Google Scholar]
- 19.Tanner J. Growth and adolescence. 2nd ed. Oxford (United Kingdom): Blackwell Scientific, 1962. [Google Scholar]
- 20.Laing EM, Wilson AR, Modlesky CM, O’Connor PJ, Hall DB, Lewis RD. Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year-old females. J Bone Miner Res 2005;20:509–19. [DOI] [PubMed] [Google Scholar]
- 21.King JC. Assessment of zinc status. J Nutr 1990;120 Suppl 11:1474–9. [DOI] [PubMed] [Google Scholar]
- 22.Schosinsky KH, Lehmann HP, Beeler MF. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem 1974;20:1556–63. [PubMed] [Google Scholar]
- 23.Breen ME, Laing EM, Hall DB, Hausman DB, Taylor RG, Isales CM, Ding KH, Pollock NK, Hamrick MW, Baile CA, et al. 25-hydroxyvitamin D, insulin-like growth factor-I, and bone mineral accrual during growth. J Clin Endocrinol Metab 2011;96:E89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem 1984;137:380–8. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine Food and Nutrition Board. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academy Press, 2010. [Google Scholar]
- 26.Institute of Medicine Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academy Press, 2001. [PubMed] [Google Scholar]
- 27.Ninh NX, Thissen JP, Collette L, Gerard G, Khoi HH, Ketelslegers JM. Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. Am J Clin Nutr 1996;63:514–9. [DOI] [PubMed] [Google Scholar]
- 28.Kikafunda JK, Walker AF, Allan EF, Tumwine JK. Effect of zinc supplementation on growth and body composition of Ugandan preschool children: a randomized, controlled, intervention trial. Am J Clin Nutr 1998;68:1261–6. [DOI] [PubMed] [Google Scholar]
- 29.Hamza RT, Hamed AI, Sallam MT. Effect of zinc supplementation on growth hormone-insulin growth factor axis in short Egyptian children with zinc deficiency. Ital J Pediatr 2012;38:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butrimovitz GP, Purdy WC. Zinc nutrition and growth in a childhood population. Am J Clin Nutr 1978;31:1409–12. [DOI] [PubMed] [Google Scholar]
- 31.Rocha ED, de Brito NJ, Dantas MM, Silva Ade A, Almeida Md, Brandão-Neto J. Effect of zinc supplementation on GH, IGF1, IGFBP3, OCN, and ALP in non-zinc-deficient children. J Am Coll Nutr 2015;34:290–9. [DOI] [PubMed] [Google Scholar]
- 32.Gundberg CM, Weinstein RS. Multiple immunoreactive forms of osteocalcin in uremic serum. J Clin Invest 1986;77:1762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivaska KK, Hentunen TA, Vaaraniemi J, Ylipahkala H, Pettersson K, Vaananen HK. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J Biol Chem 2004;279:18361–9. [DOI] [PubMed] [Google Scholar]
- 34.Stokes FJ, Ivanov P, Bailey LM, Fraser WD. The effects of sampling procedures and storage conditions on short-term stability of blood-based biochemical markers of bone metabolism. Clin Chem 2011;57:138–40. [DOI] [PubMed] [Google Scholar]
- 35.Brandt J, Krogh TN, Jensen CH, Frederiksen JK, Teisner B. Thermal instability of the trimeric structure of the N-terminal propeptide of human procollagen type I in relation to assay technology. Clin Chem 1999;45:47–53. [PubMed] [Google Scholar]
- 36.Lee AJ, Hodges S, Eastell R. Measurement of osteocalcin. Ann Clin Biochem 2000;37:432–46. [DOI] [PubMed] [Google Scholar]
- 37.Ohlsson C, Nilsson A, Isaksson O, Lindahl A. Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc Natl Acad Sci USA 1992;89:9826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest 1989;83:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hershkovitz E, Printzman L, Segev Y, Levy J, Phillip M. Zinc supplementation increases the level of serum insulin-like growth factor-I but does not promote growth in infants with nonorganic failure to thrive. Horm Res 1999;52:200–4. [DOI] [PubMed] [Google Scholar]
- 40.Kanbur NO, Derman O, Kinik E. The relationships between pubertal development, IGF-1 axis, and bone formation in healthy adolescents. J Bone Miner Metab 2005;23:76–83. [DOI] [PubMed] [Google Scholar]
- 41.Knapen MH, Hamulyak K, Vermeer C. The effect of vitamin K supplementation on circulating osteocalcin (bone Gla protein) and urinary calcium excretion. Ann Intern Med 1989;111:1001–5. [DOI] [PubMed] [Google Scholar]
- 42.Douglas AS, Robins SP, Hutchison JD, Porter RW, Stewart A, Reid DM. Carboxylation of osteocalcin in post-menopausal osteoporotic women following vitamin K and D supplementation. Bone 1995;17:15–20. [DOI] [PubMed] [Google Scholar]
- 43.Chevalley T, Hoffmeyer P, Bonjour JP, Rizzoli R. Early serum IGF-I response to oral protein supplements in elderly women with a recent hip fracture. Clin Nutr 2010;29:78–83. [DOI] [PubMed] [Google Scholar]
- 44.Johansen JS, Giwercman A, Hartwell D, Nielsen CT, Price PA, Christiansen C, Skakkebaek NE. Serum bone Gla-protein as a marker of bone growth in children and adolescents: correlation with age, height, serum insulin-like growth factor I, and serum testosterone. J Clin Endocrinol Metab 1988;67:273–8. [DOI] [PubMed] [Google Scholar]