Abstract
Introduction:
Recent studies suggest that glucocorticoids modulate memory reconsolidation. Moreover, cholinergic system is involved in memory reconsolidation. Since glucocorticoids interact with brain cholinergic system in modulating memory processing, we investigated whether glucocorticoid influences on the reconsolidation of emotionally arousing training depend on the cholinergic system.
Methods:
Mice were trained (1mA, 3s footshock) in an inhibitory avoidance task. Forty-eight hours after training, memory reactivation was occurred (Test 1), and different treatments were given. Two (Test 2), five (Test 3), and seven days (Test 4) after memory reactivation (Test 1), animals were retested for fear memory retention.
Results:
In the first experiment, we observed that administration of corticosterone (CORT, 0.3, 1 and 3 mg/kg) following memory reactivation impaired subsequent expression of memory in a dose-dependent manner. In the second experiment, we found that CORT-induced impairment of memory reconsolidation was reversed by the muscarinic receptor antagonist atropine (0.5 and 2 mg/kg). In the third experiment, the nicotinic receptor antagonist mecaylamine (0.5 or 2 mg/kg) was not able to block the corticosterone response.
Discussion:
These findings indicate that glucocorticoids impair memory reconsolidation by a muscarinic cholinergic mechanism.
Keywords: Memory reconsolidation, Glucocorticoids, Cholinergic agents
1. Introduction
Memory is a complex process that has multiple phases, including acquisition, consolidation, retrieval, reconsolidation or extinction. While it was traditionally accepted that once consolidation is complete memories become stable (McGaugh, 2000), a growing body of knowledge indicates that when a well consolidated memory is reactivated, it again becomes sensitive to disruption (Besnard, Caboche, & Laroche, 2012; Tronson & Taylor, 2007). Most treatments affecting memory consolidation when given after training are also able to modulate memories when given after its reactivation. The period of sensitivity triggered after memory retrieval was named re-consolidation (Nader & Einarsson, 2010). During reconsolidation that requires protein synthesis, the original memory is thought to update or integrate new information into long-term memories (Nader & Einarsson, 2010)
Glucocorticoid hormones (corticosterone in rodents, and cortisol in humans), released from the adrenal cortex during stressful episodes, and regulate a variety of physiological functions. Recent studies showed that stress and glucocorticoids modulate the reconsolidation of memory (I. Akirav & M. Maroun, 2012; Irit Akirav & Mouna Maroun, 2012). Administration of corticosterone immediately after reactivation of a contextual fear memory disrupts subsequent recall in mice (Cai, Blundell, Han, Greene, & Powell, 2006) and rats (Abrari, Rashidy-Pour, Semnanian, & Fathollahi, 2008). On the other hand, systemic as well as intra-amygdala or intrahippocampal injections of RU38486, the GR antagonist also impaired the reconsolidation of fear memory (Jin, Lu, Yang, Ma, & Li, 2007; Nikzad, Vafaei, Rashidy-Pour, & Haghighi, 2011; Tronel & Alberini, 2007).
It is well known that brain cholinergic systems are involved in mnemonic processes (Micheau & Marighetto, 2011). In most cases, central or systemic administration of anti-cholinergic drugs and lesions of the cholinergic system cause memory impairments while drugs that enhance cholinergic activity improve memory (Graef, Schönknecht, Sabri, & Hegerl, 2011). Two different families of receptors are recognized by Ach, muscarinic receptors (a G protein-coupled receptor), and nicotinic receptors (a ligand-gated ion channel). Both receptors are expressed in central and peripheral nervous systems and are implicated in many fundamental physiological processes such as learning and memory (Albuquerque, Pereira, Alkondon, & Rogers, 2009; Leach, Simms, Sexton, & Christopoulos, 2012).
Although, it is well established that cholinergic system plays an important role in memory consolidation (Decker & McGaugh, 2004), a few recent studies have also addressed the role of this system in memory reconsolidation (Blake, Boccia, Krawczyk, Delorenzi, & Baratti, 2012; Boccia, Blake, Krawczyk, & Baratti, 2010, 2011). A recent study has reported that memory consolidation and reconsolidation of an inhibitory avoidance are impaired when Ach synthesis is disrupted by intra-ventricular administration of the reversible inhibitor of the sodium-dependent high-affinity choline up-take hemicholinium (Boccia, Acosta, Blake, & Baratti, 2004; Boccia et al., 2011) suggesting the role of cholinergic system in both consolidation and reconsolidation of memory.
As mentioned above, both glucocorticoids and the cholinergic system play an important role in memory reconsolidation. Previous studies have shown that stressful stimuli can alter cholinergic activity in the brain, promote the release of acetylcholine in the hippocampus (Mark, Rada, & Shors, 1996), and regulate the expression of both nicotinic (Takita & Muramatsu, 1995) and muscarinic receptors (Kaufer, Friedman, Seidman, & Soreq, 1998). These findings suggest that cholinergic system may mediate the effects of glucocorticoids on memory processes. Thus, we investigated whether blockade of muscarinic or nicotinic cholinergic receptors would influence glucocorticoid effects on memory reconsolidation.
2. Methods
2.1. Animals
Adult male mice (25–30 g, n=180) were used in this study. Animals were housed five per cage in a room under 12-h light/dark cycles (6 am lights on–6 pm lights off) and constant temperature (23 ± 2°c). Food and water were available ad libitum. All experiments were performed between 10:00 and 14:00 h during the light cycle. All procedures were conducted in agreement with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
2.2. Drugs
Corticosterone (0.3, 1 or 3 mg/kg, Sigma) was dissolved in 100% propylene glycol (vehicle, VEH). Atropine (ATR, 0.5 or 2 mg/kg, Sigma) and mecamylamine (MEC, 0.5 or 2 mg/kg, Sigma) were dissolved in 0.9% saline. All drugs were injected intraperitoneally at a volume of 6 ml/kg. The drug doses were derived from pilot studies.
2.3. Inhibitory avoidance task
The experimental apparatus was a shuttle box (UgoBasile, Spain) divided into dark and light compartments. Both compartments had a grid floor (2 mm stainless steel rods spaced at 6 mm) connected to a shock generator. An automated apparatus registered the latency of passage from the light to the dark side of the box. The apparatus was located in the sound attenuated room.
2.4. Memory reconsolidation protocol
2.4.1. Training
The animals were trained following the protocol mentioned above. The animals were received a 50 Hz, 1mA constant current shock for 3s immediately after entering into the dark compartment. The mouse was removed from the dark compartment about 10s after receiving the shock, and returned to his home cage.
2.4.2. Memory Reactivation
Forty-eight hours after training, memory reactivation was occurred (Test 1). The mice were again placed in the illuminated compartment and the guillotine door was opened. The mice that entered the dark compartment were removed, given different treatments as mentioned below, and returned to their home cages. For the mice that did not enter the dark side, the test was terminated at 540 sec. Foot shock was not delivered during memory reactivation.
2.4.3. Memory Retention Tests
Two (Test 2), five (Test 3), and seven days (Test 4) after memory reactivation (Test 1), animals were retested for fear memory retention. To determine whether memory could re-emerge, immediately after Test 3, mice were exposed to a reminder shock of (0.5 mA, 1.5 s) in a different box and re-tested 24 hours later (Test 4). All retention tests (Tests 1–4) were done as described for test 1.
2.5. Statistical analysis
Data were compared by with two-way or three-way ANOVA for repeated measure (day). ANOVAs followed by Tukey’s test to determine the source of detected significant differences. Student’s t test was used to compare two independent groups. Values of P<0.05 were considered significant.
3. Results
3.1. Experiment 1
This experiment examined the effects of CORT (0.3, 1 and 3 mg/kg) on memory reconsolidation.
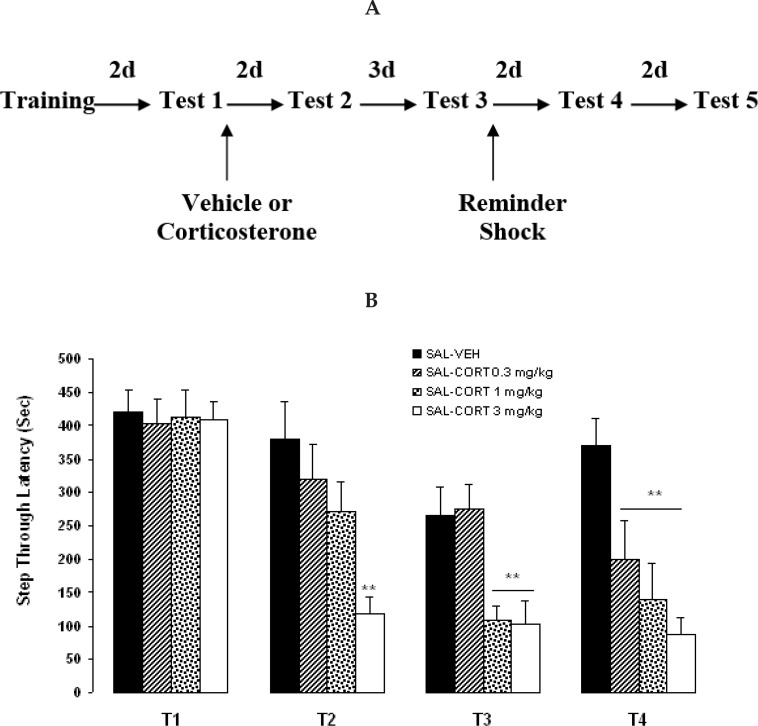
Mice were randomly divided into 4 groups (n=10 in each group) and trained and tested by procedures described above. Immediately after reactivation, the animals received two injections IP (Figure 1A). The 4 pairs of injections were saline (SAL) - VEH; SAL - CORT (0.3 mg/kg), SAL - CORT (1 mg/kg), and SAL - CORT (3 mg/kg). The first injection was followed immediately by the second injection.
Figure 1.
Effects of corticosterone administration following memory reactivation on fear memory reconsolidation. A: passive avoidance training/testing and drug administration schedule. B: Mean latencies ± SEM of groups of mice systemically injected with 0.3, 1 or 3 mg/kg of corticosterone or vehicle immediately after Test 1 and retested two days (Test 2), 5 days (Test 3) and 2 days after a remainder shock (Test 4). **P< 0.01 as compared with SAL - VEH group. VEH: Vehicle; CORT: Corticosterone. N=10 for each group.
When systemic administration of CORT (0.3, 1 and 3 mg/kg) was done following memory reactivation (Test 1), memory impaired significantly at subsequent tests (Figure 1B). A two–way repeated measurement ANOVA on latencies data indicated a significant effect of groups (F3, 119=5.08, P=0.0049), a significant effect of tests (F2, 119=56.4, P<0.0001) and a significant interaction between both factors (F6, 119=4.74, P=0.0004). Post-hoc comparison indicated that there is a significant difference between the vehicle group and CORT group (3 mg/kg) (P<0.01) in Tests 2 and 3, indicating a permanent disrupting effects of CORT on memory retention. Also, application of a weak reminder shock after Test 3 strengthened the memory in vehicle-injected, but not in the CORT-injected group. Since the effective dose of CORT was 3 mg/kg, subsequent experiments were done with this dosage.
3.2. Experiment 2
This experiment examined the effects of CORT (3 mg/kg) on memory reconsolidation in the presence or absence of the cholinergic muscarinic receptor antagonist ATR.
The data of the control group from the experiment 1 given saline together with vehicle and from group given saline and the most effective dose of CORT (3 mg/kg) were used in this experiment. Mice were randomly divided into the six following groups (n=10 in each group): SAL - VEH; SAL - CORT; ATR (0.5 mg/kg) - VEH; ATR (2 mg/kg) - VEH; ATR (0.5 mg/kg) - CORT or ATR (2 mg/kg) - CORT. The training and testing procedures were the same as those mentioned above (Figure 2A).
Figure 2.
Step-through latencies (mean±SEM) for a 48 h inhibitory avoidance test. A: Passive avoidance training/testing and drug administration schedule. B and C: Effects of CORT (3 mg/kg) on the reconsolidation of long-term memory in the presence or absence of the muscarinic receptors antagonist atropine (ATR, 0.5 and 2 mg/kg, B) or nicotinic receptors antagonist mecamylamine (MEC, 0.5 and 2 mg/kg, C). Testing intervals are the same as mentioned in the legend of Figure 1. *P<0.05 as compared with the corresponding SAL-VEH group. **P<0.05 as compared with the corresponding SAL - CORT group. N=10 for each group.
The results of Experiment 2 are shown in Figure 2B. A three-way repeated measurement ANOVA on retention latencies data revealed a significant interaction between CORT, ATR and Test (F4, 179=4.59, P=0.0018). There was a main effect of test (F2, 179=59.57, P<0.0001), and ATR (F2, 179=3.43, P=0.039), but not CORT (F1, 179=2.23, P=0.141). The interaction between CORT and ATR (F2, 179=3.07, P<0.05), CORT and Tests (F2, 179=8.42, P<0.0001), and ATR and Tests (F4, 179=6.11, P=0.0002) were all significant. Post-hoc comparisons showed that step-through latencies of animals receiving SAL-CORT were significantly shorter than those of groups receiving SAL-VEH (P<0.01), ATR (0.5 mg/kg) - CORT (P<0.05) and ATR (2 mg/kg) - CORT (P<0.05). The step-through latencies of animals receiving VEH - VEH were not significantly longer than those of ATR (0.5 mg/kg) - VEH, and ATR (2 mg/kg) - VEH.
3.3. Experiment 3
This experiment examined the effects of CORT (3 mg/kg) on memory reconsolidation in the presence or absence of the cholinergic nicotinic receptor antagonist MEC.
The data of the control group from the experiment 1 given saline together with vehicle and from group given saline and the most effective dose of CORT (3 mg/kg) were used in this experiment. Mice were randomly divided into the six following groups (n=10 in each group): SAL -VEH; SAL - CORT; MEC (0.5 mg/kg) - VEH; MEC (2 mg/kg) - VEH; MEC (0.5 mg/kg) - CORT or MEC (2 mg/kg) - CORT. The training and testing procedures were the same as those mentioned above.
The results of Experiment 3 are shown in Figure 2C. A three-way repeated measurement ANOVA on retention latencies data showed no significant interaction between CORT, MEC and Tests (F4, 179=1.1, P=0.358). There was a main effect of Test (F2, 179=105,88, P<0.0001), and CORT (F1, 179=21.51, P<0.0001), but not MEC (F2, 179=0.179, P=0.835). The interaction between CORT and MEC (F2, 179=1.19, P=0.30) and MEC and Test (F4, 179=0.7, P=0.59) were not significant, but the interaction between CORT and Test was significant (F2, 179=0.58, P<0.0001). Post-hoc comparisons showed that step-through latencies of animals receiving VEH - CORT, MEC (0.5 mg/kg) - CORT (P<0.05) and MEC (2 mg/kg) - CORT (P<0.05) were significantly shorter than those of groups receiving SAL-VEH (P<0.01). The step-through latencies of animals receiving SAL - VEH were not significantly longer than those of MEC (0.5 mg/kg) -VEH, MEC (2 mg/kg) -VEH, and there was no significant interaction between both factors (F2, 54=1.82; P=0.17).
3.4. Experiment 4
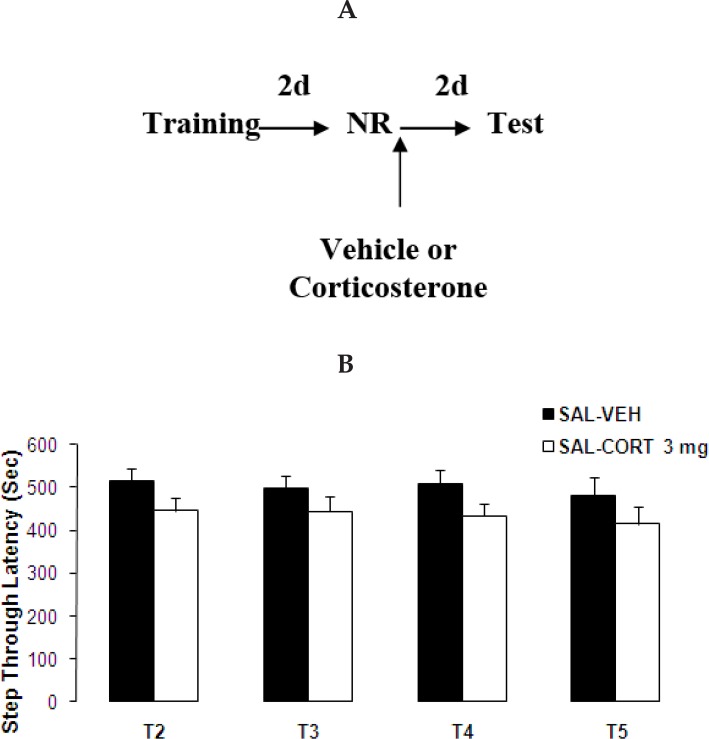
This experiment examined whether the effect of CORT on memory reconsolidation depends on memory reactivation, Two additional groups of mice (n=10 in each group) were given saline or CORT (3 mg/kg) 2 days after training in the absence of memory reactivation (Figure 3A) and re-tested two days later.
Figure 3.
Effects of CORT administration in the absence of memory reactivation (NR) on fear memory reconsolidation. A: Passive avoidance training/testing and drug administration schedule. B: Mean latencies ±SEM of groups of mice systemically injected with CORT (3 mg/kg) or vehicle (VEH; n=10) in the absence of memory reactivation and tested two days. VEH: vehicle, NR: No memory reactivation.
The results of experiment 4 are shown in Figure 3B. Student t-test showed that retention latency of mice injected with the vehicle or CORT 48 hours after training in the absence of memory reactivation (test 1), did not differ during retention test which was done two days (Figure 3B) (t16=1.74, P=0.17).
4. Discussion
The purpose of this study was to investigate the role of cholinergic system in the effects of glucocorticoids on memory reconsolidation of an inhibitory avoidance in mice. Our findings indicate that systemic administration of corticosterone impairs memory reconsolidation. These effects of corticosterone on memory reconsolidation were blocked by the muscarinic receptor antagonist atropine, but not by the nicotinic receptor antagonist mecamylamine. These findings provide evidence for the view that the impairing effects of glucocorticoids on memory reconsolidation might be mediated by muscarinic cholinergic mechanism.
4.1. Corticosterone impairs memory reconsolidation of an inhibitory avoidance
We found that corticosterone administration after memory reactivation produced a deficit in subsequent expression of memory. This impairment is only seen after reactivation of memory and not in the absence of memory reactivation, indicating that adequate memory reactivation must occur for corticosterone to alter post-reactivation memory processes. Our findings are in agreement with other studies showing that post-reactivation systemic administration of corticosterone impairs subsequent expression of contextual fear in rats and mice and also drug-related memory in rat (Abrari et al., 2008; Cai et al., 2006; Wang, Zhao, Ghitza, Li, & Lu, 2008).
Surprisingly, systemic and intra-cerebral injections of the glucocorticoid receptor antagonist RU34486 also impair the reconsolidation of auditory fear memory (Jin et al., 2007) and inhibitory avoidance (Nikzad et al., 2011; Taubenfeld, Rice-berg, New, & Alberini, 2009) in experimental animals. This paradox effect in which agonist and antagonist have the same effect on memory is also observed in studies on glucocorticoids modulation of consolidation. For example, acute administration of glucocorticoids enhances memory consolidation of spatial/contextual learning in a variety of appetitively or aversively motivated tasks (Roozendaal, 2002). Similarly, intra-cerebral GR antagonist enhanced memory consolidation for a reduction in reward magnitude task in an alley maze (Conrad et al., 2004; Ramot & Akirav, 2012), spatial learning and memory (Oitzl, Fluttert, Sutanto, & De Kloet, 2001), and fear conditioning (Conrad et al., 2004). These findings suggest that there is a dual modulatroy function of glucocorticoids on memory consolidation and reconsolidation, which, in turn, probably is determined by several factors such as the nature of task, and the animal’s level of arousal.
4.2. Atropine but not mecamylamine blocks the corticosterone-induced deficit of reconsolidation memory
We found that blockade of cholinergic muscarinic receptors by atropine, but not nicotinic cholinergic receptors by mecamlamine prevented the impairing effects of corticosterone on reconsolidation. Moreover, the antagonists alone did not alter memory processing. These findings indicate that glucocorticoids impair memory reconsolidation by a muscarinic cholinergic mechanism. Cholinergic system via muscarinic receptors plays an important role in the consolidation and reconsolidation of some kinds of memory. For example, intra-cerebroventricular injections with hemicholinium, a choline uptake inhibitor, may impair long-term expression of inhibitory avoidance memory in mice when administered immediately after training. Furthermore, inhibitory avoidance memory was impaired when hemicholinium was given after memory reactivation, but not in hemicholinium-treated mice not receiving reactivation (Boccia et al., 2004).
Post-training intra-basolateral amygdala (BLA) injections of the muscarinic receptor antagonist scopolamine impair contextual fear memory consolidation. However, post-reactivation intra-BLA injections of scopolamine did not affect the reconsolidation of fear memory (Bucherelli, Baldi, Mariottini, Passani, & Blandina, 2006). Post-retrieval systemic injections of scopolamine one week after training disrupted the expression of a conditioned place preference for cocaine, suggesting that post-retrieval stabilization of cocaine-associated contextual memory may indeed involve activation of muscarinergic receptors (Kelley, Anderson, & Itzhak, 2007).
The anatomical sites of the interaction between the muscarinic receptor antagonist atropine and glucocorticoids on memory reconsolidation are not clear. Hippocampus is a possible site of interaction between glucocorticoids and cholinergic system. This area plays an important role in memory reconsolidation. A recent study in our laboratory has shown that intra-hippocampal administration of RU38486 immediately following memory reactivation of an inhibitory avoidance produced a long-lasting deficit in long-term memory, suggesting that hippocampal glucocorticoid receptors are involved in the reconsolidation of fear–based memory (Nikzad et al., 2011). Stressful stimulation can alter cholinergic activity in the brain, promote the release of acetylcholine in the hippocampus (Mark, Rada, & Shors, 1996), and regulate the genetic expression of nicotinic (Takita & Muramatsu, 1995) and muscarinic receptors (Kaufer, Friedman, Seidman, & Soreq, 1998). The removal of the cholinergic innervation to the hippocampus via selective immune-lesions of septohippocampal cholinergic neurons induces dysfunction of the hypothalamic–pituitary–adrenocortical axis and decreases glucocorticoid receptor (GR) mRNA (Lim et al., 2012). More studies are needed to determine how these interactions between glucocorticoids and hippocampal cholinergic system may contribute to the effects of glucocorticoids on memory reconsolidation.
The BLA is another possible site of interaction between glucocorticoids and cholinergic system in regulating memory reconsolidation. Some evidence suggest that cholinergic system within the BLA plays a critical role in the enhancement of memory consolidation induced by systemic as well as intra-BLA injections of glucocorticoids. For example, memory enhancement induced by post-training systemic or intra-BLA administration of GR agonist is blocked by concurrent intra-BLA infusions of atropine after training (Power, Roozendaal, & McGaugh, 2001), suggesting that muscarinic cholinergic activation within the BLA is critical for enabling glucocorticoid enhancement of memory consolidation. GRs located in the BLA following memory reactivation of inhibitory avoidance impaired subsequent expression of memory (Taubenfeld et al., 2009; Tronel & Alberini, 2007). Thus, it is likely that cholinergic muscarinic receptors in the BLA interact with glucocorticoids in impairing memory reconsolidation.
The importance of impairing effects of glucocorticoids on memory reconsolidation is not clear. Previous studies have reported similar impairing effects on memory retrieval, and it has been suggested that this apparent detrimental effect on retrieval may favor memory consolidation, allowing a more appropriate response (Roozendaal, 2002). Although it is not clear what role may play glucocorticoids in impairing memory reconsolidation in physiological conditions, but by inhibiting memory reconsolidation, glucocorticoids may weaken pathologic memories such as those seen in post-traumatic stress disorders (PTSD) and phobia patients, and, thus might have therapeutic implication for treatment of these patients which usually suffer from retrieval of aversive memory (traumatic memory in PTSD and fear memory in phobias).
In conclusion, we observed that glucocorticoids have impairing effects on memory reconsolidation of an inhibitory avoidance via a cholinergic muscarinic mechanism. Further studies are required to determine the brain sites of interaction between glucocorticoids and cholinergic system on these cognitive processes. The ability of glucocorticoids to disrupt memory reconsolidation has important implications for the treatment or erasing pathogenic memories such as those seen in pathological conditions such as PTSD, and phobia disorders.
Acknowledgments
This work was supported by a grant from Semnan University of Medical Sciences to Dr. A. Rashidy-Pour and Dr. A. A. Vafaei. In addition, Mrs. S. Amiri and Z. Jafarian carried out this work in partial project fulfillment of the requirements to obtain the general physician degree.
References
- Abrari K., Rashidy-Pour A., Semnanian S., Fathollahi Y. (2008). Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: Dependence upon training intensity. Neurobiology of Learning and Memory, 89(2), 178– 184. [DOI] [PubMed] [Google Scholar]
- Akirav I., Maroun M. (2012). Stress modulation of reconsolidation. Psychopharmacology, 1– 15. doi: 10.1007/s00213-012-2887-6 [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Pereira E. F. R., Alkondon M., Rogers S. W. (2009). Mammalian nicotinic acetylcholine receptors: from structure to function. Physiological Reviews, 89(1), 73– 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard A., Caboche J., Laroche S. (2012). Reconsolidation of memory: a decade of debate. Progress in Neurobiology, 99( 1), 61– 80. doi: 10.1016/j.pneurobio.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Blake M., Boccia M., Krawczyk M., Delorenzi A., Baratti C. (2012). Choline reverses scopolamine-induced memory impairment by improving memory reconsolidation. Neurobiology of Learning and Memory, 98, 112– 21. [DOI] [PubMed] [Google Scholar]
- Boccia M., Acosta G., Blake M., Baratti C. (2004). Memory consolidation and reconsolidation of an inhibitory avoidance response in mice: effects of icv injections of hemicholinium-3. Neuroscience, 124(4), 735– 741. [DOI] [PubMed] [Google Scholar]
- Boccia M., Blake M., Krawczyk M., Baratti C. (2010). Hippocampal alpha 7 nicotinic receptors modulate memory reconsolidation of an inhibitory avoidance task in mice. Neuroscience, 171(2), 531– 543. [DOI] [PubMed] [Google Scholar]
- Boccia M., Blake M., Krawczyk M., Baratti C. (2011). Sildenafil, a selective phosphodiesterase type 5 inhibitor, enhances memory reconsolidation of an inhibitory avoidance task in mice. Behavioural Brain Research, 220(2), 319– 324. [DOI] [PubMed] [Google Scholar]
- Bucherelli C., Baldi E., Mariottini C., Passani M. B., Blandina P. (2006). Aversive memory reactivation engages in the amygdala only some neurotransmitters involved in consolidation. Learning & Memory, 13(4), 426– 430. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Blundell J., Han J., Greene R. W., Powell C. M. (2006). Postreactivation glucocorticoids impair recall of established fear memory. The Journal of Neuroscience, 26(37), 9560– 9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C. D., Macmillan D. D., Ii, Tsekhanov S., Wright R. L., Baran S. E., Fuchs R. A. (2004). Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiology of Learning and Memory, 81(3), 185– 199. [DOI] [PubMed] [Google Scholar]
- Decker M. W., McGaugh J. L. (2004). The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse, 7(2), 151– 168. [DOI] [PubMed] [Google Scholar]
- Graef S., Schönknecht P., Sabri O., Hegerl U. (2011). Cholinergic receptor subtypes and their role in cognition, emotion, and vigilance control: an overview of preclinical and clinical findings. Psychopharmacology, 215(2), 205– 229. [DOI] [PubMed] [Google Scholar]
- Jin X. C., Lu Y. F., Yang X. F., Ma L., Li B. M. (2007). Glucocorticoid receptors in the basolateral nucleus of amygdala are required for postreactivation reconsolidation of auditory fear memory. European Journal of Neuroscience, 25(12), 3702– 3712. [DOI] [PubMed] [Google Scholar]
- Kaufer D., Friedman A., Seidman S., Soreq H. (1998). Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature, 393(6683), 373– 377. [DOI] [PubMed] [Google Scholar]
- Kelley J. B., Anderson K. L., Itzhak Y. (2007). Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport, 18(8), 777– 780. [DOI] [PubMed] [Google Scholar]
- Leach K., Simms J., Sexton P. M., Christopoulos A. (2012). Structure–function studies of muscarinic acetylcholine receptors. Muscarinic Receptors, 29– 48. [DOI] [PubMed] [Google Scholar]
- Lim C. S., Kim Y. J., Hwang Y. K., Bañuelos C., Bizon J. L., Han J. S. (2012). Decreased interactions in protein kinase A–Glucocorticoid receptor signalling in the hippocampus after selective removal of the basal forebrain cholinergic input. Hippocampus, 22(3), 455– 465. [DOI] [PubMed] [Google Scholar]
- Mark G., Rada P., Shors T. J. (1996). Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience, 74(3), 767– 774. [DOI] [PubMed] [Google Scholar]
- McGaugh J. L. (2000). Memory--a century of consolidation. Science, 287(5451), 248– 251. [DOI] [PubMed] [Google Scholar]
- Micheau J., Marighetto A. (2011). Acetylcholine and memory: a long, complex and chaotic but still living relationship. Behavioural Brain Research, 221(2), 424– 429. [DOI] [PubMed] [Google Scholar]
- Nader K., Einarsson E. Ö. (2010). Memory reconsolidation: an update. Annals of the New York Academy of Sciences, 1191(1), 27– 41. [DOI] [PubMed] [Google Scholar]
- Nikzad S., Vafaei A. A., Rashidy-Pour A., Haghighi S. (2011). Systemic and intrahippocampal administrations of the glucocorticoid receptor antagonist RU38486 impairs fear memory reconsolidation in rats. Stress, 14(4), 459– 464. [DOI] [PubMed] [Google Scholar]
- Oitzl M. S., Fluttert M., Sutanto W., De Kloet E. R. (2001). Continuous blockade of brain glucocorticoid receptors facilitates spatial learning and memory in rats. European Journal of Neuroscience, 10(12), 3759– 3766. [DOI] [PubMed] [Google Scholar]
- Power A. E., Roozendaal B., McGaugh J. L. (2001). Glucocorticoid enhancement of memory consolidation in the rat is blocked by muscarinic receptor antagonism in the basolateral amygdala. European Journal of Neuroscience, 12(10), 3481– 3487. [DOI] [PubMed] [Google Scholar]
- Ramot A., Akirav I. (2012). Cannabinoid receptors activation and glucocorticoid receptors deactivation in the amygdala prevent the stress-induced enhancement of a negative learning experience. Neurobiology of Learning and Memory, 97(4), 393– 401. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. (2002). Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory, 78(3), 578– 595. [DOI] [PubMed] [Google Scholar]
- Takita M., Muramatsu I. (1995). Alteration of brain nicotinic receptors induced by immobilization stress and nicotine in rats. Brain Research, 681(1), 190– 192. [DOI] [PubMed] [Google Scholar]
- Taubenfeld S. M., Riceberg J. S., New A. S., Alberini C. M. (2009). Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors. Biological Psychiatry, 65(3), 249– 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S., Alberini C. M. (2007). Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biological Psychiatry, 62(1), 33– 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson N. C., Taylor J. R. (2007). Molecular mechanisms of memory reconsolidation. Nature Reviews Neuroscience, 8(4), 262– 275. [DOI] [PubMed] [Google Scholar]
- Wang X. Y., Zhao M., Ghitza U. E., Li Y. Q., Lu L. (2008). Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. The Journal of Neuroscience, 28( 21), 5602– 5610. [DOI] [PMC free article] [PubMed] [Google Scholar]