Abstract
Introduction:
The formalin test is the most accepted chemical test for evaluation of nociception. It requires the injection of an adequate amount of formalin into the surface of the hindpaw. Formalin test consists of phase 1 (0–7 min) and phase 2 (15–60) in which the animal shows painful behaviors. These phases are separated with a quiet phase named interphase, in which the nociceptive responses are decreased or completely disappeared.
Methods:
The goal of the current study was to evaluate the effects of swim stress at different heights of water on different phases of the formalin test in male rats.
Results:
Swim stress decreased nociceptive behaviors in first phase and prolonged interphase or delayed the start of second phase in a water height dependent manner. Swim stress in 25 and 50cm completely abolished the nociceptive behaviors in phase 1.
Discussion:
The present results showed different pain modulation during different phases of the formalin test and elucidated the impact of swim stress on duration of interphase. Interphase considered as an inactive period, but a recent research has shown that active inhibitory mechanisms are involved in the modulation of pain during this period. Therefore, swim stress may be considered as a useful tool for study of the basic inhibitory mechanisms underlying attenuation of nociceptive behaviors between phase 1 and 2 of the formalin test.
Keywords: Swim stress, Interphase, Formalin test, Pain
1. Introduction
Swim stress induced analgesia (SSIA) can reduce the formalin-induced nociceptive responses. This form of analgesia is considered to be mediated through opioid and non-opioid mechanism ( Hayati et al., 2008). Mogil (1993) showed that swim stress in 15°C induced an analgesia which was insensitive to naloxone and preventable by NMDA antagonists, while swim stress in 20°C water was partially mediated by opioidergic and non-opioidergic mechanisms ( Mogil, Sternberg, Kest, Marek & Liebeskind, 1993). The major advantage of the formalin assay over other models of inflammatory pain is the limited duration of assay (approximately 1 hour). Additionally, this assay produces a response in two discrete stages, allowing researchers to model both acute and tonic pain using a single noxious chemical. Moreover, this test is continuous and tonic, rather than a transient test such as tail flick or hot plate and these traits make formalin test an appropriate tool for pain research ( Abbott et al., 1995; Dubuisson et al., 1977). Immediately after the formalin injection, animals show early or acute painful responses (0–7 min) which imitate the direct activation of nociceptors. These painful responses followed by attenuation or quiescent of nociceptive responses in an interphase which per se followed by a long lasting period of nociceptive behaviors which might last for more than 45 minutes ( Hunskaar, Berge & Hole, 1986; Hunskaar & Hole, 1987). Although interphase was previously considered as an inactive phase, but Henry et al. (1999) showed that active inhibitory mechanisms are involved in this period ( Franklin & Abbott, 1993; Henry, Yashpal, Pitcher & Coderre, 1999).
In the present study, a formalin test performed following the swim stress in different heights of water in male rats. Considering that swimming in 20 °C induces SSIA that is the result of opioid and non-opioid mechanism (partially reversible by naloxone) ( Mogil et al., 1993), we used 20°C water for swim stress. Any study regarding the effects of different height of water on nociceptive responses to stressors in formalin test has not reported until date. We are thinking that some factor might be effect on nociceptive responses to stress-ors in different height; including contact of rat’s tail to container floor or gravity and so on. Moreover, since it suggested that active mechanisms are involved in the interphase of the formalin test, we decided to not only look at phase 1 and 2, but also look at the interphase to see the probable changes that might occur.
2. Methods
2.1. Subjects
All Experiments were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996) and were approved by the Research and Ethics Committee of Sabzevar University of Medical Sciences, Sabzevar, Iran. Maximum effort was made throughout the experiments to minimize discomfort for the animals and to use minimum number of animals. Adult male, Sprague- Dawley rats (80–120 g) were purchased from Razi Institute (Hesarak, Karaj, Iran). Rats were housed in groups of three in a temperature controlled room with a 12/12 light-dark cycle with lights on at 7:00 to 19:00. Food and water was provided ad libitum. Maximum attention was paid to the handling of animals to minimize environmental stress and discomfort.
2.2. Swim Stress-Induced Analgesia (Ssia)
Rats were placed in the testing room for at least one hour before the start of the experiment. To evaluate the effect of swim stress-induced pain modulation during the formalin test in rats, animals were allowed to acclimate to formalin test box for 30 minutes and then were subjected to forced swim stress for 3 min in a cylinder plastic pool (that contained 10, 25 and 50cm height of water with 50cm diameter) filled with 20°C water. Animals with sham swim stress were placed in the same cylinder that contained only 5cm of water at 20°C. After swimming sessions, each rat carefully dried with a new towel and placed into formalin test box for 5 minutes to acclimate, and then the formalin injected into the plantar surface of right hind paw. Animals in the control group did not receive any stress procedure. The water was clear and 2 rats were simultaneously used for swim stress paradigm.
2.3. Formalin Test
After swim stress, animals were transferred into clear plastic boxes (H: 25× W: 25 ×L: 25 cm) and allowed to habituate for 5 minutes. A mirror was placed underneath at a 45° angle to allow an unimpeded view of the paws. 50 μL of different concentrations of formalin (2%) were injected SC into either the plantar surface of the hind paw or the hairy area of the hind leg (using a 30 gauge needle inserted under the skin and advanced approximately 5–7 mm at an angle of 15 – 20 degrees). Then, each rat was immediately returned to the testing chamber and pain-like behaviors were recorded using a digital video camera (DV, Sony, Japan) for 90 min, for offline analysis of the behaviors. Experimenters left the testing room during the recording period. Pain behaviors were scored as follows: 0, normal weight bearing on injected paw; 1, the injected paw has little or no weight placed on it; 2, the injected paw is elevated and is not in contact with any surface; and 3, the injected paw is licked or bitten ( Erami, Azhdari-Zarmehri, Ghasemi-Dashkhasan, Esmaeili, & Semnanian, 2012). Recording of nociceptive behaviors began immediately following formalin injections (time 0) and was continued for 90 minutes. The score of pain-like behaviours for each 3- minute interval was calculated as the weighted average of the number of seconds spent in each posture. As a control, the scores were also recorded in normal rats, who received saline injection in different areas. Behavioral responses of each rat during the first phase (1–7 minutes), inter-phase (8–14) and the second phase (15–90) were separately evaluated ( Azhdari Zarmehri et al., 2011; Azhdari Zarmehri, Semnanian, & Fathollahi, 2008).
2.4. Data Analysis
Data presented as mean ± SEM, and analyzed by oneway analysis of variance and t-test between groups. The mean of nociceptive score in each phases (1, interphase, 2) of the formalin test for the groups receiving swim stress was analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. Phase 1 (1–7 minutes), inter-phase (8–14 minutes) and phase 2 (2A, 15–60 minutes and 2B, 60–90 minutes) of the formalin test were analyzed separately. The defined level for statistical significance was P<0.05.
2.5. Experimental Protocols
The formalin test was the experimental procedure used in our study to examine the effect of SIA in different phase. Three sets of experiments in the formalin test were considered as follows: (1) Rats were given formalin injection; (2) Rats were given formalin injection following performing the swim stress procedures (6 min at 20 ± 1 °C, 5 cm); (3) Rats were given formalin injection following performing the swim stress procedures (6 min at 20 ± 1 °C, 10 cm); (4) Rats were given formalin injection following performing the swim stress procedures (6 min at 20 ± 1 °C,2 5 cm); and (5) Rats were given formalin injection following performing the swim stress procedures (6 min at 20 ± 1 °C, 50 cm) .
3. Results
3.1. Effect of Different Heights of Water on the Nociceptive Behaviors
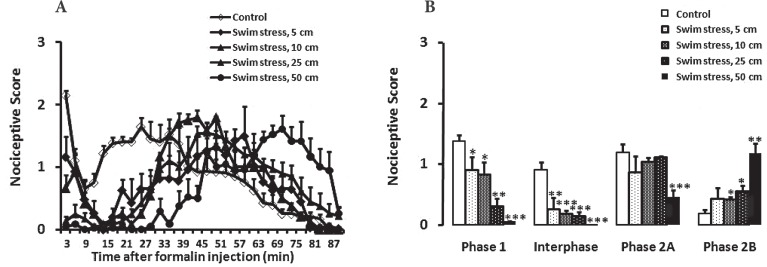
Swim stress in all heights of water decreased the nociceptive behaviors of animals in the first phase (Figure 1. A, B; for 5 cm height P<0.05 and 10 cm height P<0.05; for 25 cm height P<0.01 and 50 cm height P<0.001) and the interphase (Figure 1.B; for 5 cm height P<0.01 and 10 cm height P<0.001; for 25 cm height P<0.001 and 50 cm height P<0.001). In the case of 50 cm height of water, the nociceptive responses were nearly abolished in the first phase and the interphase. In first part of the phase 2, no significant change was seen in the nociceptive behaviors in 5, 10 and 25 cm (Figure 1.B; P>0.05) heights of water. In contrast, swim stress in 50 cm height of water (Figure 1.B; P<0.001) significantly reduced the nociceptive behaviors of animals in comparison with the control group. In second part of phase 2, no significant change was seen in the nociceptive behaviors of animals in 5 cm (Figure 1.B; P>0.05) height of water. However, swim stress in 10 (Figure 1.B; P<0.05) 25 (Figure 1.B; P<0.05) and 50 cm (Figure 1.B; P<0.01) heights of water significantly reduced the nociceptive behaviors in comparison with the control group.
Figure 1.
Time scores of formalin induced nociceptive behaviours (mean ± S.E.M. of 8 rats per group) following swim stress in different high of water measured every 3 minutes for 90 minutes (A) and bar chart for formalin test after swim stress in male rats (B). The columns represent the mean of nociceptive score in each phase: phase 1 (minutes 1–7), interphase (minutes 8–14) and first part (minutes 15–60) and second part (minutes 61–90) of phase 2 (B). For compression between swim stress in different high and control in male rats, all groups in the different high of water and control plotted in one graph. * P<0.05; ** P<0.01 and *** P<0.001 in comparison with male group.
4. Discussion
In the present study, we have shown that swimming stress in different heights of water modulates pain responses during different phases of formalin test. Mogil et al., (1993) showed that swimming stress in 20°C water induced an analgesia that was naloxone sensitive in male rats, while antagonizing opioid receptors were not effective in inhibiting SSIA in female rats, suggesting the involvement of other possible mechanisms ( Mogil et al., 1993). In the previous study, the author indicated that acute restraint stress can produce short-term and long-term SIA for tonic pain. The short-term SIA is reflected as a decreasing in nociceptive behaviors during phase 1, whereas the long-term SIA is reflected as a decreasing in nociceptive behaviors during phase 2 ( Heidari-Oranjaghi et al., 2012a). The antinociceptive effect with either restraint stress or swim stress prevented by OX1R antagonist (SB-334867) and Orexin antagonist injection prevent from the reduction of pain behaviors caused by acute food deprivation. Also, the opioid receptor antagonist naloxone could not totally reverse the antinociception effect with either form of stress. It suggested that OX1R might be involved in antinociception behaviors induced by these two forms of stress ( Ghasemi et al., 2013; Sofi-Abadi et al., 2011; Heidari-Oranjaghi et al., 2012b). Naloxone administration blocked the pronociceptive effect of 48 h food deprivation in the inter-phase of male rats and in the first phase of female rats (Saroukhani, Erami, Hosseini, & Azhdari-Zarmehri, 2013). The intra- rostral ventromedial medulla (RVM) and nucleus of paragigantocellularis lateralis injection of lidocaine was reduced pain in the first phase of formalin test, but it had no effect on the interphase or second Phase. In addition, injection of lidocaine into RVM, before inducing swim stress, potentiated the anti-nociceptive effects of swim stress in phase 1 and phase 2 A (Soleimani, Erami, Abbasnejad & Azhdari-Zarmehri, 2013; Azhdari-Zarmehri, Heidari-Oranjaghi, Soleimani & Sofi-Abadi, 2013; Azhdari-Zarmehri, Rahmani, Puzesh, Erami & Emamjomeh, 2013). Interestingly, swim stress prolonged interphase or/and delayed the start of second phase compared to control group in a water height dependent manner. Interphase was considered to be an inactive phase, but some evidences showed the involvement of active mechanisms in this quiescent phase ( Henry et al., 1999). Height of water seems to be an important factor in the SSIA, since swimming stress in 5cm water showed different effects on nociception in comparison with swimming stress in 50 cm water. Therefore, we used different heights of water for SSIA, and our results indicated that swimming in different heights of water had different effects on nociception in different phases of formalin test. Forced swimming in different heights of water decreased nociceptive score in phase 1, interphase and phase 2. In the present study, we showed that male rats were sensitive to SSIA in phase 1, inter-phase and phase 2 and other inhibitory system might involved in pain responses during phase 1 and 2. These findings suggest that other mechanisms including opioid or NMDA might be involved in SSIA, and different heights of water may employ different inhibitory mechanisms (especially considerable for 5cm water).
Therefore, comparable neurotransmitter or/and cellular mechanisms might be responsible for appearance of different inhibitory mechanisms by swim stress, however further in-depth studies are needed to investigate the presence of possible mechanisms. Furthermore, a deeper understanding of the mechanisms by which swim stress modulates different phases of the formalin test may be considers as a useful tool for study of the basic inhibitory mechanisms underlying attenuation of nociceptive behaviors in interphase.
Duration of different phases of the formalin test was calculated to evaluate the effect of SSIA in different heights of water on nociception periods in formalin test. Phase 1 duration was decreased in male rats, a finding in line with Gaumond (2005) study, which indicated the role of sex hormones on duration of phase 1 ( Gaumond, Arsenault & Marchand, 2005). The duration of interphase was increased in swim stress groups compared to the control group. It was of interest that this increase was height-dependent, so that with the increase in water height, duration of interphase augments which showing recruitment of novel mechanisms for the modulation of pain during this period. The differences between our study and other studies might be due to different methods of scoring and defined intervals for formalin test. Moreover, age, weight and genetics of animals that used in different studies could affect the results too.
As a conclusion, in this study we have shown the effects of SSIA in different heights of water on the intensity and duration of different phases of formalin test. Height of water plays an important role in modulation of pain by SSIA. More studies are needed to be designed for elucidate the exact mechanisms involved, and it seems that swim stress is an appropriate tool for the study of interphase mechanisms.
Acknowledgment
This research was supported by a grant from neuroscience Research Center, Kerman University of Medical Sciences, Kerman, Iran. The authors wish to express their appreciation to Elmira Ghasemi for her assistance and support.
Footnotes
Conflict of interest
We have no financial or other conflicts of interest.
References
- Abbott F. V., Franklin K. B., Westbrook R. F. (1995). The formalin test: scoring properties of the first and second phases of the pain response in rats. Journal of Pain, 60(1), 91– 102. [DOI] [PubMed] [Google Scholar]
- Azhdari Zarmehri H., Semnanian S., Fathollahi Y., Erami E., Khakpay R., Azizi H., et al. (2011). Intra-periaqueductal gray matter microinjection of orexin-A decreases formalin-induced nociceptive behaviours in adult male rats. Journal of Pain, 12(2), 280– 287. [DOI] [PubMed] [Google Scholar]
- Azhdari Zarmehri H., Semnanian S., Fathollahi Y. (2008). [Comparing the analgesic effects of periaqueductal gray matter injection of orexin A and morphine on formalin-induced nociceptive behaviours (Persian)]. Physiology and Pharmacology, 12(3), 188– 193. [Google Scholar]
- Azhdari-Zarmehri H., Heidari-Oranjaghi N., Soleimani N., Sofi-Abadi M. (2013). Effects of lidocaine injections into the rostral ventromedial medulla on nociceptive behviours in hot-plate and formalin tests in rats. Koomesh, 14(4), 490– 496. [Google Scholar]
- Azhdari-Zarmehri H., Rahmani A., Puzesh S., Erami E., Emamjomeh M. M. (2013). [Assessing the effect of lidocaine injection into the nucleus paragigantocellularis lateralis on formalin test and hot plate test induced nociceptive behaviours in rats (Persian)]. Journal of Zanjan University of Medical Sciences, 21(85), 10– 29. [Google Scholar]
- Dubuisson D., Dennis S. G. (1977). The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Journal of Pain, 4, 161– 174. [DOI] [PubMed] [Google Scholar]
- Erami E., Azhdari-Zarmehri H., Ghasemi-Dashkhasan E., Esmaeili M. H., Semnanian S. (2012). Intra-paragigantocellularis lateralis injection of orexin-A has an antinociceptive effect on hot plate and formalin tests in rat. Brain Research, 1478, 16(23), 16– 23 . [DOI] [PubMed] [Google Scholar]
- Franklin K. B., Abbott F. V. (1993). Pentobarbital, diazepam, and ethanol abolish the interphase diminution of pain in the formalin test: evidence for pain modulation by GABAA receptors. Pharmacology Biochemistry & Behaviour, 46(3), 661– 666. [DOI] [PubMed] [Google Scholar]
- Gaumond I., Arsenault P., Marchand S. (2005). Specificity of female and male sex hormones on excitatory and inhibitory phases of formalin-induced nociceptive responses. Brain Research, 1052(1), 105– 111. [DOI] [PubMed] [Google Scholar]
- Ghasemi E., Salehi B., Nakhost H., Sofiabdi M., Erami E., zhdari Zarmehri H. (2013). Effect of Orexin receptor-1 antagonist in acute food deprivation on formalin test. Journal of Qazvin University of Medical Sciences, 17, 26– 34. [Google Scholar]
- Hayati A. A., Zalina I., Myo T., Badariah A. A., Azhar A., Idris L. (2008). Modulation of formalin-induced fos-like immunoreactivity in the spinal cord by swim stress-induced analgesia, morphine and ketamine. Journal of German Medical Science, 6. [PMC free article] [PubMed] [Google Scholar]
- Heidari-Oranjaghi N., Ghasemi E., Mahdipour H., Salehi B., Sofiabadi M., Erami E., et al. (2012a). [Effects of acute and chronic immobilization stress on formalin test on the male rat (Persian)]. Journal of Rafsanjan University of Medical Sciences, 11(4), 391– 402. [Google Scholar]
- Heidari-Oranjaghi N., Ghasemi E., Mahdipour H., Salehi B., Sofiabadi M., Erami E., et al. (2012b). The effects of different acute and chronic restraint stress on formalin test in male rats. Pharmacology Biochemistry and Behaviour, 103, 299– 307. [DOI] [PubMed] [Google Scholar]
- Henry J. L., Yashpal K., Pitcher G. M., Coderre T. J. (1999). Physiological evidence that the ‘interphase’ in the formalin test is due to active inhibition. Journal of Pain, 82(1), 57– 63. [DOI] [PubMed] [Google Scholar]
- Hunskaar S., Berge O. G., Hole K. (1986). Dissociation between antinociceptive and anti-inflammatory effects of acetylsalicylic acid and indomethacin in the formalin test. Journal of Pain, 25(1), 125– 132. [DOI] [PubMed] [Google Scholar]
- Hunskaar S., Hole K. (1987). The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Journal of Pain, 30(1), 103– 114. [DOI] [PubMed] [Google Scholar]
- Mogil J. S., Sternberg W. F., Kest B., Marek P., Liebeskind J. C. (1993). Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Journal of Pain, 53(1), 17– 25. [DOI] [PubMed] [Google Scholar]
- Saroukhani M. R., Erami E., Hosseini S. S., Azhdari-Zarmehri H. (2013). [Involvement of endogenous opioidergic system in effects of food deprivation on formalin-induced nociceptive behaviours in rat (Persian)]. Journal of Kerman University of Medical Sciences, 20(3), 244– 251. [Google Scholar]
- Sofi-Abadi M., Heidari-Oranjaghi N., Ghasemi E., Esmaeili M. H., Haghdoost-Yazdi H., Erami E., et al. (2011). Assessment of orexin receptor 1 in stress attenuated nociceptive behaviours in formalin test. Physiology and Pharmacology, 12, 188– 193. [Google Scholar]
- Soleimani N., Erami E., Abbasnejad M., Shamsizadeh A., Azhdari-Zarmehri H. (2013). Effect of transient inactivation of rostral ventromedial medulla on swim stress induced analgesia in formalin test in rats. Physiology and Pharmacology, 17, 116– 124. [Google Scholar]