Abstract
Exposure to environmental stressors during embryo development can have long-term effects on the adult organism. This study used the thioredoxin reductase inhibitor auranofin to investigate the consequences of oxidative stress during zebrafish development. Auranofin at low doses triggered upregulation of the antioxidant genes gstp1 and prdx1. As the dose was increased, acute developmental abnormalities, including cerebral hemorrhaging and jaw malformation, were observed. To determine whether transient disruption of redox homeostasis during development could have long-term consequences, zebrafish embryos were exposed to a low dose of auranofin from 6–24 hours post fertilization, and then raised to adulthood. The adult fish were outwardly normal in their appearance with no gross physical differences compared to the control group. However, these adult fish had reduced odds of breeding and a lower incidence of egg fertilization. This study shows that a suboptimal early life environment can reduce the chances of reproductive success in adulthood.
Keywords: Oxidative stress, Reproductive toxicity, Auranofin, Zebrafish, Longitudinal cohort
Graphical abstract
Highlights
-
•
We exposed zebrafish embryos to the oxidative stress-inducing compound auranofin.
-
•
Embryos showed a dose-dependent increase in developmental abnormalities.
-
•
Exposed embryos responded by upregulating oxidative stress-responsive genes.
-
•
Embryos transiently exposed to a low dose of auranofin were raised into adults.
-
•
The resulting adults had reduced fertility compared with controls.
1. Introduction
Embryonic development can be influenced by exposure to environmental toxins and by a variety of pathologies, including oxygen deprivation, inflammation and metabolic disease [10], [24]. Oxidative stress underlies many of these conditions, and excessive oxidative stress can cause direct cell damage and directly compromise the health of surviving animals. Cell culture experiments have revealed that more subtle disturbances of cellular redox homeostasis modulate signaling pathways and trigger adaptive responses [11], [13]. For example, redox-dependent activation of the transcription factor Nrf2 increases expression of genes in the glutathione and thioredoxin systems, protecting the cells from subsequent stress [17], [38]. However, there is little understanding of how redox disruption in the embryo affects the phenotype of the resultant organism [36].
Animal models allow the developmental consequences of environmental insults to be investigated in a way not possible in human studies [3]. The zebrafish, Danio rerio, is a vertebrate model of development that is increasingly used in biomedical science [25]. Advantages to using zebrafish include the well-characterized embryonic development and the ease with which toxic effects of chemicals can be studied [21], [33]. In zebrafish, the chemical exposure of large numbers of developmentally synchronized embryos can be performed simply by dissolving the chemical of interest in the aquatic media [33].
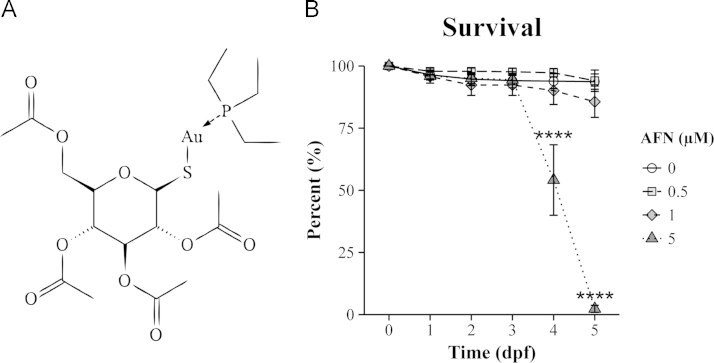
Oxidative stress can be triggered by promoting oxidant generation or compromising antioxidant defences. Auranofin (AFN) is a gold-based compound (Fig. 1A) that compromises antioxidant defence by targeting selenol and thiol proteins, in particular thioredoxin reductase (TrxR), involved in maintaining the reductive capacity of a cell [5]. In cell culture systems, TrxR has been found to be completely inhibited by 1 µM AFN, with significant mitochondrial oxidative stress and apoptosis occurring as the dose is increased [12], [9]. Lower doses, between 0.1–1 µM AFN, which partially inhibit TrxR activity, increase H2O2 levels, and activate Nrf2 [12], [20], [29]. In zebrafish larvae, exposure to 1 µM AFN increased transcription of the Nrf2-target gene gstp1 in the larval gill tissue, indicating that these animals have plastic chemical defence systems capable of responding to AFN [22].
Fig. 1.
Embryonic toxicity of AFN. (A) Schematic of the structural formula of C20H34AuO9PS. The compound consists of an acetoxythioglucose group linked to triethylphosphine by a gold atom. (B) Survival following static AFN exposure beginning at 6 hpf, given as the percentage of larvae alive at each time point out of the number of embryos at 0 dpf. The values represent the mean percentage±SEM for four biological replicates with each treatment group containing at least 30 embryos. Statistically significant differences are noted as ****p ≤0.0001.
In this study we sought to model the long-term consequences of environmental oxidative stress during development by transiently exposing zebrafish embryos to AFN. While this stressor is not representative of all forms of oxidative stress, AFN provides a useful pharmacological model of disrupted thiol redox homeostasis and an endogenous antioxidant stress response. First, the acute toxicity of AFN was assessed in embryos as an understanding of the developmental effects of AFN-induced oxidative stress in zebrafish is lacking. This revealed that continuous AFN exposure resulted in dose-dependent embryonic toxicity and the upregulation of antioxidant pathways. We then chose a low AFN dose to disrupt redox homeostasis during early embryogenesis, and observed reduced fertility in the resulting adults.
2. Materials and methods
2.1. Animal husbandry
Mature zebrafish were maintained under standard conditions [39]. All zebrafish research was approved by the University of Otago Animal Ethics Committee. The ABPS in-house wild type line was used for the embryonic viability experiments. To visualize blood flow, the Tg(gata1:DsRed) line with fluorescent red blood cells was used [37]. To visualize the craniofacial structures, the Tg(sox10:EGFP) line with fluorescent green neural crest cells was used [18]. For the cohort experiments, the Tg(vas:EGFP) line was used [23].
AFN exposed embryos (see next section) were reared using normal husbandry methods to generate three tanks of fish within each treatment arm of each cohort. As adults, the zebrafish were kept at a stocking density of approximately three fish per litre and bred as frequently as once a week. Two cohorts were produced from multiple founder pairs and raised sequentially. As such, at the time data were gathered, the first cohort was grown out to an older age than the second cohort that followed it. Cohort 1 was bred between 0.77 and 1.24 years post-fertilization (ypf). Cohort 2 was bred between 0.35 and 0.56 ypf. On average three pairs from each of three tanks were used in three spawning experiments.
Spawning was induced in the morning with a water change and the removal of the barrier between the pairs. A successful breeding event was one where at least one egg was released. As such, breeding success for each pair was classified as a binomial outcome, either successful or not. Eggs were collected with a sieve, transferred into a dish containing E3 embryo media, and incubated at 28 °C. The numbers of fertilized and unfertilized eggs were counted from each breeding pair in order to calculate the fertilization rate. The fertilization rate was classified as a Poisson count normalized by the size of the clutch produced by that pair.
2.2. Auranofin exposure
AFN (Sigma-Aldrich, cat. A6733) was made up in DMSO and kept as a 10 mM stock solution, 100 μM working stocks were made fresh in E3 media for each experiment. Final test solutions were made in a volume of 20 ml.
At 6 hours post-fertilization (hpf), embryos were randomly distributed into petri dishes. The excess media was removed and replaced with the AFN test solution or the solvent control, which contained the equivalent volume of DMSO. For embryo viability experiments there were typically 20 embryos in each dish. In the static (continuous) exposures, the test solution remained unchanged throughout the duration of the test. In the transient exposures used in the cohort experiments, the test solution was washed out with E3 media at 1 day post-fertilization (dpf). For the long-term experiment there were 50 embryos in each dish which were subsequently transferred to tanks at 5 dpf.
2.3. Scoring for developmental abnormalities
Mortality was identified in early embryos by the lack of any discernible heartbeat. Hemorrhaging was observed in the head of the larvae as a dense red region. Jaw defects were noted when the angle of the lower jaw was obviously altered in comparison to larvae exposed to the solvent control.
2.4. Measurement of transcript levels
Zebrafish embryo RNA extracts were prepared from 50 pooled embryos from each treatment group using the NucleoSpin RNA kit (Macherey-Nagel, cat. 740955.250) according to the manufacturer's instructions. RNA concentrations and purity were verified on a Nanodrop spectrophotometer. The SuperScript III First-Strand Synthesis System (Invitrogen, cat. 18080-051) was used for cDNA synthesis from 1 μg total RNA using oligo dT primers according to the manufacturer's instructions.
Primers used for quantitative real-time PCR analysis of gene expression are listed in Table S1. The primers were validated by checking the product size, sequencing the product to confirm its identity, determining the amplification efficiency and checking the melt curve for a single peak. Platinum SYBR Green qPCR SuperMix-UDG with ROX (Invitrogen, cat. 11744-500) was used to amplify cDNA in duplicate with the LightCycler 480 Instrument II (Roche, cat. 05015278001).
The cycle threshold (Ct) was calculated by the LightCycler 480 SW1.5 software and converted into a fold change relative to the reference genes (eif1a and rpl13a) using qBase (Biogazelle). The final values were normalized by the control treatment group.
2.5. Analysis of sperm motility
To test the quality of the male gametes, fish were set up as pairs the night before the analysis. In the morning, the males were anaesthetised one at a time in 40 μg/mL tricaine, pH 7.0, for 2 min. The fish were then transferred to a saline zebrafish sperm immobilizer solution, blotted dry, and transferred to a foam block. Blunt forceps were used to squeeze the male's abdomen gently and a capillary was used to collect the sperm and transfer the sample to sperm immobilizer solution.
Sperm was loaded onto a chamber slide (Leja, cat. SC-20-01-02-B) and flooded with 3 μL of reverse osmosis water to activate the sperm. Sperm motility was recorded by video and analysed using computer assisted sperm analysis (CASA) software (CEROS, Hamilton Thorne) 11 s after activation. Duplicate readings were taken for each sample.
2.6. Necropsy
After the fertility measurements were made, the fish were euthanized by hypothermia using incubation in an ice-slurry. The fish were then transferred from the ice, blotted dry, and examined for any gross external abnormalities. The weight was measured using a fine balance. To determine the length, each fish was photographed from above next to a ruler and measured using ImageJ [32]. The body mass index (BMI) was calculated as the weight (kg)/standard length2 (m2). The standard length, from the snout to the base of the tail, was used in the BMI calculation. Gonads were dissected out of the fish and weighed using a fine balance. The gonadosomatic index (GSI) was calculated as the gonad weight (mg)/total weight (mg)×100.
2.7. Data analysis
Statistical analysis was carried out using R statistics [26]. For the comparison of two means the Student's unpaired t-test was used. For the comparison of more than two samples one-way analysis of variance (ANOVA) was used. Two-way ANOVA was used to analyse the influence of two variables. When determining statistical significance a p value of ≤0.05 was considered significant. Unless otherwise noted, error bars indicate the standard error of the mean. Generalized linear models were fitted to the cohort fertility data; the R code used to run this analysis is provided as a Supplementary method.
3. Results
3.1. Auranofin treatment elicits dose-dependent toxicity in zebrafish embryos
Investigating the long-term consequences of oxidative stress requires a well-defined stress input. Since the developmental toxicity of AFN in zebrafish had not yet been characterised, we first recorded the effects of increasing doses of AFN (Fig. 1A) on the development of zebrafish embryos. We started the embryonic AFN exposure at 6 hpf, after zygotic genome activation, and found that there was a dose-dependent effect on early development. Exposure to AFN was associated with a range of gross developmental abnormalities including reduced pigmentation, a kinked tail, pericardial edema, hemorrhaging, and jaw malformation.
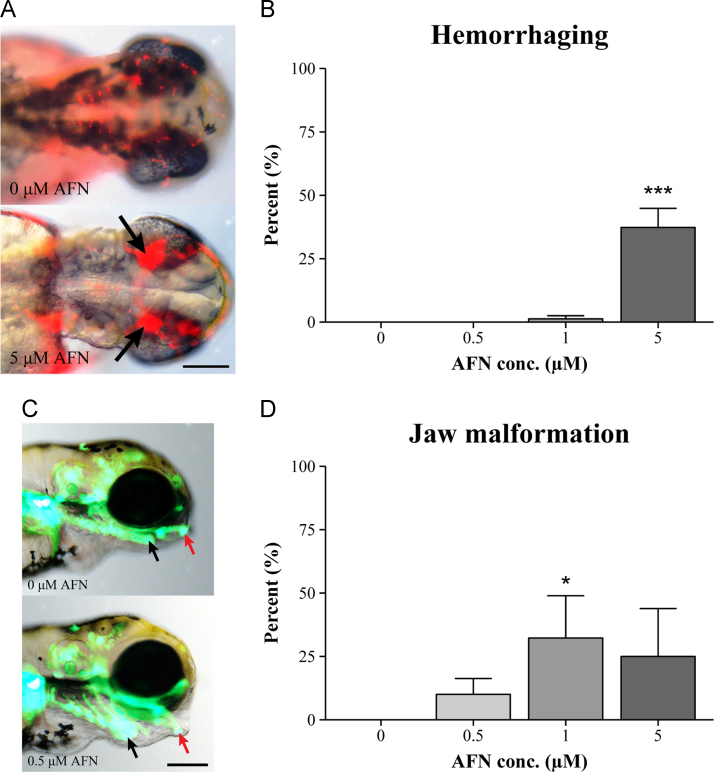
The developmental effects of AFN were observed as early as 2 dpf. At this time point, in addition to the reduced pigmentation, there was a notable hemorrhaging phenotype (Fig. 2A) observed following exposure to the 5 µM dose of AFN. By 3 dpf (Fig. 2B), this phenotype was observed in 37±9% of embryos (103/248 embryos, n=5 replicate experiments), a significant increase in comparison to the solvent control (p=0.0002, post-hoc Tukey). Using the Tg(gata1:DsRed) line, it was found that the cerebral hemorrhaging was located both medially and dorsally to the eyes of the larvae (Fig. 2A). By 4 dpf it was apparent that the 5 µM dose of AFN was acutely toxic to embryos as there was a significant reduction (p<0.0001, post-hoc Tukey) in embryonic survival (Fig. 1B) to 54±14% (n=4 replicate experiments) .
Fig. 2.
AFN-induced developmental defects. (A) Images of Tg(gata1:DsRed) embryos at 2 dpf (dorsal view) in which the blood is labeled by DsRed fluorescence, DMSO control is top and 5 µM AFN is bottom. Arrows indicate the increased fluorescent signal indicating hemorrhaging. Scale bar: 100 µm. (B) Hemorrhaging at 3 dpf following static AFN exposure beginning at 6 hpf, given as the percentage of larvae with the phenotype out of the total number of larvae at each time point. (C) Images of Tg(sox10:EGFP) embryos at 4 dpf (lateral view), DMSO control is top and 0.5 µM AFN is bottom. Arrows indicate malformation of the ceratohyal (black) and Meckel's (red) facial cartilage. Scale bar: 100 µm. (D) Jaw defects at 4 dpf following static AFN exposure beginning at 6 hpf, given as the percentage of larvae with the phenotype out of the total number of larvae at each time point. The values in B and D represent the mean percentage±SEM for five biological replicates with each treatment group containing on average 40 embryos. Statistically significant differences are noted as *p≤0.05 (1 µM), and ***p≤0.001.
The lower doses of AFN still impacted on development without the acute toxicity observed for the high 5 µM dose. The hemorrhaging that was frequently produced with the high 5 µM AFN dose was never observed with the 0.5 µM dose of AFN (Fig. 2B). Furthermore, with the 1 µM dose there was only a non-significant reduction in survival to 86±6% at 5 dpf (Fig. 1B). Survival for embryos in the 0.5 µM AFN group and the solvent control remained at 94±3% for the 5 days following fertilization. However, the 0.5 and 1 µM doses frequently resulted in larvae with a jaw malformation phenotype (Fig. 2D). Visualization of craniofacial development using the Tg(sox10:EGFP) line revealed that the jaw defect consisted of a ventral extension of the ceratohyal and Meckel's cartilage structures (Fig. 2C). This phenotype was never found in the solvent control but by 4 dpf was observed in 32±17% of embryos (102/230 embryos, n=5 replicate experiments, p=0.01, post-hoc Tukey) exposed to 1 µM AFN group and 10±6% of embryos (19/246 embryos, n=5 replicate experiments) in the 0.5 µM AFN group.
In addition to observing the gross phenotypic abnormalities that followed AFN exposure, AFN toxicity was also determined by the induction of DNA damage. Using a ‘comet' assay (Fig. S1), DNA in 2 dpf larvae was found to be significantly more fragmented (n=3 replicate experiments, p=0.02, t-test) following exposure to the high 5 µM dose such that 3.0±0.8% more DNA had migrated out of the cells after electrophoresis. Exposed embryos also showed a dose-dependent increase in the amount of TUNEL-positive cells in the head and neural tube of 2 dpf larvae (Fig. S2). The 1 and 5 µM doses of AFN had on average 13±1 (p<0.0001, post-hoc Tukey) and 18±1 (p<0.0001, post-hoc Tukey) TUNEL-positive cells in the head, respectively, significantly more than the 2±1 found in the solvent control (n=5 embryos). Interestingly, the 0.5 µM treatment did not elicit the excessive apoptosis observed with the higher doses, but did show a trend towards an increase, with 4±1 TUNEL-positive cells in the head.
3.2. Auranofin exposure leads to upregulation of antioxidant pathways in zebrafish embryos
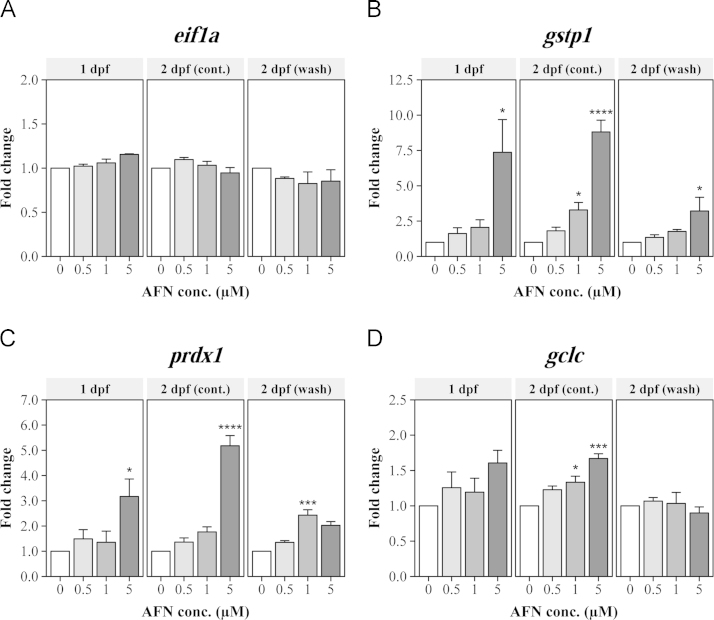
Exposure to AFN from 6 hpf induced a rapid stress response as early as 1 dpf (Fig. 3B), most notably with the marked transcriptional upregulation of gstp1. At 1 dpf, embryos that had been exposed to the 5 µM dose were found to have 7.4±2.3-fold more gstp1 mRNA than embryos in the solvent control (p=0.02, post-hoc Bonferroni). Continuing the static AFN exposure out to 2 dpf increased the level of gstp1 further to 8.8±0.8-fold more than that in the solvent control embryos (p<0.0001, post-hoc Bonferroni). Furthermore, when the AFN was washed out of the media at 1 dpf, the amount of gstp1 mRNA in embryos exposed to the 5 µM dose was still 3.2±1.0 fold higher than the solvent control (p=0.04, post-hoc Bonferroni).
Fig. 3.
AFN exposure increases antioxidant gene expression in zebrafish embryos. The levels of (A) eif1a (reference), (B) gstp1, (C) prdx1, and (D) gclc mRNA was determined from pools of 50 embryos in each treatment group at 1 and 2 dpf. AFN exposure began at 6 hpf and was either continued or washed out at 1 dpf. The Ct was normalized to the reference genes and scaled to the solvent control at each time point as a fold change. The values represent the mean ± SEM from three biological replicates. Statistically significant differences are noted as *p≤0.05, **p≤0.01, ***p≤0.001, and ****p≤0.0001.
Embryos exposed to the high 5 µM dose of AFN were also found to have altered levels of prdx1 and gclc. The increase in the expression of these genes was most notable at 2 dpf after continuous AFN exposure. At this time, prdx1 was significantly increased 5.2±0.7 fold (p<0.0001, post-hoc Bonferroni) following exposure to 5 µM AFN. The level of gclc was also significantly increased with this dose (p=0.0001, post-hoc Bonferroni), to levels 1.7±0.1 fold higher than in the solvent control. Although significant, the changes in gclc were not as large as those observed for gstp1 and prdx1.
The transcriptional upregulation of antioxidant genes following AFN exposure was dose-dependent. At 2 dpf, after continuous AFN exposure, the levels of gstp1 were increased 1.8±0.4 fold with the 0.5 µM dose and significantly increased 3.3±0.5 fold (p=0.04, post-hoc Bonferroni) with the 1 µM dose. Similarly, at this time, the levels of prdx1 were increased 1.4±0.1 fold with the 0.5 µM dose and increased 2.4±0.2 fold (p=0.0002, post-hoc Bonferroni) with the 1 µM dose. Although not statistically significant after correcting for multiple comparisons, the response to AFN had begun to emerge by 1 dpf in the lowest dose tested. At this early time point, the 0.5 µM dose had induced 1.6±0.4-fold more gstp1 and 1.5±0.4-fold more prdx1 in whole embryonic tissue samples. Note that the increase in gstp1 following exposure to the 0.5 µM dose was significant when other doses were ignored (Fig. S3).
3.3. Transient exposure to a low dose of auranofin during embryogenesis reduced reproductive capacity
The toxicity testing identified a dose of AFN that could be used to test the consequences of an altered developmental environment on adult health. We chose 0.5 µM AFN as a dose that would only cause minor disturbances in redox homeostasis. The low 0.5 µM dose of AFN did not affect survival by 5 dpf (Fig. 1), yet it was capable of inducing low grade oxidative stress-related phenotypes such as jaw malformation (Fig. 2C). Furthermore, assays of apoptosis (Fig. S2) and the antioxidant stress response (Fig. 3) showed a trend consistent with a loss of redox homeostasis without the excessive cellular damage observed at higher doses (Fig. S1).
Two sequential cohorts of embryos were exposed to either 0.5 µM AFN or the equivalent 0.005% v/v DMSO as a solvent control, between 6 hpf and 1 dpf, and raised to adulthood. Zebrafish produce many offspring that have a low probability of survival, this was evident in the solvent arm of the cohorts. By 3 months post-fertilization (mpf), only 31±6% (cohort 1) and 47±7% (cohort 2) of the eggs exposed to the solvent control had survived to adulthood. However, there was a trend toward lower long-term survival in the AFN treatment arms of each cohort, suggesting that the transient exposure to AFN had a long-term effect on animal viability. Survival at the same time in the treatment arms was 21±5% and 31±2% for cohorts 1 and 2, respectively. The low background survival meant that from 50 embryos there was on average 13 (cohort 1) and 15 (cohort 2) fish remaining at 3 mpf in tanks of the solvent arm and 10 (cohort 1) and 12 (cohort 2) fish in the tanks of the treatment arm.
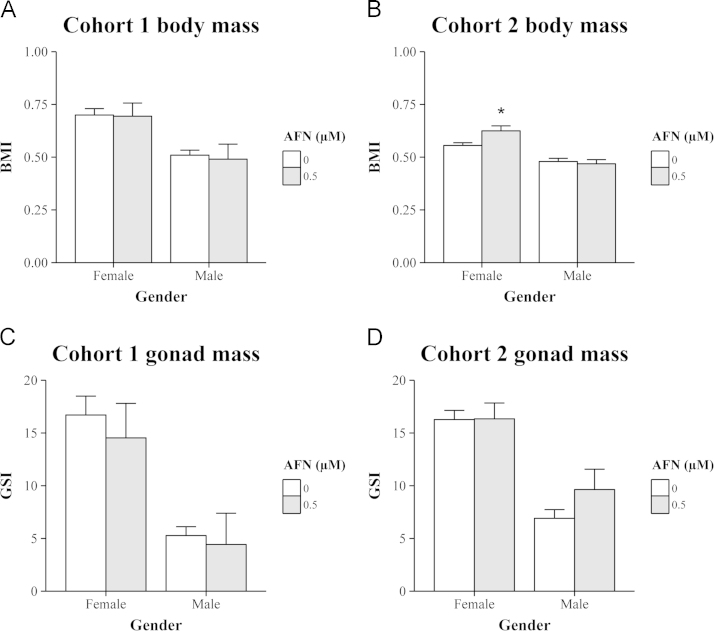
After the fertility measurements (below) were made, a necropsy was performed where phenotypic parameters were measured. At this time, fish from the first cohort were 22 months old while those from the second cohort were 10 months old. Consistent between the cohorts was a marked sexual dimorphism in both the body mass index (BMI) (Fig. 4A and B) and the proportion of weight dedicated to the reproductive tissues (Fig. 4C and D). In comparing the two cohorts, the older fish of cohort 1 had both a higher BMI and a higher degree of sexual divergence in BMI (Fig. 4A and B), consistent with weight tracking with age. However, in comparing the solvent and treatment arm there were few notable differences. One exception might be the significantly higher BMI measured for the young females of cohort 2, this was 0.63±0.03 in the treatment arm and 0.56±0.01 in the solvent arm (p=0.01, two-way ANOVA with Sidak's multiple comparisons).
Fig. 4.
Transient exposure to a sublethal dose of AFN during early life did not affect the adult phenotype. (A, B) The body mass index (BMI) is given for fish from (A) cohort 1 and (B) cohort 2. (C, D) The gonadosomatic index (GSI) is also given for fish from (C) cohort 1 and (D) cohort 2. Values represent the mean±SEM for (A, C) solvent: 17 females and 14 males; AFN: 6 females and 3 males; (B, D) solvent: 22 females and 21 males; AFN: 17 females and 11 males.
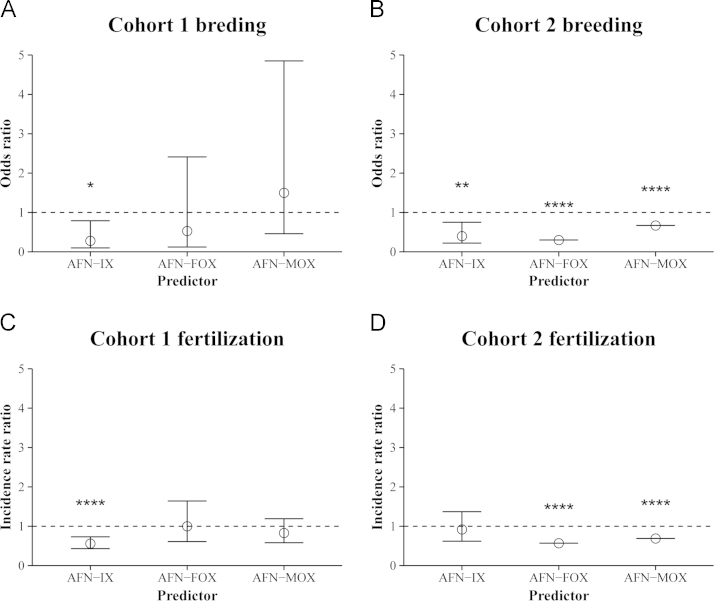
Despite being outwardly normal in their appearance relative to the solvent control, the fish that had been transiently exposed to AFN as embryos showed reduced fertility. Crossing the fish with single partners from within each tank revealed that pairs of fish from the AFN treatment arm were less likely to produce any eggs (Fig. 5A and B). For cohort 1, it was found that the odds of an incross resulting in successful breeding were 72% lower in the 0.5 µM AFN treatment arm (OR=0.28, 95% CI: 0.10–0.79, p=0.02). Similarly, for cohort 2, the odds of breeding success were 60% lower in the 0.5 µM AFN treatment arm (OR=0.40, 95% CI: 0.22–0.75, p=0.004).
Fig. 5.
Reproductive toxicity of AFN in adulthood following transient sublethal exposure during embryogenesis. (A, B) The breeding success and (C, D) egg fertilization rate is given for fish from (A, C) cohort 1 and (B, D) cohort 2, respectfully. Using a nested design, the effect of AFN exposure was compared to the solvent control within incrosses of the treated fish (IX), and outcrosses of treated females (FOX) and treated males (MOX) to untreated fish. Values represent the odds ratio (A, B) or incidence rate ratio (C, D) from an average of three pairs from each of three tanks in three spawning experiments. Note, only one tank was used in the outcrosses of cohort 2. Robust standard errors were calculated with respect to the tank clusters and are presented as a ±95% CI. Statistically significant differences (see Supplementary information for R script) are noted as *p≤0.05, **p≤0.01, and ****p≤0.0001.
Counting the number of fertilized eggs among the clutch of each pair revealed a reduced rate of egg fertilization in the AFN treatment arm of both cohorts (Fig. 5C and D). A significant reduction in the fertilization rate was observed in cohort 1 where fertilization was 44% lower than in the solvent arm (IRR=0.56, 95% CI: 0.43–0.73, p<0.0001). A small decrease was also apparent in cohort 2 where the fertilization rate was 8% lower in the 0.5 µM AFN treatment arm. Both cohorts showed decreased breeding success and egg fertilization in the AFN treatment arm; the larger effect observed in cohort 1 suggest that AFN-induced infertility is enhanced with increasing age.
Outcrosses of the experimental fish from the treatment arms to untreated wild type fish revealed sex-specific contributions to the reproductive toxicity of AFN. Crosses between females that had been exposed to AFN as embryos and untreated male partners trended towards having reduced breeding success with odds 47% (Fig. 5A) and 70% (Fig. 5B) lower than in the solvent controls for both cohort 1 and 2, respectively. In contrast, males out-crossed to untreated female partners did not show a consistent trend in breeding success, but did show a trend in fertilization rates which were 17% (Fig. 5C) and 31% (Fig. 5D) lower than in the solvent controls for both cohort 1 and 2, respectively.
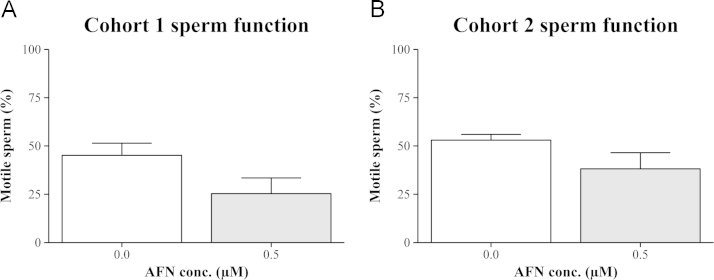
The males from the AFN treatment arms showed a trend towards reduced sperm motility (Fig. 6). In cohort 1, 45±6% of sperm cells from control males was scored as progressively motile, this was reduced to 25±8% in the semen of the males that had been exposed to AFN as embryos (p=0.06, one-tailed t-test). Similarly, in cohort 2, 53±3% of sperm from control males was progressively motile, whereas 38±8% of sperm from AFN-exposed males was (p=0.09, one-tailed t-test). It was noted that of those sperm that were motile there was no effect of AFN treatment on their speed (Supplementary video).
Fig. 6.
Exposure to AFN during embryogenesis reduces sperm motility in adult males. Shown is the percent of sperm that were progressively motile in semen samples from males in (A) cohort 1 and (B) cohort 2. Values represent the mean±SEM of 3 males from each treatment group.
Supplementary material related to this article can be found online at doi:10.1016/j.redox.2015.10.010.
The following is the Supplementary material related to this article Video S1.
Transient exposure to a low dose of AFN during embryogenesis reduces sperm motility in adult males. Representative videos of sperm after activation from a male in the solvent control arm (left) and a male in the 0.5 µM AFN arm (right).
4. Discussion
Transient disruption of embryonic redox homeostasis by AFN reduced the reproductive fitness of adult zebrafish without disrupting the gross physical traits of the fish. Based on embryonic toxicity testing, AFN doses were categorized as high (5 µM), moderate (1 µM), and low (0.5 µM). The low, 0.5 µM, dose of AFN was one that dependably elicited subtle signs of stress without overt toxicity. Following transient embryonic exposure (6–24 hpf) to this low grade stress, the fish in the AFN treatment arm were not visibly affected. But upon assessment of fertility, statistically significant differences to the solvent control were noted. The AFN treatment arm consistently showed impaired reproductive function in terms of breeding success, egg fertilization, and sperm motility over two experimental cohorts. From this evidence we conclude that the exposure to an abnormal early life environment had long-term consequences for reproductive health.
Several retrospective epidemiological studies have shown that a poor early life environment can have later life consequences, such as exposure to famine in utero leading to metabolic syndrome in adulthood [27]. Some theoretical frameworks have emerged in order to understand the epidemiological observations. The ‘silver spoon' hypothesis suggests exposure to a favorable early life environment provides a general fitness advantage while other theories, such as the ‘predictive adaptive response', propose an interaction between the developmental and adult environments [24]. Our study saw a reduction in fitness following an abnormal early life environment but did not test for any fitness advantage in a poor adult environment.
Successful fertilization could be hampered by damage to the germline, changes in gamete maturation, and changes in gamete structure and function [6]. There is a strong precedence for oxidative stress influencing the reproductive biology of males [2] and females [1]. In this study, developing germ cells were only exposed to the oxidative stress transiently, during the embryonic period when primordial germ cells migrate to gonad [28]. DNA damage in the germline, such as that which occurs during ageing, is known to impact fertility and increase the burden of disease in the offspring [2]. While the delayed reproductive toxicity of AFN could be caused by germline damage, the dose of AFN was very low, so other changes in the germline, as well as somatic/hormonal influences, should also be considered.
AFN has a history of use in the clinic for the control of rheumatoid arthritis, with the oral availability more preferable than other auro-/chryso-therapeutic anti-arthritic gold salts that required intramuscular injection. The doses of AFN used in this study are comparable to those found in a trial in children, aged between 1½ and 17 years, treated for juvenile rheumatoid arthritis where the average steady state concentration measured in the blood was 0.5 µg/mL (0.7 µM) [14]. The prescribing rate of AFN declined through the 1990s, a notable clinical side effect of AFN was diarrhea, observed in approximately 40% of patients, and other disease modifying anti-arthritic drugs, most notably methotrexate and sulfasalazine, became available [15].
Although AFN was prescribed to adults and considered safe, human fetuses could be exposed via the placenta, and exposure after birth could also occur through the milk [19]. While a small number of case studies with limited sample sizes found no increase in congenital malformations, there is one concerning case report [31]. The otherwise healthy mother was receiving gold therapy for arthritis throughout the first and second trimester of pregnancy and her baby died on the fifth day with an occipital encephalocele and a notable left sided cleft lip and palate [31]. Gold-containing compounds have been assessed in pregnant rats and rabbits where they were observed to produce abnormalities of the head, face, brain, heart, abdomen, and skeleton [34], [35].
One explanation for the abnormalities observed in exposed embryos could be an effect on the neural crest. The crest is a developmental population of cells that contributes to the craniofacial cartilage and bone, the enteric nervous system, the heart, and the pigmentation [16]. The neural crest has been found to be vulnerable to oxidative stress-induced apoptosis following in utero ethanol exposure: a risk factor for mental retardation and cleft palate [7]. Migration of the neural crest has also been shown to be disrupted by oxidative stress as exogenous H2O2 and glucose have been found to result in malformation of the enteric nervous system and cardiac outflow tract, respectively [30], [4].
All of the AFN doses examined produced correlating levels of craniofacial dysmorphology, as evidenced by abnormal positioning of sox10-GFP-positive cells (Fig. 2 C and D); the expression of sox10 denoting that these cells are of neural crest origin. While the AFN-exposed embryos produced an antioxidant stress response, as determined by transcription of stress response genes (Fig. 3), this was evidently not sufficient to prevent the acute developmental abnormalities. Intriguingly, it has been shown that ethanol-induced apoptosis in neural crest cells can be rescued by Nrf2 over-expression [7], or induction with sulforaphane [8], and glucose-induced congenital heart defects can be limited by the antioxidant N-acetylcysteine [30]. Correcting the redox imbalance in the neural crest then might represent a new therapeutic approach for the amelioration of environmental insults.
Funding
This work was supported by the Dunedin School of Medicine Research Development Investment Award (JAH, MH); Maurice Wilkins Centre Flexible Research Seeding Programme (JAH, MH); Maurice and Phyllis Paykel Trust (JAH, MH); University of Otago Doctoral Scholarship (TN); Gravida National Centre for Growth and Development (JAH, MH).
Acknowledgments
The authors would like to thank Noel Jhinku for zebrafish management and Sheri Johnson for technical assistance regarding zebrafish sperm analysis. We would also like to thank Andrew Grey for support with statistical analysis.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2015.10.010.
Appendix A. Supplementary material
Supplementary material
References
- 1.Agarwal A., Aponte-Mellado A., Premkumar B.J., Shaman A., Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitken R.J., Baker M.A. Oxidative stress and male reproductive biology. Reprod. Fertil. Dev. 2004;16:581–588. doi: 10.10371/RD03089. [DOI] [PubMed] [Google Scholar]
- 3.Baker T.R., Peterson R.E., Heideman W. Using zebrafish as a model system for studying the transgenerational effects of dioxin. Toxicol. Sci. 2014;138:403–411. doi: 10.1093/toxsci/kfu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow A.J., Dixon J., Dixon M., Trainor P.A. Balancing neural crest cell intrinsic processes with those of the microenvironment in Tcof1 haploinsufficient mice enables complete enteric nervous system formation. Hum. Mol. Genet. 2012;21:1782–1793. doi: 10.1093/hmg/ddr611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berners-Price S., Filipovska A. Gold compounds as therapeutic agents for human diseases. Metallomics. 2011;3:863–873. doi: 10.1039/c1mt00062d. [DOI] [PubMed] [Google Scholar]
- 6.Bobe J., Labbé C. Egg and sperm quality in fish. Gen. Comp. Endocrinol. 2010;165:535–548. doi: 10.1016/j.ygcen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Chen X., Liu J., Chen S. Over-expression of Nrf2 diminishes ethanol-induced oxidative stress and apoptosis in neural crest cells by inducing an antioxidant response. Reprod. Toxicol. 2013;42:102–109. doi: 10.1016/j.reprotox.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Liu J., Chen S. Sulforaphane protects against ethanol induced oxidative stress and apoptosis in neural crest cells by the induction of Nrf2 mediated antioxidant response. Br. J. Pharmacol. 2013;169:437–448. doi: 10.1111/bph.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox A.G., Brown K.K., Arner E.S.J., Hampton M.B. The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem. Pharmacol. 2008;76:1097–1109. doi: 10.1016/j.bcp.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Dennery P.A. Oxidative stress in development: nature or nurture? Free Radic. Biol. Med. 2010;49:1147–1151. doi: 10.1016/j.freeradbiomed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson B.C., Chang C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011;7:504–510. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drechsel D.A., Patel M. Respiration-dependent H2O2 removal in brain mitochondria via the thioredoxin/peroxiredoxin system. J. Biol. Chem. 2010;285:27850–27858. doi: 10.1074/jbc.M110.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkel T. Signal transdution by reactive oxygen species. J. Cell. Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannini E., Brewer E., Person D. Blood gold concentrations in children with juvenile rheumatoid arthritis undergoing long-term oral gold therapy. Ann. Rheum. Dis. 1984;43:228–231. doi: 10.1136/ard.43.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grove M., Hassell A., Hay E., Shadforth M. Adverse reactions to disease-modifying anti-rheumatic drugs in clinical practice. QJM. 2001;94:309–319. doi: 10.1093/qjmed/94.6.309. [DOI] [PubMed] [Google Scholar]
- 16.Halloran M.C., Berndt J.D. Current progress in neural crest cell motility and migration and future prospects for the zebrafish model system. Dev. Dyn. 2003;228:497–513. doi: 10.1002/dvdy.10374. [DOI] [PubMed] [Google Scholar]
- 17.Hayes J., Dinkova-Kostova A. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman T.L., Javier A.L., Campeau S.A., Knight R.D., Schilling T.F. Tfap2 transcription factors in zebrafish neural crest development and ectodermal evolution. J. Exp. Zool. B Mol. Dev. Evol. 2007;308:679–691. doi: 10.1002/jez.b.21189. [DOI] [PubMed] [Google Scholar]
- 19.Kean W., Hart L., Buchanan W. Auranofin. Rheumatology. 1997;36:560–572. doi: 10.1093/rheumatology/36.5.560. [DOI] [PubMed] [Google Scholar]
- 20.Kim N.-H., Oh M.-K., Park H.J., Kim I.-S. Auranofin, a gold(I)-containing antirheumatic compound, activates Keap1/Nrf2 signaling via Rac1/iNOS signal and mitogen-activated protein kinase activation. J. Pharmacol. Sci. 2010;113:246. doi: 10.1254/jphs.09330fp. [DOI] [PubMed] [Google Scholar]
- 21.Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krøvel A.V., Olsen L.C. Expression of a vas:: EGFP transgene in primordial germ cells of the zebrafish. Mech. Dev. 2002;116:141–150. doi: 10.1016/s0925-4773(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 24.Monaghan P. Early growth conditions, phenotypic development and environmental change. Philos. Trans. R. Soc. B Biol. Sci. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips J.B., Westerfield M. Zebrafish models in translational research: tipping the scales toward advancements in human health. Dis. Model. Mech. 2014;7:739–743. doi: 10.1242/dmm.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2012. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 27.Ravelli A.C.J., van der Meulen J.H.P., Michels R.P.J., Osmond C., Barker D.J.P., Hales C.N., Bleker O.P. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 28.Raz E. Primordial germ-cell development: the zebrafish perspective. Nat. Rev. Genet. 2003;4:690–700. doi: 10.1038/nrg1154. [DOI] [PubMed] [Google Scholar]
- 29.Rigobello M.P., Folda A., Baldoin M.C., Scutari G., Bindoli A. Effect of Auronofin on the mitochondrial generation of hydrogen peroxide. Role of thioredoxin reductase. Free Radic. Res. 2005;39:687–695. doi: 10.1080/10715760500135391. [DOI] [PubMed] [Google Scholar]
- 30.Roest P.A.M., van Iperen L., Vis S., Wisse L.J., Poelmann R.E., SteegersTheunissen R.P.M., Molin D.G.M., Eriksson U.J., Groot G., Adriana C. Exposure of neural crest cells to elevated glucose leads to congenital heart defects, an effect that can be prevented by N-acetylcysteine. Birth Defects Res. A Clin. Mol. Teratol. 2007;79:231–235. doi: 10.1002/bdra.20341. [DOI] [PubMed] [Google Scholar]
- 31.Rogers J.G., Anderson R.M., Chow C.W., Gillam G.L., Markman L. Possible teratogenic effects of gold. Aust. Paediatr. J. 1980;16:194–195. doi: 10.1111/j.1440-1754.1980.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 32.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholz S., Fischer S., Gündel U. The zebrafish embryo model in environmental risk assessment—applications beyond acute toxicity testing. Environ. Sci. Pollut. Res. 2008;15:394–404. doi: 10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- 34.Szabo K., DiFebbo M., Phelan D. The effects of gold-containing compounds on pregnant rabbits and their fetuses. Vet. Pathol. 1978;15:97–102. doi: 10.1177/0300985878015s0509. [DOI] [PubMed] [Google Scholar]
- 35.Szabo K., Guerriero F., Kang Y. The effects of gold-containing compounds on pregnant rats and their fetuses. Vet. Pathol. 1978;15:89–96. doi: 10.1177/0300985878015s0508. [DOI] [PubMed] [Google Scholar]
- 36.Timme-Laragy A.R., Goldstone J.V., Imhoff B.R., Stegeman J.J., Hahn M.E., Hansen J.M. Glutathione redox dynamics and expression of glutathione-related genes in the developing embryo. Free Radic. Biol. Med. 2013;65:89–101. doi: 10.1016/j.freeradbiomed.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traver D., Paw B., Poss K., Penberthy W. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 38.Wakabayashi N., Slocum S. When NRF2 talks, who׳s listening? Antioxid. Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerfield M. University of Oregon Press; Eugene: 2000. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transient exposure to a low dose of AFN during embryogenesis reduces sperm motility in adult males. Representative videos of sperm after activation from a male in the solvent control arm (left) and a male in the 0.5 µM AFN arm (right).
Supplementary material