Abstract
Introduction:
Spreading depression (SD) is a propagating wave of depolarization followed by depression of the neuroglial activities and can modulate extracellular dopamine concentrations in the neocortex. It has been shown that the dopaminergic system plays a role in migraine. SD has been suggested as a critical phenomenon in the pathophysiology of migraine. The aim of this study was to investigate the effect of dopamine D2 receptors on the characteristic features of SD in rat neocortical tissues.
Methods:
The effect of dopamine D2 receptor agonist quinpirole and D2 receptor antagonist sulpiride was tested on different characteristic features (amplitude, duration and velocity) of KCl-induced SD in somatosensory neocortical slices of adult rats. The effect of above-mentioned substances on production of long-term potentiation (LTP) in the neocortex was also evaluated.
Results:
The present data revealed a dose-dependent suppression of the amplitude and duration of SD in the presence of the dopamine D2 receptor antagonist sulpiride in the neocortex. D2 dopamine receptor agonist quinpirole dose-dependently enhanced the amplitude and duration of the neocortical SD. Furthermore, application of D2 receptor antagonist significantly suppressed induction of LTP.
Discussion:
These results indicate that D2 receptors modulate the initiation of SD in the neocortex. This finding refers to the potential role of D2 receptor antagonist in treatment of migraine pain.
Keywords: Spreading depolarization, Migraine, Stroke, Synapse, In vitro
1. Introduction
Spreading depression (SD) is a self-propagating depolarization wave fallowed by depression of the bioelectrical activity of the neurons and glia for a period of minutes (Somjen, 2001). SD is involved in different clinical neurological disorders, such as migraine, cerebrovascular diseases (including brain hemorrhage, subarachnoid hemorrhage and head injury), and epilepsy (Gorji, 2001; Bartholini et al., 1997). Processes similar to SD in animal cortex take place in numerous pathological conditions in humans. Direct alterations of electrical activity of cortical neurons by the locally spreading wave can lead to clinical symptoms (Dreier et al., 2010; Eickhoff et al., 2013). SD can also change the neurochemistry as well as synaptic plasticity of subcortical and cortical structures and modulate oxygen redistribution as well as neuronal survival (Gorji et al., 2001; Jafarian et al., 2010) in different animal models of SD (Eikermann-Haerter & Moskowitz, 2008).
Dopamine is a crucial neurotransmitter in the mammalian brain and has different functions, such as regulatory effects on behavior and cognition, motor activity, motivation, prolactin production and secretion, sleep, attention, mood as well as learning and memory. Dopaminergic neurons are primarily found in the ventral tegmental area, the substantianigra and the hypothalamus. Dopamine activates five subtypes of dopamine receptors (D1–D5; Stanwood, 2008). The distributions of the transcripts encoding the five dopamine sub-receptors have been characterized in the striatum and selected regions of the neocortex in human brain. In the prefrontal and temporal neocortices expression of D1 and D4 receptor, mRNAs are higher than other subtypes whereas in the occipital neocortex, D1 receptor mRNA is the most abundant sub-type (Meador-Woodruff et al., 1996).
It has been shown that change of neuronal activities induced by SD, changed modulated extracellular dopamine in the prefrontal cortex (Taber and Fibiger, 1995; Karreman and Moghaddam, 1996). During SD, the sharp increase of potassium concentration is accompanied by a dopamine release in the striatum (Moghaddam et al., 1987). In keeping with neuronal depression by SD, blockade of the prefrontal cortex activity by tetrodotoxin (Karreman and Moghaddam, 1996) or by local anesthetics (Murase et al., 1993) decreased basal dopamine values in the nucleus accumbens septi. The mechanisms by which SD modulates the dopaminergic synaptic network in the striatum are unknown. There is evidence that cortex can enhance dopamine release in striatum via activation of glutamatergic neurotransmission (Cheramy et al., 1990). Another study indicates an elevation of evoked dopamine release in the nucleus accumbens and a decrease in the nucleus caudatus resulting from depression of the cortical activity induced by SD. These findings suggest that in the nucleus caudatus dopaminergic presynaptic terminals are under cortical tonic activating control (Seghatoleslam et al., 2014). Therefore, SD in the cortex may modulate neurotransmitter release in subcortical structures. In spite of these investigations, the exact role of dopamine in initiation and propagation of neocortical SD still needed to be elucidated.
The dopaminergic system has been explored for a potential role in different neurological diseases. Several dopaminergic candidate genes have been studied in several migraine case control cohorts (for example see; Del Zompo et al., 1998). There is dopamine receptor hypersensitivity in patients with migraine (Gorji, 2001). In a large subgroup of patients, dopamine acts as an endogenous protagonist in the pathophysiology of migraine. Antagonism of this protagonist neurotransmitter, therefore, results in symptomatic relief of both the headache and associated symptoms (Peroutka, 1997). Prochlorperazine and Domperidone, D2 receptor antagonists, have a high degree of efficacy in treatment of migraine cephalalgia (Marmura, 2012; Kanis and Timm, 2014). The purpose of this study was to investigate the effect of D2 receptors on the characteristic features of cortical SD. Therefore, we investigated the effects of modulation of both D1 and D2 receptors on SD in rat neocortical tissues.
2. Methods
The experiments were performed on adult rat (250–350g) somatosensory cortical slices. The brain was removed under deep methohexital anesthesia and placed in cold (1–4°C) artificial cerebrospinal fluid (ACSF) preequilibrated with 5% CO2 in O2 to give a pH of 7.4. The ACSF contained (in mM): NaCl 124, KCl 4, CaCl2 1.0, NaH2PO4 1.24, MgSO4 1.3, NaHCO3 26 and glucose 10. The somatosensory neocortices were dissected and cut into slices of 500 μm thickness. The slices were incubated in ACSF solution for more than 1 h at 28°C. After 30 min incubation, CaCl2 was increased to 2.0 mM. Slices were transferred to an interphase chamber and superfused with ACSF at 32°C (1.5–2 ml/min). The investigations were approved by the local ethics committee in Münster University. Extracellular field potentials were recorded with glass micropipettes (150 mmol/l NaCl; 2–10 MΩ) connected to an amplifier by an Ag/AgCl–KCl bridge in the third and fifth layers of the neocortical tissues. Field potentials were traced by an ink-writer and recorded by a digital oscilloscope.
2.1. Induction of Neocortical SD
SD was elicited by KCl microinjection. A glass electrode filled with 2 M KCl was fixed in a special holder connected with plastic tube to a pressure injector and the tip inserted into the sixth layer of the neocortical slices. A high-pressure pulse was applied to inject an amount of K+ in the tissue sufficient to induce a cortical SD (tip diameter: 2 microm; injection pressure 0.5–1.0 bar applied for 200–300 ms, two separate injections, 1–3 nl per pulse). Neocortical SD-like deflection was evaluated regarding its amplitude, duration and velocity. SD duration was defined as the interval between the time of half-maximal voltage shift during onset and recovery of the negative DC potential deflection.
2.2. Long-term Potentiation (LTP)
Single pulses of electrical stimulation were applied through a bipolar platinum electrode attached to the white matter perpendicular to the recording electrodes. Evoked field excitatory postsynaptic potentials (fEPSP) were recorded in the third layer of neocortical slices. The fEPSP was elicited by adjusting the intensity of stimulation to ∼50% of that at which population spikes after fEPSP began to appear. The amplitude of fEPSP 1 ms after the onset was measured for data analysis. In long-term potentiation experiments, the cortex was sequentially stimulated once every minute. Ten trains of four pulses (pulse duration 0.1 msec; interpulse interval 50 msec; intensity 5 V) were repeated at intervals of 10 msec. LTP was defined as the mean change in fEPSP amplitude in response to five stimuli given 30 min after tetanic stimulation compared with the mean response to five test pulses applied immediately before the stimulation. Tetanic stimulation was applied 60 min after application of different dopamine modulators.
2.3. Experimental Protocols
The experimental protocol consisted of four periods as follows:
(i) control period, neocortical slices were superfused with ACSF (30 min), tested for spontaneous SD; (ii) KCl injection, induction of SD (SD1); (iii) application of D2 dopamine receptor agonist quinpirole (10-200 micromol/l) or D2 dopamine receptor antagonist sulpiride (0.1–10 micromol/l, 60 min) before the second injection of KCl (SD2); (iv) washout of quinpirole or sulpiride with ASCF (45 min, second control period); and third injection of KCl (SD3). Only a single concentration of quinpirole or sulpiride was used in a given slice. In control experiments, DMSO (0.5%) was added to the bath solution after the first KCl injection (60 min) and washed with ASCF (45 min) after the second and before the third KCl application.
2.4. Drugs and Statistical Analysis
Sulpiride and quinpirole were purchased from Sigma-Aldrich, Germany. The data were statistically analyzed using the Mann–Whitney Rank Sum test. Multiple comparisons were performed by analysis of variance test (ANOVA) for repeated measures followed by a Holm-Sidak’s test. The probability values less than 0.05 were considered as significant values. All results are given as mean ± SEM.
3. Results
3.1. The Effect of D2 Dopamine Receptor Agonist on SD
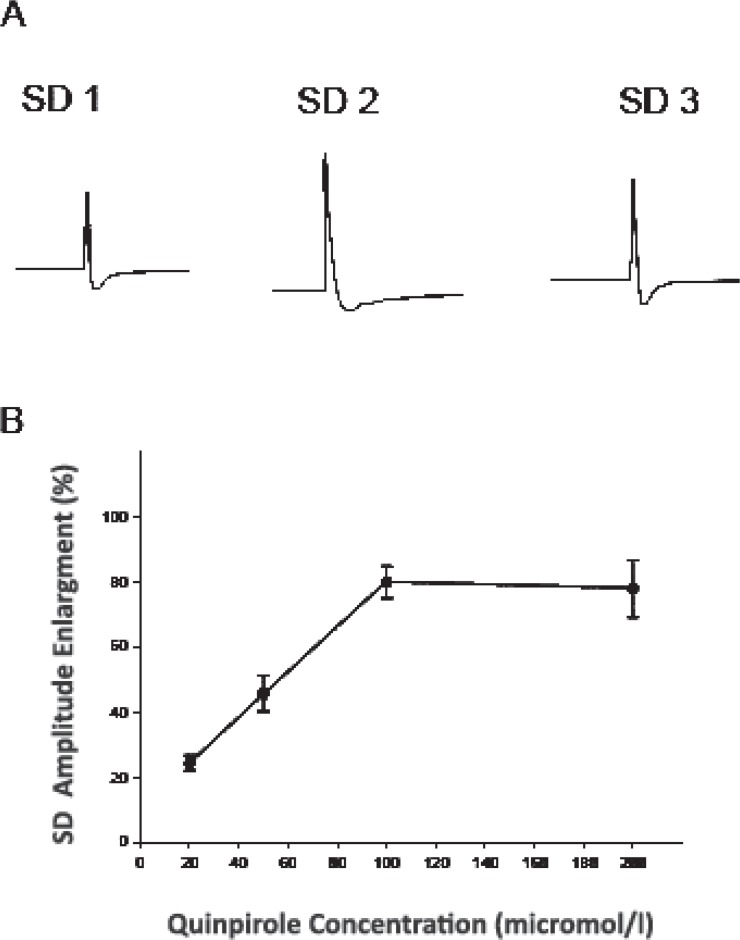
Local microinjection of KCl in the sixth layer of neocortical tissues induced negative DC waves followed by positive fluctuations (amplitude of 15.6±1.9 mV; duration of 113±5 sec). Negative DC-fluctuations were sometimes preceded by small positive waves. These cortical SD waves propagated opposite to the direction of the ACSF flow at propagation velocity of 3.1±0.1 mm / min. The effect of five different concentrations of D2 dopamine receptor agonist quinpirole (10, 20, 50, 100, 200 micro mol/l; n: 6 for each concentration) was investigated on KCl-induced negative depolarization shifts in the neocortical tissues. The ratio between the second and the first DC potential waves (SD2/SD1) was calculated in control slices and slices treated with quinpirole. Sixty minutes of quinpirole application at 10 μM did not significantly change different characteristics of SD, i.e. amplitude, duration, and propagation velocity. Quinpirole at higher concentrations, dose-dependently increased the amplitude and the duration of negative potential deflections appeared after the second KCl application (SD2). The amplitude as well as the duration of SD2 and the SD2/SD1 ratio significantly increased after application of quinpirole at concentration of 20–200 μM (Figure 1; P≤0.001; ANOVA test, F[4,100]=21.54). Quinpirole enhanced the SD amplitude between 23 ± 4 to 80±7 % and prolonged the duration between 18±5 to 59±5% of the baseline levels. Quinpirole at all different concentrations did not change the velocity of negative DC wave. After washout of the drug, the amplitude, duration, and velocity of the propagation of SD-like events (SD3) returned close to the initial levels (SD1; Figure 1).
Figure 1.
Effects of quinpirole on spreading depression (SD) in the rat somatosensory neocortical tissues. A: Recording of negative DC potential deflections in the third layer of a neocortical slice before (SD1), during (SD2), and after (SD3) superfusion of quinpirole (50 μM). Field potentials were recorded by an ink-writer. SD was elicited by KCl microinjection. B: The curve indicates the plot of percentage enlargement of SD amplitude vs. quinpirole concentrations (n: 6 for each concentration). Quinpirole dose-dependently enhanced the amplitude of SD in the neocortex. The percentage of SD amplitude enlargement was measured by division of the amplitude of SD induced after application of quinpirole to the amplitude of SD elicited before superfusion of the compound. Values represent mean ± SEM.
3.2. The Effect of D2 Dopamine Receptor Antagonist on SD
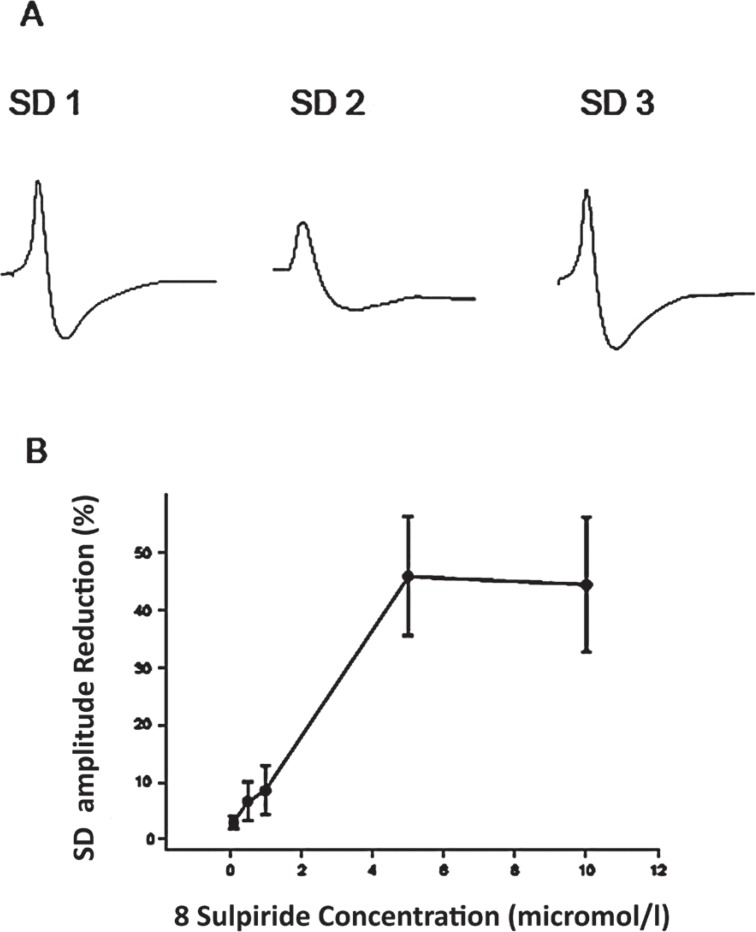
Sulpiride at 1–10 μM dose-dependently reduced the amplitude of negative DC potentials appeared after the second KCl microinjection (SD2; Figure 2; P≤0.001; ANOVA test, F [2, 42] = 48.36). Sulpiride at 0.1 μM did not affect SD significantly. Superfusion of sulpiride for 60 minutes at higher concentrations (0.5, 1, 5 and 10 micromol/l) decreased the SD amplitude to 38 ± 5% of the initial levels (SD2/SD1 ratio). Sulpiride at these concentrations also significantly and dose-dependently reduced the mean duration of SD to 48 ± 6% of the baseline values. Sulpiride at all different concentration did not alter the velocity of SD-like wave propagation. After washout of the compound, the amplitude of the negative deflection of DC potentials (SD3) returned close to the initial levels (SD1; Figure 2).
Figure 2.
Effects of sulpiride on spreading depression (SD) in the rat somatosensory neocortical slices. A: Recording of negative DC potential shifts in the third layer of a neocortical slice before (SD1), during (SD2), and after (SD3) application of sulpiride (5 μM). Field potentials were recorded by an ink-writer. SD was elicited by KCl application. B: The curve indicates the plot of percentage reduction of SD amplitude vs. sulpiride concentrations (n: 6 for each concentration). Sulpiride dose-dependently decreased the amplitude of SD. The percentage of SD amplitude reduction was measured by division of the amplitude of SD induced after application of sulpiride to the amplitude of SD induced before application of the drug. Values represent mean ± SEM.
3.3. The Effect of Quinpirole and Sulpiride on Synaptic Plasticity
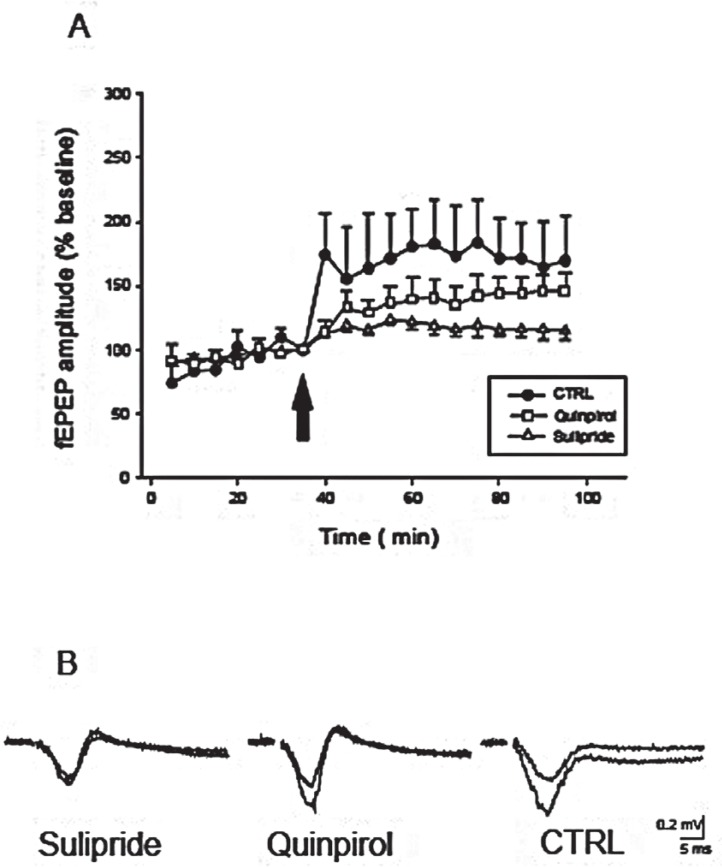
A conditioning tetanic stimulation was delivered to the white substance of the neocortical slices followed by pulses with stimulation parameters identical to control values. The evoked fEPSP was stable for at least 30 min before application of tetanic stimulation (less than 10% variation; Figure 3). Application of tetanic stimulation produced a rapid and stable enhancement of the amplitude of the fEPSP in all tested preparations (n: 6, 164±12% control; Figure 3). LTP lasted as long as the fEPSP were recorded (at least for 90 min). The potentiation rose within 1–2 min and stabilized within five minutes after the train of stimulations. Application of sulpiride (5 micromol/l; n: 10) sixty min before tetanic stimulation significantly suppressed LTP induction in all tested slices (122±3% baseline, P≤0.001, Figure 3). However, application of quinpirole (50 micromol/l; n: 10) sixty minutes before tetanic stimulation did not significantly alter the LTP compared to control slices (147±6% baseline, Mann–Whitney Rank Sum test; P=0.08, Figure 3).
Figure 3.
The effect of D2 dopamine receptor- agonist quinpirole and antagonist sulpiride on long-term potentiation (LTP) of the evoked field excitatory postsynaptic potentials (fEPSP) in the rat neocortical preparations. A: Tetanic stimulation. (Ten trains of four pulses; pulse duration 0.1 msec; interpulse interval 50 msec; intensity 5 V) produces a rapid and stable potentiation in the amplitude of the evoked field potentials, calculated as a percentage of baseline mean response amplitude. Open triangles, open square and closed circles show fEPSP after application of sulpiride (5 μmol/l), quinpirole (50 μmol/l), and ACSF (artificial cerebrospinal fluid; control), respectively. Arrow shows the time of tetanic stimulation, 60 min after application of substances. Application of sulpiride significantly inhibited LTP of the evoked field potentials, calculated as a percentage of baseline mean response amplitude. B: Representative samples of fEPSP before and after tetanic stimulation in sulpiride, quinpirole, and ACSF (control) slices.
4. Discussion
The present data revealed a dose-dependent suppression and enhancement of the amplitude and duration of the neocortical SD in the presence of the D2 dopamine receptor antagonist sulpiride and D2 dopamine receptor agonist quinpirole, respectively. The data point to the involvement of D2 dopamine receptor in the initiation of SD in the somatosensory neocortex. In addition, administration of D2 receptor antagonist significantly suppressed synaptic plasticity, whereas D2 receptor agonist did not change the induction of LTP. This indicates the modulatory effect of D2 dopamine receptor in the somatosensory neocortical synaptic transmission.
Dysfunction of dopaminergic system plays an important role in several neurological and psychiatric disorders, such as schizophrenia, Parkinson’s disease, attention-deficit and hyperactivity disorders as well as drug addiction (Biederman and Faraone 2005; Kalivas et al., 2005). The receptor of D2 Dopamine has been one of the most extensively investigated receptor in neurological as well as psychological disorders. A higher D2 allelic frequency and prevalence was reported in alcoholics when compared to controls (Gelernter and Kranzle, 1999). The D2 gene has been implicated in schizophrenia, posttraumatic stress disorder, movement disorders and migraine (Noble, 2003). It has been suggested that activation of dopaminergic system is a primary pathophysiologic component in certain forms of migraine (Peroutka, 1997). The NcoI D2 C to T polymorphism located in exon 6 was assessed in individuals having migraine with and without aura (Peroutka, 1997; Peroutka et al., 1998). Patients having migraine with aura had a higher frequency of the D2C allele than healthy individuals as well as patients suffering from migraine without aura. The dinucleotide repeat alleles within intron 2 of the D2 gene were tested in patients affected by migraine without aura (Del Zompo et al., 1998).
SD is believed to play a crucial role in migraine with aura (Leão and Morison, 1945; Welch et al., 1993). SD-like waves were recorded from human neocortex during the aura phase of migraine attacks as well as human brain tissue resected during epilepsy surgery (Gorji et al., 2001; Hadjikhani et al., 2001). Furthermore, increasing evidence suggesting the intense perturbations generate the cellular, molecular, and vascular changes in brain akin to SD could cause the headaches of aura-induced migraine (Moskowitz et al., 1993; Bolay et al.,2002; Gorji et al., 2004; Seghatoleslam et al., 2014). It has been suggested that the unbalanced levels of dopamine in the brain may activate the trigeminal system, induce the inflammatory sensitization of trigeminal neuron and lead to the migraine attack (D’Andrea and Leon, 2010). In the present study, sulpiride suppressed the amplitude and the duration of SD. According to our results, sulpiride is an effective drug in treatment of headache in migraine attacks (Siniachkin et al., 1997; Nitsche et al., 2006). Our data suggest that blocking effects of D2 dopamine receptor sulpiride on SD may be the mechanism for its efficacy in migraine headache. Several other anti-migraine substances, such as topiramate, valproate, propranolol, amitriptyline and methysergide, have inhibitory actions on the cortical SD (Ayata et al., 2006).
LTP is an experimental phenomenon, which can be used to demonstrate the repertoire of long-lasting modifications of which individual synapses are capable (Malenka and Bear, 2004). In the present experiments, neocortical slices perfused with sulpiride exhibited a significant suppression of LTP. Induction of LTP in the synaptic pathway from the basolateral amygdala to the dentate gyrus is regulated by D2 dopamine receptors (Abe et al., 2009). Consistent with our data, it has been reported that blocking of D2 receptors lead to inhibition of LTP (Abe et al., 2008). It has been suggested that dopamine D2 receptors in the induction of LTP have modulatory effect and depend on GABAergic inhibition (Abe et al., 2009). SD produced an LTP-like effect in rat neocortical slices (Footitt and Newberry, 1998) and increased LTP induction in human neocortical tissues (Berger et al., 2008). Both inhibition of LTP and SD generation were observed by drug manipulation in rat neocortical tissues (Müller et al., 2006). On the other hand, enhancement of LTP and facilitation of SD was reported under female hormones application in rat somatosensory neocortical slices (Sachs et al., 2007). Dopamine D2 receptors are involved in the enhancement of LTP in the lateral amygdala affected by cortical SD (Dehbandi et al., 2008). Modulatory effect of SD on LTP responses was also observed away from the SD site in the hippocampus (Wernsmann et al., 2006). The inhibition of synaptic plasticity by administration of dopamine D2 receptors may be responsible for its suppressive effect on SD.
In conclusion, D2 dopamine receptors seem to play a role in SD initiation in the rat neocortex. The therapeutic effects of inhibition of D2 dopamine receptors in migraine attacks may be due to its inhibitory action on SD. Furthermore, the present results reveal the importance of D2 receptors in neocortical synaptic plasticity, which may be involved in its inhibitory action on SD.
Acknowledgment
Authors would like to thank Ms Herrenpoth for her technical assistance. This work was performed as Doctoral thesis of AMH in Münster University.
References
- Abe K., Niikura Y., Fujimoto T., Akaishi T., Misawa M. (2008). Involvement of dopamine D2 receptors in the induction of long-term potentiation in the basolateral amygdaladentate gyrus pathway of anesthetized rats. Neuropharmacology, 55(8), 1419– 24. [DOI] [PubMed] [Google Scholar]
- Abe K., Fujimoto T., Akaishi T., Misawa M. (2009). Stimulation of basolateral amygdaloid serotonin 5-HT2C receptors promotes the induction of long-term potentiation in the dentate gyrus of anesthetized rats. Neuroscience Letters, 451(1), 65– 8. [DOI] [PubMed] [Google Scholar]
- Ayata C., Jin H., Kudo C., Dalkara T., Moskowitz M. A. (2006). Suppression of cortical spreading depression in migraine prophylaxis. Annals of Neurology, 59(4), 652– 61 [DOI] [PubMed] [Google Scholar]
- Bartholini LPG, Keller H H, Pletscher A. (1972) Effect of spreading depression on electrical activity and dopamine turnover in the striatum of rats. Expriemtia, 29(4), 452– 454 [DOI] [PubMed] [Google Scholar]
- Berger M., Speckmann E. J., Pape H. C., Gorji A. (2008). Spreading depression enhances human neocortical excitability in vitro. Cephalalgia, 28 (5), 558– 62. [DOI] [PubMed] [Google Scholar]
- Biederman J., Faraone S. V. (2005). Attention-deficit hyperactivity disorder. Lancet, 366(9481), 237– 48. [DOI] [PubMed] [Google Scholar]
- Bolay H., Reuter U., Dunn A. K., Huang Z., Boas D. A., Moskowitz M. A. (2002). Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Journal of Natural Medicines, 8(2), 136– 42. [DOI] [PubMed] [Google Scholar]
- Cheramy A., Barbeito L., Godeheu G., Desce J. M., Pittaluga A., Galli T., Artaud F., Glowinski J. (1990). Respective contributions of neuronal activity and presynaptic mechanisms in the control of the in vivo release of dopamine. Journal of Neural Transmission, 29, 183– 93. [DOI] [PubMed] [Google Scholar]
- D’Andrea G., Leon A. (2010). Pathogenesis of migraine: from neurotransmitters to neuromodulators and beyond. Neurological Sciences, 31(Suppl 1), S1– 7. [DOI] [PubMed] [Google Scholar]
- Dehbandi S., Speckmann E. J., Pape H. C., Gorji A. (2008). Cortical spreading depression modulates synaptic transmission of the rat lateral amygdala. European Journal of Neuroscience, 27(8), 2057– 65. [DOI] [PubMed] [Google Scholar]
- Del Zompo M., Cherchi A., Palmas M. A., Ponti M., Bocchetta A., Gessa G. L., Piccardi M. P. (1998). Association between dopamine receptor genes and migraine without aura in a Sardinian sample. Neurology, 51(3), 781– 6. [DOI] [PubMed] [Google Scholar]
- Dreier J. P., Major S., Pannek H. W., Woitzik J., Scheel M., Wiesenthal D., Martus P., Winkler M. K., Hartings J. A., Fabricius M., Speckmann E. J., Gorji A., COSBID study group . (2012). Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain, 135(Pt 1), 259– 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff M., Kovac S., Shahabi P., Ghadiri M. K., Dreier J. P., Stummer W., Speckmann E. J., Pape H. C., Gorji A. (2014). Spreading depression triggers ictaform activity in partially disinhibited neuronal tissues. Experimental Neurology, 253, 1– 15. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K., Moskowitz M. A. (2008). Animal models of migraine headache and aura. Current Opinion in Neurology, 21(3), 294– 300. [DOI] [PubMed] [Google Scholar]
- Footitt D. R., Newberry N. R. (1998). Cortical spreading depression induces an LTP-like effect in rat neocortex in vitro. Brain Research, 781(1–2), 339– 42. [DOI] [PubMed] [Google Scholar]
- Gelernter J., Kranzler H. (1999). D2 dopamine receptor gene (DRD2) allele and haplotype frequencies in alcohol dependent and control subjects: no association with phenotype or severity of phenotype. Neuropsychopharmacology, 20(6), 640– 9. [DOI] [PubMed] [Google Scholar]
- Gorji A. (2001) Spreading depression: a review of the clinical relevance. Brain Research Review, 38, 33– 60. [DOI] [PubMed] [Google Scholar]
- Gorji A., Scheller D., Straub H., Tegtmeier F., Köhling R., Höhling J. M., Tuxhorn I., Ebner A., Wolf P., Werner Panneck H., Oppel F., Speckmann E. J. (2001). Spreading depression in human neocortical slices. Brain Research, 906(1–2), 74– 83. [DOI] [PubMed] [Google Scholar]
- Gorji A., Zahn P. K., Pogatzki E. M., Speckmann E. J. (2004). Spinal and cortical spreading depression enhance spinal cord activity. Neurobiology of Disease, 15(1), 70– 9. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N., Sanchez Del Rio M., Wu O., Schwartz D., Bakker D., Fischl B., Kwong K. K., Cutrer F. M., Rosen B. R., Tootell R. B., Sorensen A. G., Moskowitz M. A. (2001). Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 98(8), 4687– 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarian M., Rahimi S., Behnan F., Hosseini M., Haghir H., Sadeghzadeh B., Gorji A. (2010). The effect of repetitive spreading depression on neuronal damage in juvenile rat brain. Journal of Neuroscience, 169(1), 388– 394. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W., Volkow N., Seamans J. (2005). Unmanageable motivation in addiction: pathology in prefrontal-accumbens glutamate transmission. Neuron, 45(4), 647– 50. [DOI] [PubMed] [Google Scholar]
- Kanis J. M., Timm N. L. (2014). Chlorpromazine for the treatment of migraine in a pediatric emergency department. Headache, 54(4), 335– 42. [DOI] [PubMed] [Google Scholar]
- Karreman M., Moghaddam B. (1996). The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. Journal of Neurochemistry, 66(2), 589– 98. [DOI] [PubMed] [Google Scholar]
- Leão A. A. P., Morison R. S. (1945). Propagation of spreading cortical depression. Journal of Neurophysiology, 8(1), 33– 45. [Google Scholar]
- Malenka R. C., Bear M. F. (2004). LTP and LTD: an embarrassment of riches. Neuron, 44(1), 5– 21. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff J. H., Damask S. P., Wang J., Haroutunian V., Davis K. L., Watson S. J. (1996). Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology, 15(1), 17– 29. [DOI] [PubMed] [Google Scholar]
- Moghaddam B., Schenk J. O., Stewart W. B., Hansen A. J. (1987). Temporal relationship between neurotransmitter release and ion flux during spreading depression and anoxia. Canadian Journal of Physiology and Pharmacology, 65(5), 1105– 10. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. A., Macfarlane R. (1993). Neurovascular and molecular mechanisms in migraine headaches. Cerebrovascular and brain metabolism reviews Journal, 5(3), 159– 77. [PubMed] [Google Scholar]
- Müller M., Pape H. C., Speckmann E. J., Gorji A. (2006). Effect of eugenol on spreading depression and epileptiform discharges in rat neocortical and hippocampal tissues. Neuroscience, 140(2), 743– 51. [DOI] [PubMed] [Google Scholar]
- Murase S., Grenhoff J., Chouvet G., Gonon F. G., Svensson T. H. (1993). Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neuroscience Letters, 157(1), 53– 6. [DOI] [PubMed] [Google Scholar]
- Marmura M. J. (2012). Use of dopamine antagonists in treatment of migraine. Current Treatment Options in Neurology, 14(1), 27– 35. [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Lampe C., Antal A., Liebetanz D., Lang N., Tergau F., Paulus W. (2006). Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. European Journal of Neuroscience, 23(6), 1651– 7. [DOI] [PubMed] [Google Scholar]
- Noble E. P. (2003). D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 116B(1), 103– 25. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. (1997). Dopamine and migraine. Neurology, 49(3), 650– 6. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Price S. C., Wilhoit T. L., Jones K. W. (1998). Comorbid migraine with aura, anxiety, and depression is associated with dopamine D2 receptor (DRD2) NcoI alleles. Journal of Molecular Medicine, 4(1), 14– 21. [PMC free article] [PubMed] [Google Scholar]
- Sachs M., Pape H. C., Speckmann E. J., Gorji A. (2007). The effect of estrogen and progesterone on spreading depression in rat neocortical tissues. Neurobiology of Disease, 25(1), 27– 34. [DOI] [PubMed] [Google Scholar]
- Seghatoleslam M., Ghadiri M. K., Ghaffarian N., Speckmann E. J., Gorji A. (2014). Cortical spreading depression modulates the caudate nucleus activity. Neuroscience, 267C, 83– 90. [DOI] [PubMed] [Google Scholar]
- Siniachkin M. S., Vein A. M., Voznesenskaia T. G., Gerber V. D. (1997). Sulpiride in the prophylactic treatment of migraine. Zh Nevrol Psikhiatr Im S S Korsakova, 97(11), 28– 32. [PubMed] [Google Scholar]
- Somjen G. G. (2001). Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiological Reviews, 81(3), 1065– 96. [DOI] [PubMed] [Google Scholar]
- Stanwood G. D. (2008). Protein-protein interactions and dopamine D2 receptor signalling: a calcium connection. Molecular Pharmacology, 74(2), 317– 9. [DOI] [PubMed] [Google Scholar]
- Taber M. T., Fibiger H. C. (1995). Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. Journal of Neuroscience, (5 Pt 2), 3896– 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K. M., Barkley G. L., Tepley N., Ramadan N. M. (1993). Central neurogenic mechanisms of migraine. Neurology, (6 Suppl 3), S21– 5. [PubMed] [Google Scholar]
- Wernsmann B., Pape H. C., Speckmann E. J., Gorji A. (2006). Effect of cortical spreading depression on synaptic transmission of rat hippocampal tissues. European Journal of Neuroscience, 23(5), 1103– 10. [DOI] [PubMed] [Google Scholar]