Abstract
Introduction:
Previous studies have shown that cannabinoidergic system is involved in anxiety. However, there are controversial reports in the experimental studies. The aim of this study is to evaluate the effect of pharmacological stimulation or blocking of CB1 receptors and inhibition of endocannabinoid degradation in anxiety like behavior in elevated plus-maze (EPM) test in rat. The EPM is one of the most widely used animal models of anxiety.
Methods:
Male Wistar rats were randomly allocated to ten groups. Different groups of animals intraperitoneally received Win-55212 (0.3, 1 and 5 mg/kg) as CB1 receptor agonist, AM-251 (0.3, 1 and 5 mg/kg) as CB1 receptor antagonist, URB-597 (0.03, 0.1 and 0.3 mg/kg) as endocannabinoid breakdown inhibitor or saline (as control group) 30 min before submitting into EPM test.
Results:
The results showed that compared to the control group, Win-55212 (1 and 5 mg/kg) and URB-597 (0.1 and 0.3 mg/kg) significantly increased both of the time and percentage of entries into open arms. AM-251 (1 and 5 mg/kg) significantly decreased the time and percentage of entries into open arms in the EPM test. These substances have no effects on the total distance covered by animals and number of closed arm entries.
Discussion:
It is concluded that activation of cannabinoid receptor exert anxiolytic effect while blocking of cannabinoid receptor resulted in anxiety behavior. The locomotor activity was not significantly changed by cannabinoid system. It is suggested that potentiation of cannabinoid system may be therapeutic strategy for the anxiety behavior.
Keywords: Anxiety, Cannabinoids, URB 597, Rat
1. Introduction
Anxiety is among the most common mental disorders. To date, this disorder is highly prevalent globally (Steel et al., 2014). The neurochemistry of anxiety disorders is an imbalance in neurotransmitter such as GABA, serotonin and dopamine function in the central nervous system. A variety of neurotransmitter mechanisms contribute in the regulation of anxiety behavior such as GABAergic, serotonergic (Chegini, Nasehi, & Zarrindast, 2014), noradrenergic, dopaminergic (Zarrindast, Mahboobi, Sadat-Shirazi, & Ahmadi, 2011) and endocannabinoid systems (Gobira, Aguiar, & Moreira, 2013; Viveros, Marco, & File, 2005).
There is some evidence related to the effects of cannabinoid system in anxiety related behavior (Patel & Hillard, 2006; Urigüen, Pérez-Rial, Ledent, Palomo, & Manzanares, 2004; Viveros et al., 2005). Cannabinoid system is affected with cannabinoid drugs derived from Cannabis sativa and exogenesis cannabinoid agents (Crippa et al., 2011). The psychoactive constituents are hashish, 9-tetrahydrocannabinol (THC), cannabidiol, and Marijuana (Adams et al., 1996).
Cannabinoids act on a specific receptor in the brain regions which is involved in mood and anxiety (Adams et al., 1996; Breivogel & Childers, 1998; Crippa et al., 2011; Herkenham et al., 1990). Endogenous ligands, such as anandamide bind to these receptors and it is less potent with a shorter duration than THC. The fatty acid amid hydrolase (FAAH) is an enzyme responsible for the degradation of endocannabinoids (Falenski et al., 2010; Pertwee, 2000). Some substances increased the endocannabinoid level by inhibition of breakdown or reuptake in neuronal synaptic (Gaetani, Cuomo, & Piomelli, 2003; Gaetani et al., 2009; Fabrício A Moreira, Aguiar, & Guimarães, 2006).
One of the unpleasant side-effects of cannabis use is anxiety and panic reactions (Adams et al., 1996; Hall, 1994), that contribute to the relatively some study of endocannabinoid in the understanding of anxiety disorders. There are controversies reporting about cannabinoids agent on the anxiety like behavior. Exposure to cannabis derivatives resulted anxiolytic (Crippa et al., 2011) emotional (Hollister, 1986) or anxiogenic responses in humans (Zuardi et al., 1982).
Some of the cannabinoid agonists and antagonist can both suppress and improve anxiety-like behaviors in animal model testes (Bisogno & Di Marzo, 2007; Lafenêtre, Chaouloff, & Marsicano, 2007; Navarro et al., 1997). In addition, some substances block the enzyme fatty acid amide and they result in elevation of cannabinoid level. Delta 9-THC produces anxiogenic effects in the higher doses (Long et al., 2010). However, elevation of cannabinoid exerts anxiolytic effects (Kathuria et al., 2003; Moreira et al., 2006; Moreira, Kaiser, Monory, & Lutz, 2008; Naidu et al., 2007; Patel et al., 2006). Genetic and pharmacological inhibition of FAAH alleviates the anxiety in mice (Gaetani et al., 2009; Fabricio et al., 2008).
On the basis of above considerations, this work was undertaken to determine the role of CB1 receptor in anxiety. Therefore, the aim of the present study was to examine the pharmacological stimulation or blocking of CB1 receptors and inhibition of endocannabinoid degradation in anxiety like behavior in rat.
2. Methods
2.1. Animals
Male Wistar rats weighting 250–350 were obtained from the Pasteur Institute (Tehran, Iran). They were maintained at 20±2°C in a 12_h light/12_h dark cycle with food and water supply ad libitum. Animals were acclimated to laboratory conditions for one week before the experiments were done. Each rat was used only once. All experiments were performed in accordance to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85–23, revised 1985).
2.2. Drugs
The following agents were used: CB1 receptor agonist, Win-55212 (0.3, 1 and 5 mg/kg, i.p.; Tocris, Biosciences); CB1 receptor antagonist, AM-251 (0.3, 1 and 5 mg/kg, i.p.; Tocris, Biosciences); endocannabinoid breakdown inhibitor, URB-597 (0.03, 0.1 and 0.3 mg/kg, i.p.; Tocris, Biosciences) in the study. Physiological saline (0.9% sodium chloride) was used as the vehicle. All drugs were prepared freshly and administered intraperitoneally (i.p.) in a volume of 0.1 mL per 10 g of body weight of the rats. All substances were dissolved in physiological saline and were administrated 30 min before elevated plus-maze test.
2.3. Elevated Plus-Maze
The black metal apparatus had equal arms. Its two arms were closed by walls (40×50×10 cm) and arranged in line with two opposite open arms (50×10 cm). The maze was elevated 50 cm above the floor. Rats were placed in the center of the maze facing the open arms. The Rats were excluded, and their behaviors monitored by digital camera above the maze for 10 min. After each test, the apparatus was cleaned with 10% ethanol to eliminate any remaining odors. The time spent in the open arms, the number of entries into the open arms, and percentage entries into the open arms were calculated (Lister, 1987; Pellow, Chopin, File, & Briley, 1985).
2.4. Statistical Analysis
All the results are presented in terms of mean±SEM. Data were analyzed by one-way ANOVA. Post hoc comparisons were performed using the Tukey-Kramer test to determine the effects of various treatments. A p-value less than 0.05 was considered to be significant.
3. Results
3.1. Effects of CB1 receptor agonist, Win-55212
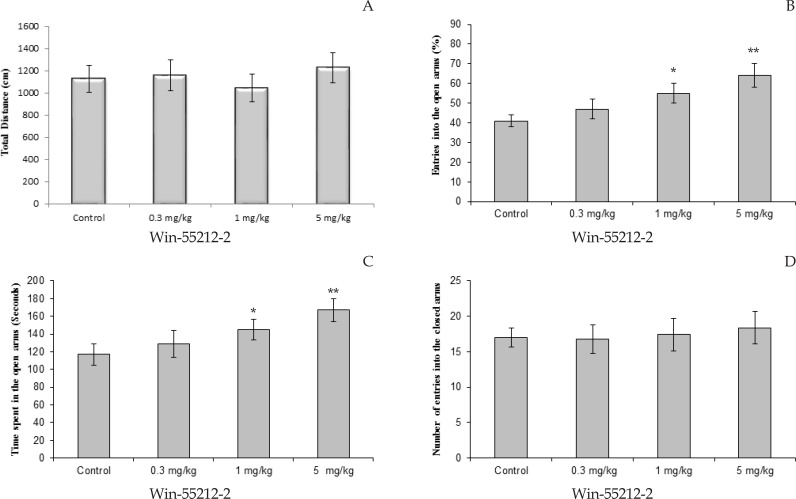
The effects of different doses of Win-55212-2 on the time spent in the open arms and percentage of entries in open arms are shown in Figure 1. The total distance covered by the Win-55212-2 groups was not significantly different from the controls (Figure 3A). One-way ANOVA showed that there was a significant difference between experimental groups in time spent in open arms, percentage of entries into open arms, but not number of entries to closed arms. One-way ANOVA showed that Win-55212-2 caused an increase in percentage of entries in open arms. Win-55212-2 significantly affected in doses 1 mg/kg (P<0.01) and 5 mg/ kg (P<0.05) compare to control rats (Figure 1B).
Figure 1.
Effect of Win-55212-2 (0.3, 1, and 5 mg/kg) treatment on elevated plus-maze performance: total distance covered by rats (A), the percentage of entries in open arms (B), time spent in open arms (C) and number of closed arms entry (D) during the 10 min test session in EPM. Data were analyzed by one-way ANOVA followed by Tukey–Kramer test (n= 10 per group). * P<0.05, and ** P<0.01 compared to control (saline).
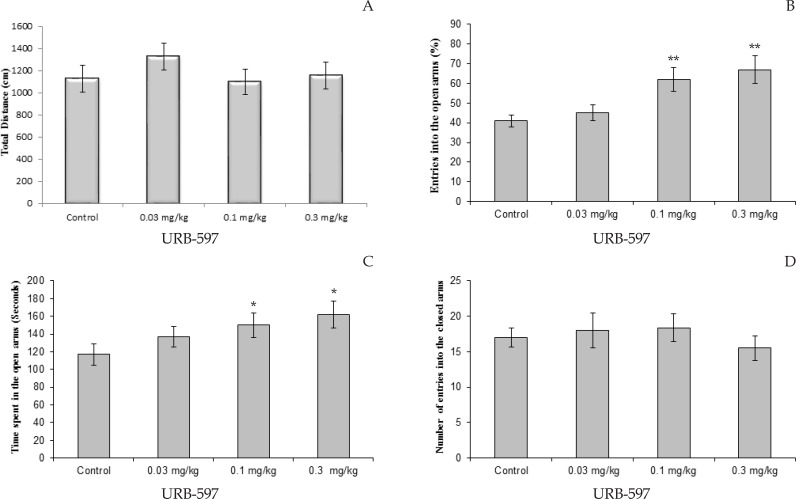
Figure 3.
Effect of URB-597 (0.03, 0.1, and 0.3 mg/kg) treatment on elevated plus-maze performance: total distance covered by rats (A), the percentage of entries in open arms (B), time spent in open arms (C) and number of closed arms entry (D) during the 10 min test session in EPM. Data were analyzed by one-way ANOVA followed by Tukey–Kramer test (n=10 per group). * P<0.05, and ** P< 0.01 compared to control (saline).
Tukey-Kramer test analysis showed that Win-55212-2 treated groups spent a significantly longer duration of time in the open arms than the control group. Win-55212-2 administered at doses 1 mg/kg (P<0.01) and 5 mg/ kg (P<0.05) exert significantly affect compare to control group (Figure 1C). The number of closed arms entries was not significantly different for groups that received Win-55212-2 (Figure 1D).
3.2. Effects of CB1 receptor antagonist, AM-251
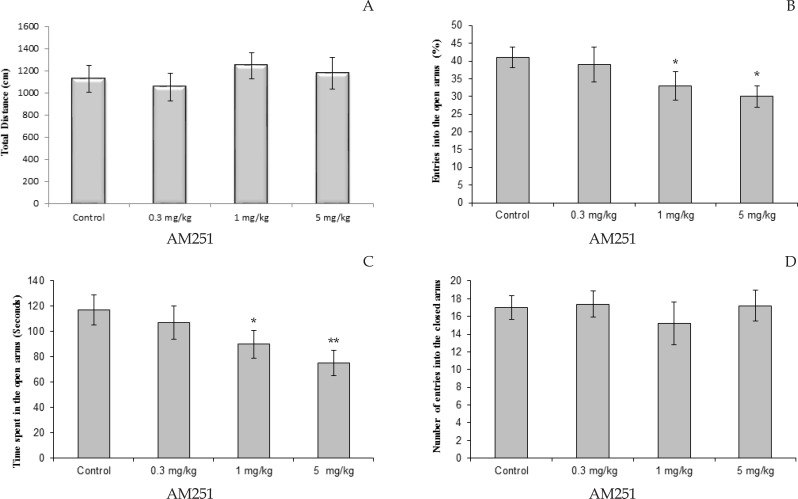
The effects of different doses of AM-251 on the percentage of entries in open arms and time spent in the open arms are shown in Figure 2. One-way ANOVA showed that AM-251 injected into the rats significantly decreased both of the percentage of entries in the open arms and time spent in the open arms, compared to controls. The Tukey-Kramer test analysis revealed a significant reduction for the doses of 1 mg/kg (P<0.05) and 5 mg/kg (P<0.01) compare to control rats in the time spent in the open arms (Figure 2A). Also, AM-251 significantly decreased percentage of entries in the open arms for the doses of 1 and 5 mg/kg (P<0.05; Figure 2B). No effect was found in the number of enclosed arms entries in experimental groups.
Figure 2.
Effect of AM251 (0.3, 1 and 5 mg/kg) administration on the elevated plus-maze performance: total distance covered by rats (A), the percentage of entries in open arms (B), time spent in open arms (C) and number of closed arms entry (D) during the 10 min test session in EPM. Data were analyzed by one-way ANOVA followed by Tukey–Kramer test (n=10 per group). * P<0.05, and ** P<0.01 compared to control (saline).
3.3. Effects of endocannabinoid breakdown inhibitor, URB-597
The effects of different doses of URB-597 on the time spent and percentage of entries in the open arms are shown in Figure 3. One-way ANOVA revealed that URB-597 treated animals exhibited significantly differences compare to control group in the time spent and percentage of entries into open arms, but not number of entries to closed arms. The Tukey-Kramer test analysis show that URB-597 at the doses of 0.1 and 0.3 mg/ kg significantly increased the time spent in open arms (P<0.05; Figure 3A) and percentage of entries into opens arms (P<0.01; Figure 3B). URB-597 administrated rat did not exhibit significantly change compare to control rat in the number of enclosed arms.
4. Discussion
The results show that administering Win-55212 and URB-597 attenuates anxiety behavior, but, rat treatment AM251 exhibited anxieogenic behavior. All of the CB1 receptor agents used did not have any effect on the locomotion of rats in the elevated plus-maze.
Our result is similar to previews investigations that the cannabinoid has anxiolytic properties in various rodent models (Haller, Varga, Ledent, Barna, & Freund, 2004; Haller, Varga, Ledent, & Freund, 2004; Kathuria et al., 2003; Marco et al., 2004; Martin, Ledent, Parmentier, Maldonado, & Valverde, 2002; Moreira et al., 2006; Moreira et al., 2008). Likewise, systemic activation of CB1 receptors produced anxiolytic effects in the elevated plus-maze (Naderi et al., 2008; Patel & Hillard, 2006). Blockade of the endogenous cannabinoid by CB1 antagonist induce anxiety-like responses in rats (Navarro et al., 1997).
However, no effect of CB1 receptors have been reported in the light dark box, fear conditioning and elevated plus-maze (Chhatwal, Davis, Maguschak, & Ressler, 2005; Crawley et al., 1993; Giuliani, Ferrari, & Ottani, 2000; Marco et al., 2004). The anxiogenic effects of CB1 receptors have been reported in both systemic and intra hippocampal in plus-maze and hole board testes (Arévalo, de Miguel, & Hernández-Tristán, 2001; Roohbakhsh, Moghaddam, Massoudi, & Zarrindast, 2007). Some of cannabinoid receptor agonists produce anxiolytic effects in the plus maze at low doses (Patel et al., 2006) and in higher doses produce an anxiogenic profile (Long et al., 2010; Viveros, Marco, Llorente, & Lopez-Gallardo, 2007).
Now, it has been demonstrated that cannabinoids exert their actions via CB1 receptors in the central nervous system (Di Marzo et al., 2000; Pertwee, 2000).These receptors are localized in brain regions (i.e. prefrontal cortex, nucleus accumbens, amygdala, and hippocampus) that are involved in emotion and anxiety behavior (Breivogel et al., 1990; Micale et al., 2009). Compounds such as cannabidiol and synthetic CB1 receptor agonists via activation canabinoide receptors produced anxiolytic behavior (Berrendero & Maldonado, 2002; Crippa et al., 2011; Fabrício Araújo Moreira, Aguiar, & Guimarães, 2007). Anxiety is increased by genetic and pharmacological inhibition of the CB1 receptor (Haller et al., 2004; Patel et al., 2006; Viveros, Marco, & File, 2005). Martin et al. showed that CB1 knockout mice were more anxious than wild type in elevated plus maze test (Martin et al., 2002).
Pharmacological block of FAHH activity attenuated anxiety via of CB1 receptors (Gaetani et al., 2003; Gaetani et al., 2009; Kathuria et al., 2003; Micale et al., 2009; Patel et al., 2006). Our results confirm the anxiolytic behavior among FAAH inhibition knocked-out mice and wild type (Fabrício Araújo Moreira et al., 2007; Moreira et al., 2008). Endogenous cannabinoids release is “on request” (Gaetani et al., 2009). They are not synthesized and stored in synaptic vesicles. While, endocannabinoids were synthesized from membrane precursors and immediately released in the synaptic cleft following neuronal activation. URB597 potently inhibit FAAH activity and induce increase anandamid levels in the brain (Di Marzo et al., 2000; Gaetani et al., 2009). URB597 indirectly enhance endocannabinoids neurotransmission and exert anxiolytic-like effect (Kathuria et al., 2003; Moreira et al., 2008; Patel et al., 2006; Rubino et al., 2008).
Controversial results have been reported under different conditions of experiments (Bisogno et al., 2007; Lafenêtre et al., 2007; Long et al., 2010; Viveros et al., 2007). It is hypothesized that the test conditions, differences in agonists, various doses and type of treatment is responsible for contentious effects of cannabinoid compounds on animal behavior. Also, various doses (Arévalo et al., 2001; Long et al., 2010; Marco et al., 2004), test conditions (Arévalo et al., 2001; Chegini et al., 2014; Chhatwal et al., 2005; Crawley et al., 1993; Giuliani et al., 2000; Marco et al., 2004; Roohbakhsh et al., 2007) and kind of knocked-out mice (Martin et al., 2002; Moreira et al., 2008) have important role in the behavior of animals in the various types of experiments.
In conclusion, CB1 receptor agonists, Win-55212 and CB1 receptor antagonists, AM-251 produced anxiolyticand anxiogenic-like effects, respectively. URB597 via inhibit FAAH activity and increasing anandamid levels potentiated anxiolytic effect of cannabinoid. This finding suggests that the potentiation of cannabinoid system may be considered as a beneficial strategy for the treatment of anxiety. In order to generalize these findings more to humans; future research using biological and pharmacological studies in rodents of both sexes are recommended.
Acknowledgments
This research was supported by a grant from the Neurophysiology Research Centre of Hamadan University of Medical Sciences.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Adams I. B., Martin B. R. (1996). Cannabis: Pharmacology and toxicology in animals and humans. Addiction, 91(11), 1585– 1614. [PubMed] [Google Scholar]
- Arévalo C., de Miguel R., Hernández-Tristán R. (2001). Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacology Biochemistry and Behavior, 70(1), 123– 131. [DOI] [PubMed] [Google Scholar]
- Berrendero F., Maldonado R. (2002). Involvement of the opioid system in the anxiolytic-like effects induced by Δ9-tetrahydrocannabinol. Psychopharmacology, 163(1), 111– 117. [DOI] [PubMed] [Google Scholar]
- Bisogno T., Di Marzo V. (2007). Short-and long-term plasticity of the endocannabinoid system in neuropsychiatric and neurological disorders. Pharmacological Research, 56(5), 428– 442. [DOI] [PubMed] [Google Scholar]
- Breivogel C. S., Childers S. R. (1998). The functional neuroanatomy of brain cannabinoid receptors. Neurobiology of Disease, 5(6), 417– 431. [DOI] [PubMed] [Google Scholar]
- Chegini H. R., Nasehi M., Zarrindast M. R. (2014). Differential role of the basolateral amygdala 5-HT3 and 5-HT4 serotonin receptors upon ACPA-induced anxiolytic-like behaviors and emotional memory deficit in mice. Behavioural Brain Research, 261, 114– 126. [DOI] [PubMed] [Google Scholar]
- Chhatwal J. P., Davis M., Maguschak K. A., Ressler K. J. (2005). Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology, 30(3), 516– 524. [DOI] [PubMed] [Google Scholar]
- Crawley J. N., Corwin R. L., Robinson J. K., Felder C. C., Devane W. A., Axelrod J. (1993). Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacology Biochemistry and Behavior, 46(4), 967– 972. [DOI] [PubMed] [Google Scholar]
- Crippa J. A. S., Derenusson G. N., Ferrari T. B., Wichert-Ana L., Duran F. L., Martin-Santos R., et al. (2011). Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. Journal of Psychopharmacology, 25(1), 121– 130. [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Breivogel C. S., Tao Q., Bridgen D. T., Razdan R. K., Zimmer A. M., et al. (2000). Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. Journal of Neurochemistry, 75(6), 2434– 2444. [DOI] [PubMed] [Google Scholar]
- Falenski K. W., Thorpe A. J., Schlosburg J. E., Cravatt B. F., Abdullah R. A., Smith T. H., et al. (2010). FAAH−/− mice display differential tolerance, dependence, and cannabinoid receptor adaptation after Δ9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology, 35(8), 1775– 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani S., Cuomo V., Piomelli D. (2003). Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends in Molecular Medicine, 9(11), 474– 478. [DOI] [PubMed] [Google Scholar]
- Gaetani S., Dipasquale P., Romano A., Righetti L., Cassano T., Piomelli D., et al. (2009). The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. International Review of Neurobiology, 85, 57– 72. [DOI] [PubMed] [Google Scholar]
- Giuliani D., Ferrari F., Ottani A. (2000). The cannabinoid agonist HU 210 modifies rat behavioural responses to novelty and stress. Pharmacological Research, 41(1), 45– 51. [DOI] [PubMed] [Google Scholar]
- Gobira P. H., Aguiar D. C., Moreira F. A. (2013). Effects of compounds that interfere with the endocannabinoid system on behaviors predictive of anxiolytic and panicolytic activities in the elevated T-maze. Pharmacology Biochemistry & Behavior, 110, 33– 39. [DOI] [PubMed] [Google Scholar]
- Hall W. (1994). Health and Psychological Effects of Cannabis Use. Current Issues in Criminal Justice, 6(2), 208. [Google Scholar]
- Haller J., Varga B., Ledent C., Barna I., Freund T. F. (2004). Context - dependent effects of CB1 cannabinoid gene disruption on anxiety - like and social behaviour in mice. European Journal of Neuroscience, 19(7), 1906– 1912. [DOI] [PubMed] [Google Scholar]
- Haller J., Varga B., Ledent C., Freund T. F. (2004). CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behavioural Pharmacology, 15(4), 299– 304. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Lynn A. B., Little M. D., Johnson M. R., Melvin L. S., De Costa B. R., et al. (1990). Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences, 87(5), 1932– 1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister L. E. (1986). Health aspects of cannabis. Pharmacological Reviews, 38(1), 1– 20. [PubMed] [Google Scholar]
- Kathuria S., Gaetani S., Fegley D., Valiño F., Duranti A., Tontini A., et al. (2003). Modulation of anxiety through blockade of anandamide hydrolysis. Nature Medicine, 9(1), 76– 81. [DOI] [PubMed] [Google Scholar]
- Lafenêtre P., Chaouloff F., Marsicano G. (2007). The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacological Research, 56(5), 367– 381. [DOI] [PubMed] [Google Scholar]
- Lister R. G. (1987). The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology, 92(2), 180– 185. [DOI] [PubMed] [Google Scholar]
- Long L. E., Chesworth R., Huang X. F., McGregor I. S., Arnold J. C., Karl T. (2010). A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. International Journal of Neuropsychopharmacology, 13(7), 861– 876. [DOI] [PubMed] [Google Scholar]
- Marco E., Perez-Alvarez L., Borcel E., Rubio M., Guaza C., Ambrosio E., et al. (2004). Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behavioural Pharmacology, 15(1), 21– 27. [DOI] [PubMed] [Google Scholar]
- Martin M., Ledent C., Parmentier M., Maldonado R., Val-verde O. (2002). Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl), 159(4), 379– 387. [DOI] [PubMed] [Google Scholar]
- Micale V., Cristino L., Tamburella A., Petrosino S., Leggio G. M., Drago F., et al. (2009). Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology, 34(3), 593– 606. [DOI] [PubMed] [Google Scholar]
- Moreira F. A., Aguiar D. C., Guimarães F. S. (2006). Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 30(8), 1466– 1471. [DOI] [PubMed] [Google Scholar]
- Moreira F. A., Aguiar D. C., Guimarães F. S. (2007). Anxiolytic-like effect of cannabinoids injected into the rat dorso-lateral periaqueductal gray. Neuropharmacology, 52(3), 958– 965. [DOI] [PubMed] [Google Scholar]
- Moreira F. A., Kaiser N., Monory K., Lutz B. (2008). Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology, 54(1), 141– 150. [DOI] [PubMed] [Google Scholar]
- Naderi N., Haghparast A., Saber-Tehrani A., Rezaii N., Alizadeh A. M., Khani A., et al. (2008). Interaction between cannabinoid compounds and diazepam on anxiety-like behaviour of mice. Pharmacology Biochemistry & Behavior, 89(1), 64– 75. [DOI] [PubMed] [Google Scholar]
- Naidu P. S., Varvel S. A., Ahn K., Cravatt B. F., Martin B. R., Lichtman A. H. (2007). Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology, 192(1), 61– 70. [DOI] [PubMed] [Google Scholar]
- Navarro M., Hernández E., Muñoz R. M., del Arco I., Villanúa M. A., Carrera M. R. A., et al. (1997). Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. NeuroReport, 8(2), 491– 496. [DOI] [PubMed] [Google Scholar]
- Patel S., Hillard C. J. (2006). Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. Journal of Pharmacology and Experimental Therapeutics, 318(1), 304– 311. [DOI] [PubMed] [Google Scholar]
- Pellow S., Chopin P., File S. E., Briley M. (1985). Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods, 14(3), 149– 167. [DOI] [PubMed] [Google Scholar]
- Pertwee R. G. (2000). Cannabinoid receptor ligands: clinical and neuropharmacological considerations, relevant to future drug discovery and development. Expert Opinion on Investigational Drugs, 9(7), 1553– 1571. [DOI] [PubMed] [Google Scholar]
- Roohbakhsh A., Moghaddam A. H., Massoudi R., Zarrindast M. R. (2007). Role of dorsal hippocampal cannabinoid receptors and nitric oxide in anxiety like behaviours in rats using the elevated plus-maze test. Clinical and Experimental Pharmacology and Physiology, 34(3), 223– 229. [DOI] [PubMed] [Google Scholar]
- Rubino T., Realini N., Castiglioni C., Guidali C., Vigano D., Marras E., et al. (2008). Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cerebral Cortex, 18(6), 1292– 1301. [DOI] [PubMed] [Google Scholar]
- Steel Z., Marnane C., Iranpour C., Chey T., Jackson J. W., Patel V., et al. (2014). The global prevalence of common mental disorders: a systematic review and meta-analysis 1980–2013. International Journal of Epidemiology, 43(2), 476– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urigüen L., Pérez-Rial S., Ledent C., Palomo T., Manzanares J. (2004). Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology, 46(7), 973– 66. [DOI] [PubMed] [Google Scholar]
- Viveros M., Marco E. M., File S. E. (2005). Endocannabinoid system and stress and anxiety responses. Pharmacology Biochemistry and Behavior, 81(2), 331– 342. [DOI] [PubMed] [Google Scholar]
- Viveros M. P., Marco E. M., File S. E. (2005). Endocannabinoid system and stress and anxiety responses. Pharmacology Biochemistry & Behavior, 81(2), 331– 342. [DOI] [PubMed] [Google Scholar]
- Viveros M. P., Marco E. M., Llorente R., Lopez-Gallardo M. (2007). Endocannabinoid system and synaptic plasticity: implications for emotional responses. Neural Plasticity, 2007, 52908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast M. R., Mahboobi S., Sadat-Shirazi M.-S., Ahmadi S. (2011). Anxiolytic-like effect induced by the cannabinoid CB1 receptor agonist, arachydonilcyclopropylamide (ACPA), in the rat amygdala is mediated through the D1 and D2 dopaminergic systems. Journal of Psychopharmacology, 25(1), 131– 140. [DOI] [PubMed] [Google Scholar]
- Zuardi A. W., Shirakawa I., Finkelfarb E., Karniol I. G. (1982). Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl), 76(3), 245– 250. [DOI] [PubMed] [Google Scholar]