Abstract
Introduction:
The neuroprotective role of opioid morphine against 6-hydroxydopamine-induced cell death has been demonstrated. However, the exact mechanism(s) underlying such neuroprotection, especially the role of subtype receptors, has not yet been fully clarified.
Methods:
Here, we investigated the effects of different opioid agonists on 6-OHDA-induced neurotoxicity in human neuroblastoma SH-SY5Y cell line as an in vitro model of Parkinson’s disease. Cell damage was induced by 150 μM 6-OHDA and the cells viability was examined by MTT assay. Intracellular calcium, reactive oxygen species and mitochondrial membrane potential were assessed by fluorescence spectrophotometry method. Immunoblot technique was used to evaluate cytochrome-c and activated caspase-3 as biochemical markers of apoptosis induction.
Results:
The data showed that 6-OHDA caused significant cell damage, loss of mitochondrial membrane potential and increase in intracellular reactive oxygen species and calcium levels as well as activated caspase-3 and cytochrome-c release. Incubation of SH-SY5Y cells with μ-opioid agonists, morphine and DAMGO, but not with δ-opioid agonist, DADLE, elicited protective effect and reduced biochemical markers of cell damage and death.
Discussion:
The results suggest that μ-opioid receptors signaling participate in the opioid neuroprotective effects against 6-OHDA-induced neurotoxicity.
Keywords: Morphine, DAMGO, μ-opioid receptors, 6-hydroxydopamine, Apoptosis, SH-SY5Y cells
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disease caused by losing dopaminergic neurons of the central nervous system. Oxidative stress, neuroinflammation, mitochondrial and lysosomal dysfunctions, and the formation of pathologic inclusions have been considered as some possible ethiology of PD (More, Kumar, & Kim, 2013). Therefore, neuroprotective strategies and prevention of neural death can be helpful in the treatment of the disease (Diogenes, M. J., Outeiro, 2010). Opioids have an important place in pharmacology and medicine due to their role in the management of pain. However, their use is restricted due to their side-effects and abuse potential (Pasternak et al., 2014). In addition, it has been documented that opioids have numerous neurophysiological functions in the peripheral nervous system and the central nervous system. Surprisingly, neurobiological studies have demonstrated neuroprotective roles for opioids (Lee et al., 2004; Zhang, Chen, & Yu, 2008; Cui et al., 2011). We have previously reported that morphine protects SH-SY5Y neuroblastoma cells against 6-Hydroxydopamine-induced toxicity and apoptosis (Elyasi, Eftekhar-Vaghefi, & Esmaeili-Mahani, 2014). However, the exact mechanisms of opioid protective effect and its receptor subtype signaling have not been completely known and are still under investigation.
Recent advances in the molecular biology of opioid receptors have confirmed that there are three types (μ, δ and κ) of opioid receptor which are coupled to intracellular signal elements via G-proteins signaling. Morphine is a partial agonist for μ-opioid receptors (MORs) and acts as a weak agonist for δ-opioid receptors (DORs). However, it has been suggested that morphine’s effect is not acting through κ-opioid receptors (KORs). DAMGO and DADEL, full agonist at MORs and DORs, have been used to specific activate of related opioid receptor in experimental studies (Spetea, Asim, & Wolber, 2013).
A growing body of evidence suggests that opioids show diverse biological (non-analgesic) effects including anti-oxidant (Gulcin, Beydemir, & Alici, 2004; Costa-Malaquias et al., 2014), anti-inflammatory (Rostami, Oryan, & Ahmadiani, 2012; Saccani et al., 2012), antiapoptotic (Iglesias, Segura, & Comella, 2003; Tamura, Monden, & Shintani, 2006) and pro-survival (Husain, Abdul, & Potter, 2012) properties.
Understanding the diversity of opioid receptors signaling and how their second messenger activation leads to regulate biological effect is a necessary and important step in opioid research. This study was designed to find which opioid receptor subtype signaling is involved in the protective effect of opioids on 6-OHDA-induced SH-SY5Y cells toxicity as an in vitro model of Parkinson’s disease.
2. Methods
2.1. Materials
Cell culture reagents, penicillin–sterptomycin solution, trypsin EDTA, Fetal bovine serum (FBS) were obtained from Biosera Co. (East Sussex, UK). Culture flasks and dishes were acquired from SPL lifesciences inc. (Gyeonggi-Do, South korea). [D-Ala(2), N mePhe(4), Gly-ol(5)] enkephalin (DAMGO), [D-Ala(2), D-Leu(5)] enkephalin (DADLE), 2-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2-tetrazolium bromide (MTT), 6-hydroxydopamine (6-OHDA), 2,7-dichlorofluorescein diacetate, rhodamine 123 and Fura 2-AM were purchased from Sigma (St Louis, MI, USA). Primary polyclonal anti-caspase 3 and primary monoclonal anti-β-actin antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Primary monoclonal anti-cytochrome-c antibodies were obtained from Santa Cruz Biotechnology, Inc. (Delaware Ave. Santa Cruz, USA). Morphine hydrochloride (TEMAD Co., Iran) was used in this study.
2.2. Cell Culture
Human neuroblastoma SH-SY5Y cells were obtained from National Cell Bank of Iran (NCBI)- Pasteur Institute of Iran (Tehran, Iran). The cells were grown with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with fetal bovine serum (10%), penicillin (100 U/mL) and streptomycin (100 μg/mL). They were maintained at 37°C in a 5% CO2 atmosphere. The cells were plated at the density of 5000 per well in a 96-well plate for the MTT, intracellular calcium and ROS assay. For protein extraction, cells were grown in a 6-well plate well and permitted to attach and grow for 24 h. Then the cells were incubated with 6-OHDA and different concentration of morphine, DAMGO and DADEL for 24 h. The drugs were added 30 min before 6-OHDA.
2.3. Cell viability analysis
Cell viability was assayed by the reduction of 2-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2-tetrazolium bromide (MTT) to formazan. MTT was added to the cell medium at a final concentration of 0.5 mg/mL for 2 h. Then, the media were carefully removed and 100 μL DMSO were added to each well, and the absorbance (OD) values were determined by spectrophotometry at 490 nm with an automatic micro plate reader (Eliza MAT 2000, DGR Instruments, GmbH, Marburg, Germany). Results were expressed as percentages of control.
2.4. Measurement of intracellular reactive oxygen species (ROS) formation
The level of intracellular ROS was determined with 2,7-dichlorofluorescein diacetate (DCFH-DA) probe. In the presence of a proper oxidant DCFH-DA is converted to the highly fluorescent dichlorofluorescein. Different groups of cells were incubated with 1 mM DCFH-DA in PBS in the dark for 10 min at 37 °C. After incubation, the cells were washed (three times) with PBS and analyzed immediately on the fluorescence plate reader (FLX 800, BioTek, USA). The fluorescence intensity of cells in 96-well plates was quantified at an excitation of 485 nm and an emission of 538 nm. Each experiment was performed six independent times. Results were expressed as fluorescence percentage of control cells.
2.5. Determination of intracellular calcium [Ca2+]i
Different groups of cells were loaded with 5 μM Fura 2-AM for 60 minutes. After washing and removal of the Fura 2-AM, the cultures were incubated in dye-free saline for at least 45 minutes to allow for the de-esterification of the Fura 2-AM. The cells were analyzed immediately on the fluorescence plate reader (FLX 800, BioTek, USA) and the fluorescence intensity of cells in 96-well plate was quantified at an excitation of 340/380 nm and an emission of 510 nm. Each experiment was performed six independent times. Results were expressed as fluorescence percentage of control cells.
2.6. Measurement of Mitochondrial Membrane Potential
The mitochondrial membrane potential was determined with rhodamine 123, which preferentially partitions into active mitochondria based on the highly negative mitochondrial membrane potential. Depolarization of mitochondrial membrane potential results in the loss of rhodamine 123 from the mitochondria and a decrease in intracellular fluorescence. Rhodamine 123 (10 μM) was added to cells after treatment with drugs, as mentioned above. After 30 min incubation at 37°C, the cells were washed twice with PBS and analyzed on the fluorescence plate reader (FLX 800, BioTek, USA). The fluorescence intensity was quantified at an excitation of 540 nm and an emission of 570 nm.
2.7. Immunoblot analysis
For detection of cytochrome-c, the cells were lysed for 30 min on ice in buffer [20 mM HEPES, pH 7.6, 20% glycerol, 500 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 2.5 μg/mL of leupeptin, 10 μg/mL of aprotinin, 0.5 m M phenylmethylsulfonyl fluoride, 1mM DTT and 0.5% sodium dodecyl sulfate (SDS)]. Lysates were centrifuged for 30 min at 14000 g at 4°C. The supernatants were removed and the pellets were re-suspended in 50 μl volume of buffer (Elyasi et al., 2014).
For detection of cleaved caspase 3 and beta-actin, the cells were homogenized in ice-cold buffer containing 10mM Tris–HCl (pH 7.4), 1mM EDTA, 0.1% SDS, 0.1% Na-deoxycholate, 1% NP-40 with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2.5 μg/mL of leupeptin, 10 μg/mL of aprotinin) and 1 mM sodium orthovanadate. The homogenate was centrifuged at 14000 rpm for 15 min at 4°C. The resulting supernatant was retained as the whole cell fraction. Protein concentrations were measured using the Bradford method. Equal amounts of protein were resolved electrophoretically on a 9% SDS-PAGE gel and transferred to PVDF membranes (Hybond ECL, GE Healthcare Bio-Sciences Corp. NJ, USA). After overnight blocking at 4°C with 5% non-fat dried milk in Tris-buffered saline with Tween 20 (blocking buffer, TBS-T, 150 mM NaCl, 20 mM Tris–HCl, pH 7.5, 0.1% Tween 20), the membranes were probed with rabbit monoclonal antibody to caspase-3 (Cell Signaling Technology, USA, 1:1000 overnight at 4°C) for three hours at room temperature. After washing in TBS-T (three times, each time 5 min), the blots were incubated for 60 min at room temperature with a horseradish peroxidase-conjugated secondary antibody (1:15000, GE Healthcare Bio-Sciences Corp. NJ, USA). The antibodies were diluted in blocking buffer. The antibody-antigen complexes were detected using the ECL system and exposed to Lumi-Film chemiluminescent detection film (Roch, Germany). Lab Work analyzing software (UVP, UK) was used to analyze the intensity of the expression. β-actin immunoblotting (antibody from Cell Signaling Technology, INC. Beverly, MA, USA; 1:1000) was used to control for loading.
2.8. Statistical Analysis
The results are expressed as mean±SEM. The differences in mean MTT, intracellular ROS and calcium and mitochondrial potential between experimental groups were determined by one-way ANOVA, followed by the Newman-Keuls test. The values of caspase-3, cytochrome-c were expressed as tested proteins/β-actin ratio band density for each sample. The averages for different groups were compared by ANOVA, followed by the Newman-Keuls test. P<0.05 was considered significant.
3. Results
3.1. Analysis of cell viability
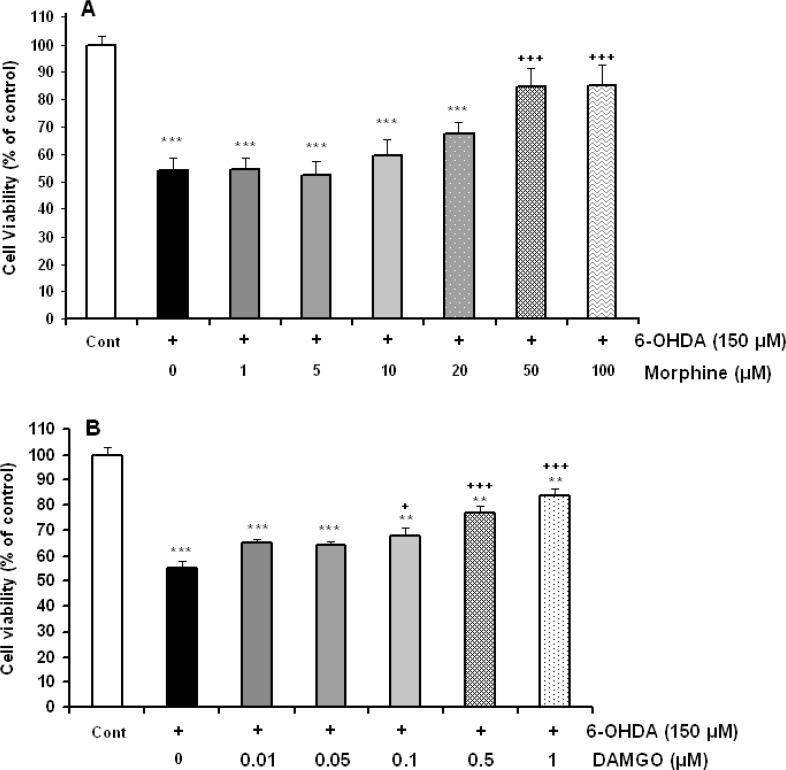
A significant toxic effect was observed in cells that were treated with 150 μM 6-OHDA.
Morphine incubation in doses of 50 and 100 μM significantly prevented 6-OHDA-induced cell toxicity [F(6,42)=6.610, P=0.0001, Figure 1A). Furthermore, 24 h treatment of cells with toxin and different concentrations (0.01–1 μM) of selective mu opioid agonist DAMGO showed the protective effects in doses of 0.1, 0.5 and 1 μM of DAMGO [F(6,35)=34.375, P=0.0001, Figure 1B).
Figure 1.
Effects of 150 μM 6-hydroxydopamine (6-OHDA) alone or accompanied with different concentration of morphine (A) and DAMGO (B) on SH-SY5Y cells viability. Data are expressed as mean±SEM; n=5–6 wells for each group; **P<0.01 and ***P<0.001 significantly different versus control cells. +P<0.05 and +++P<0.001 versus 6-OHDA-treated cells.
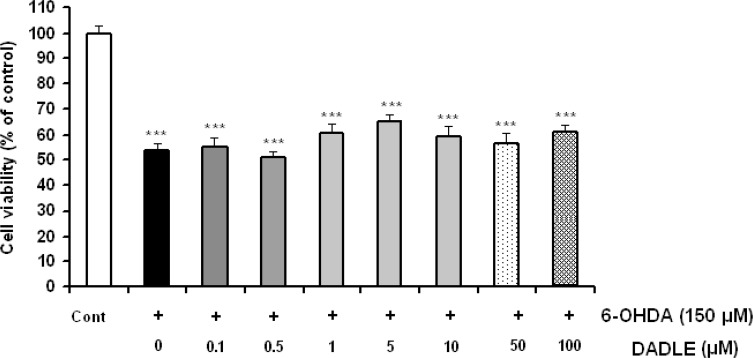
As shown in Figure 2, selective delta opioid agonist DADLE could not prevent 6-OHDA-induced cell damage in a dose rang from 0.1 to 100 μM [F(7,40)=1.872, P=0.1001].
Figure 2.
The effects of DADLE on 6-OHDA-induced SH-SY5Y cell damage. The cells were treated with 6-OHDA (150 μM) and different doses of DADLE for a 24 h period. Data are expressed as mean±SEM; n=5-6 wells for each group.
Furthermore, 24 h treatment of cells with different concentrations of morphine, DAMGO and DADEL without toxin had no significant effect on cell viability (data not shown).
3.2. Measurement of intracellular ROS in SH-SY5Y cells
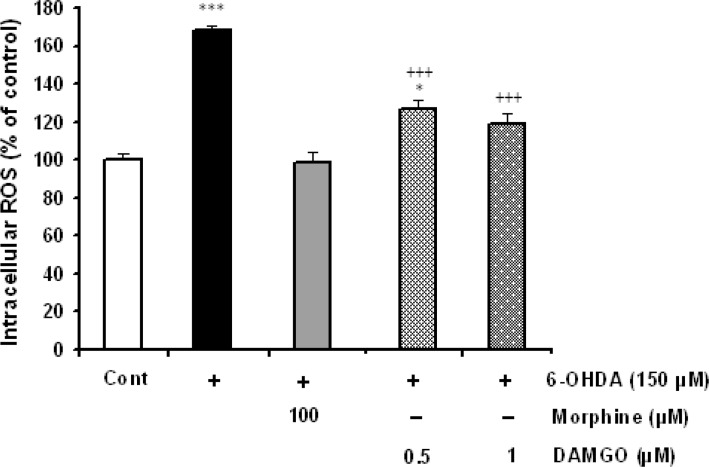
The intracellular ROS levels were determined in control, 6-OHDA-treated and 6-OHDA-treated cells that received different concentration of morphine and DAMGO. Treatment of SH-SY5Y cells with 6-OHDA (150 μM) significantly increased the levels of interacellular ROS (P<0.001). 6-OHDA-induced ROS elevation was significantly damped in morphine- (50 and 100 μM) and DAMGO- (0.5 and 1 μM) treated cells (Figure 3).
Figure 3.
The effects of μ opioid agonists morphine and DAMGO on 6-OHDA-induced intracellular ROS elevation. 24 h 6-OHDA treatment dramatically increased the intracellular level of ROS and morphine (50 μM) as well as DAMGO (0.5 and 1 μM) could suppress ROS production. Data are expressed as mean±SEM; n=5–6 wells for each group. *P<0.05 and ***P<0.001 versus control cells. +++P<0.001 versus 6-OHDA-treated cells.
3.3. Determination of intracellular calcium [Ca2+]i in SH-SY5Y cells
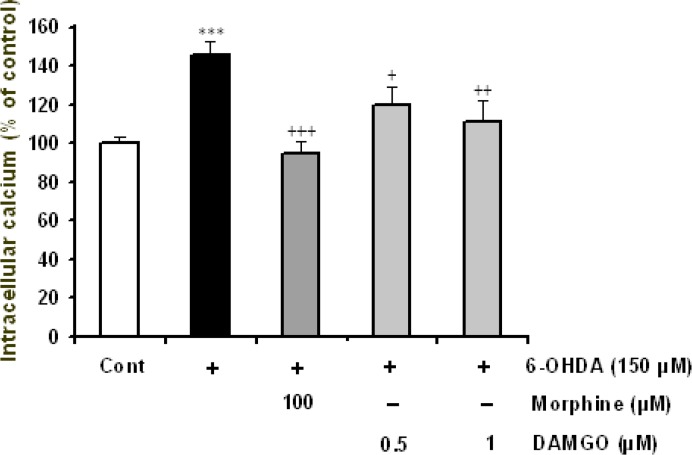
As shown in Figure 4, intracellular calcium was significantly (P<0.001) increased in toxin-treated group. However, in cells that had toxin plus 50 μM morphine, the measured calcium level was closed to that in control cells (P>0.05). DAMGO in doses of 0.5 and 1 μM could significantly (P<0.05 and P<0.001, respectively) suppress 6-OHDA-induced calcium elevation (Figure 4).
Figure 4.
The effects of morphine and DAMGO on 6-OHDA-induced intracellular calcium elevation. Data are expressed as mean±SEM; n=5–6 wells for each group. **P<0.01 and ***P<0.001 versus control cells. ++P<0.01 versus 6-OHDA treated cells.
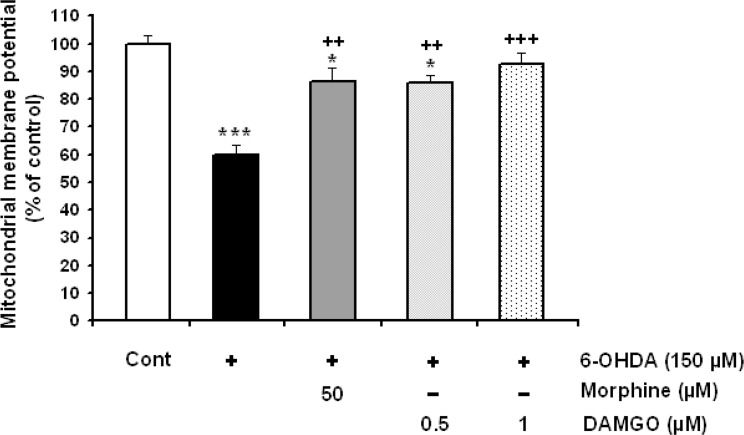
3.4. Determination of mitochondrial membrane potential in SH-SY5Y Cells
As shown in Figure 5, mitochondrial membrane potential was dramatically decreased (P<0.001) in 6-OHDA-treated SH-SY5Y cells. Decreased in mitochondrial membrane potential was attenuated in opioids-treated groups (Figure 5).
Figure 5.
The effects of morphine and DAMGO on 6-OHDA-induced depression of the mitochondrial membrane potential. Data are expressed as mean±SEM; n=5–6 wells for each group. *P<0.05 and ***P<0.001 versus control cells. ++P<0.01 and +++P<0.001 versus 6-OHDA treated cells.
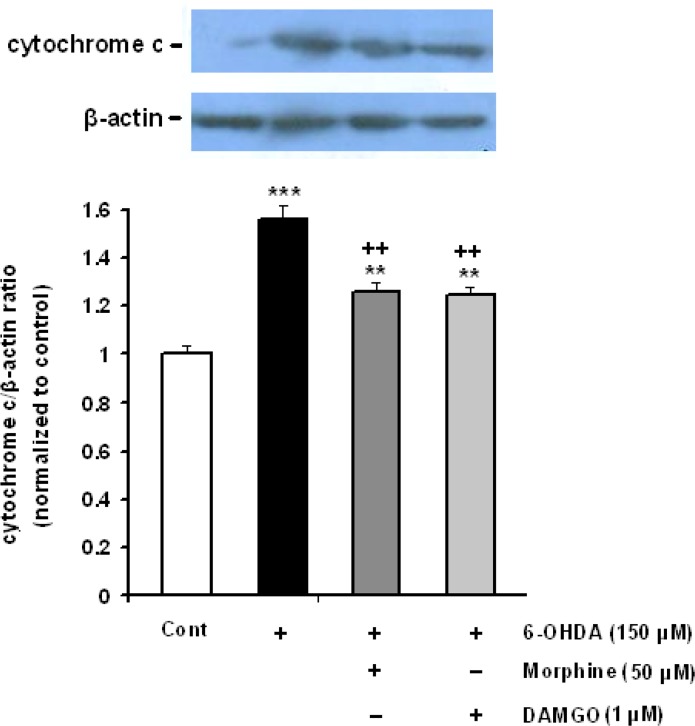
3.5. Western blot analysis of cleaved cytochrome-c and activated caspase-3 in SH-SY5Y cells
To find the effect of opioid agonists on potential mediators of 6-OHDA-induced apoptosis, the rates of cytochrome-c release and caspase 3 activation were analyzed. The SH-SY5Y cells were incubated with 6-OHDA alone or accompanied with morphine (50 μM) and DAMGO (1 μM) for 24 h. As shown in Figure 5, treatment of cells with 150 μM 6-OHDA significantly elicited cytochrome-c release from mitochondria into the cytosol. Opioids could significantly inhibit the release of cytochrome-c (Figure 6).
Figure 6.
Effect of morphine and DAMGO on the 6-OHDA-induced cytochrome-c release in SH-SY5Y cells. Each value represents the mean±SEM band density ratio for each group. β-actin was used as an internal control. **P<0.01 and ***P<0.001 significantly different versus control group. ++P<0.001 versus 6-OHDA-treated cells.
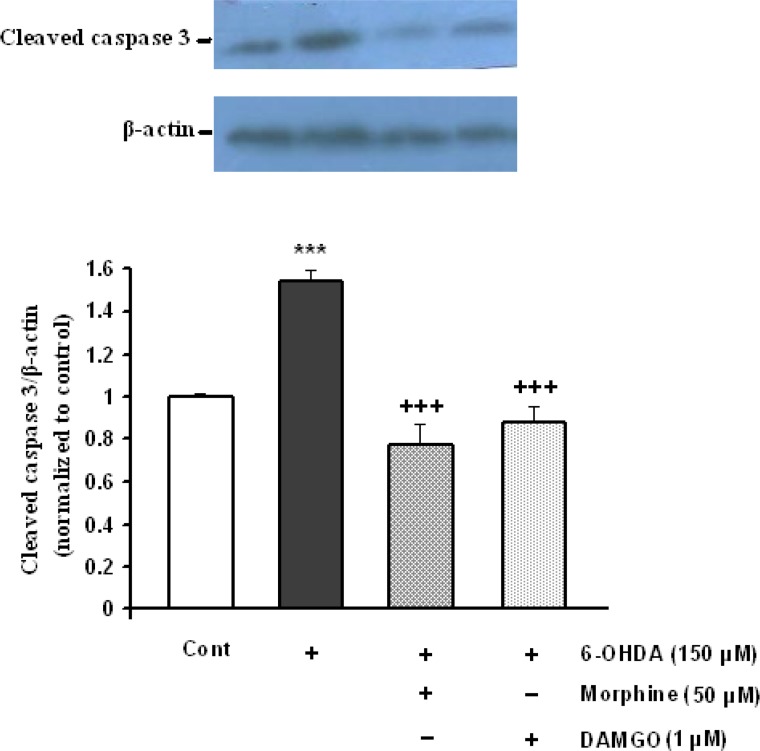
In addition, cleaved caspase-3 was increased following 6-OHDA treatment (P<0.001). Morphine and DAMGO in doses of 50 and 1 μM respectively, significantly reduced 6-OHDA-induced activation of cleaved caspase-3 (Figure 7).
Figure 7.
The activation of caspase-3 protein in SH-SY5Y cells exposed to 150 μM 6-OHDA and 6-OHDA plus protective doses of morphine for 24 h. Each value represents the mean±SEM band density ratio for each group. β-actin was used as an internal control. ***P<0.001 significantly different versus control cells. +++P<0.001 versus 6-OHDA treated cells.
4. Discussion
In this study, the possible role of opioid receptor sub-type in the protective effect of morphine was investigated in 6-OHDA-induced SH-SY5Y cells damage as an in vitro model of PD. The obtained data showed that morphine and DAMGO, as partial and selective full agonist for μ-opioid receptors respectively, can preserve the viability of dopaminergic cells and inhibit apoptotic signals.
In differentiated SH-SY5Y neuroblastoma cells, selective μ-agonist DAMGO and the alkaloid morphine promoted cell survival after serum deprivation, without inducing cell proliferation (Iglesias et al., 2003).
In addition, protective effects of DAMGO have been reported in ethanol-induced gastric mucosal damage (Gyires and Ronai, 2001), cardiac ischemia and reperfusion injury (Maslov et al., 2001), serum-deprived SHSY5Y cells (Wang et al., 2006) low temperature-induced hippocampal neurons death (Tamura et al., 2006), acute hepatic cell death (Chakass et al., 2007) HIV protein gp120-induced neuronal/glial cell damage (Avdoshina, Biggio, & Palchik, 2010).
It is documented that oxidative stress plays a critical role in the pathogenesis of parkinson’s disease which damages cell molecules and leads to the dopaminergic neurodegeneration (Gaki and Papavassiliou, 2014). Our results showed that μ-opioid receptor agonists have inhibitory effects against 6-OHDA-induced oxidative stress (Figure 3). Surprisingly, the potent antioxidant activity of opioids has been reported. In vitro antioxidant, capacity of opioids was demonstrated using different antioxidant tests, including total antioxidant activity, reducing power, free radical scavenging, superoxide anion radical scavenging, hydrogen peroxide scavenging and metal chelating activities (Gulcin et al., 2004). In addition, Lee and colleagues (2004) reported that morphine protects primary rat neonatal astrocytes from glutamate-induced cytotoxicity and shows antioxidant property. They found that morphine can inhibit the generation of H2O2 which dose not block by μ-receptor antagonist, naloxone, or mimic by selective ligand, DAMGO (Lee et al., 2004). However, our finding revealed that morphine protective effect is receptor dependent and could be blocked by naloxone (Elyasi et al., 2014) and induced by DAMGO (present data).
Numerous reports indicate that the increased calcium entry is also responsible for neurotoxin-induced neurode-generation. Changes in Ca2+ homeostasis and increased in intracellular Ca2+ have been reported in in vitro and in vivo models of PD (Surmeier and Schumacker, 2013). One aspect of opioid receptor signal transduction is their ability to modulate calcium and potassium ion channels. Following opioid receptor activation and dissociation of Gαi from Gβγ, the Gβγ protein subunit directly interacts with calcium channels and reduces voltage activation of channel pore opening (Al-Hasani and Bruchas, 2011).
Our data showed that μ-opioid agonist DAMGO could significantly suppress 6-OHDA-induced intracellular calcium elevation (Figure 4). It seems that Ca2+ channel blockade by μ-opioid receptor signaling can be, at least in part, responsible for observed neuroprotective effects in this study. However, the exact of such possible mechanism needs further investigation.
Oxidative stress disrupts the mitochondrial membrane potential which promotes apoptosis-inducing factors release, i.e. cytochrome-c, and activates caspase cascade, such as caspase-3. Caspases take part in the apoptotic process in two broad pathways: the death receptor pathway and the mitochondrial apoptotic pathway. Numerous scientific reports have demonstrated that neuronal death in PD is strikingly associated with activation of caspase-3 and the release of cytochrome- c can promote the activation of caspase-3 in cell models of PD (Tatton, Chalmers-Redman, & Brown, 2003; Esmaeili-Mahani, Vazifekhah, & Pasban-Aliabadi, 2013).
Here, we evaluated the effect of μ-opioid agonists on the release of apoptosis-inducing factors, cytochrome-c, and on caspase-3 activation as final executor of apoptosis pathway using immunoblotting in toxin- and drugs-treated SH-SY5Y cells. Our data indicated that 6-OHDA-produced SH-SY5Y cell death increased the activity of caspase-3 and cytochrome-c levels and incubation of cells with μ-opioid agonists reduced the mentioned components (Figures 6 and 7). It seems that such opioids effects may be mediated by its antioxidant, calcium blockage and anti-apoptotic activities via reduction of ROS inhibition of calcium and suppression of caspase-3 activation and cytochrome-c release.
It has been reported that the inflammatory cytokines and TNF- α levels significantly increase in the brain of parkinsonian patients (Sawada, Imamura, & Nagatsu, 2006). Targeting TNF-α can induces ameliorating effect in neuroinflammation in neurodegenerative diseases such as Parkinson’s disease (Frankola, Greig, & Luo, 2011).
Saccani et al. (2012) reported that μ-opioid agonist DAMGO significantly reduced tissue damage and TNF-α expression in rat mesenteric ischemia/reperfusion which was reversed by μ-opioid antagonist CTAP (Saccani et al., 2012). Such possible anti-inflammatory effect may be elicited by μ-opioid agonists in this study.
A central question is how morphine exerts its protective actions at the cellular and molecular level. However, further studies need to clarify the protective cascade signaling of μ-opioid receptors.
Our results reveal that μ-opioid receptor activation has a protective role in SH-SY5Y dopaminergic cells against 6-OHDA-induced apoptosis and neurodegeneration. It seems that such property is established through antioxidant, calcium blocking and anti-apoptotic strategies.
Acknowledgments
This work was supported by Kerman Neuroscience Research Center (KNRC/93).
Footnotes
Conflict of interest
No conflict to disclose.
References
- Al-Hasani R., Bruchas M. R. (2011). Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology, 115(6), 1363– 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V., Biggio F., Palchik G., Campbell L. A., Mocchetti I. (2010). Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia, 58, 1630– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Malaquias A., Almeida M. B., Souza Monteiro J. R., Macchi Bde M., do Nascimento J. L., Crespo-Lopez M. E. (2014). Morphine protects against methyl mercury intoxicaition: A role for opioid receptors in oxidative stress? PLoS One, 9(10), e110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakass D., Philippe D., Erdual E., Dharancy S., Malapel M., Dubuquoy C., Thuru X., Gay J., Gaveriaux-Ruff C., Dubus P., Mathurin P., Kieffer B. L., Desreumaux P., Chamaillard M. (2007). Micro-Opioid receptor activation prevents acute hepatic inflammation and cell death. Gut, 56(7), 974– 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Wang Y., Dong Q., Wu S., Xiao X., Hu J., Chai Z., Zhang Y. (2011). Morphine protects against intracellular amyloid toxicity by inducing estradiol release and upregulation of Hsp70. Journal of Neuroscience, 31(45), 16227– 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diógenes M. J., Outeiro T. F. (2010). Neurotrophic factors as a protective strategy in Parkinson’s disease. CNS & Neurological Disorders - Drug Targets, 9(6), 754– 63. [DOI] [PubMed] [Google Scholar]
- Elyasi L., Eftekhar-Vaghefi S. H., Esmaeili-Mahani S. (2014). Morphine protects SH-SY5Y human neuroblastoma cells against 6-hydroxydopamine-induced cell damage: involvement of anti-oxidant, calcium blocking, and anti-apoptotic properties. Rejuvenation Research, 17(3), 255– 63. [DOI] [PubMed] [Google Scholar]
- Esmaeili-Mahani S., Vazifekhah S., Pasban-Aliabadi H., Abbasnejad M., Sheibani V. (2013). Protective effect of orexin-A on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells. Neurochemistry International, 63(8), 719– 25. [DOI] [PubMed] [Google Scholar]
- Frankola K. A., Greig N. H., Luo W., Tweedie D. (2011). Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS & Neurological Disorders - Drug Targets, 10(3), 391– 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaki G. S., Papavassiliou A. G. (2014). Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolecular Medicine, 16(2), 217– 30. [DOI] [PubMed] [Google Scholar]
- Gulcin I., Beydemir S., Alici H. A., Elmastaş M., Buyukokuroglu M. E. (2004). In vitro antioxidant properties of morphine. Pharmacological Research, 49(1), 59– 66 [DOI] [PubMed] [Google Scholar]
- Gyires K., Ronai A. Z. (2001). Supraspinal δ-and μ-opioid receptors mediate gastric mucosal protection in the rat. Journal of Pharmacology and Experimental Therapeutics, 297(3), 1010– 1015. [PubMed] [Google Scholar]
- Husain S., Abdul Y., Potter D. E. (2012). Non-analgesic effects of opioids: neuroprotection in the retina. Current Pharmaceutical Design, 18(37), 6101– 8. [DOI] [PubMed] [Google Scholar]
- Iglesias M., Segura M. F., Comella J. X., Olmos G. (2003). Mu-opioid receptor activation prevents apoptosis following serum withdrawal in differentiated SH-SY5Y cells and cortical neurons via phosphatidylinositol 3-kinase. Neuropharmacology, 44(4), 482– 92. [DOI] [PubMed] [Google Scholar]
- Lee J., Kim M. S., Park C., Jung E. B., Choi D. H., Kim T. Y., Moon S. K., Park R. (2004). Morphine prevents glutamate-induced death of primary rat neonatal astrocytes through modulation of intracellular redox. Immunopharmacology and Immunotoxicology, 26(1), 17– 28. [DOI] [PubMed] [Google Scholar]
- Maslov L. N., Lasukova T. V., Solenkova N. V., Lishmanov A. Iu., Bogomaz S. A., Tam S. V., Gross G. J. (2001). Participation of K(ATP)-channels in cardioprotective effect of mu-opioid receptor agonists in acute ischemia and reperfusion of the isolated heart. Eksperimental’naia i Klinicheskaia Farmakologiia, 64(5), 23– 7. [PubMed] [Google Scholar]
- More S. V., Kumar H., Kim I. S., Song S. Y., Choi D. K. (2013). Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators of Inflammation, 2013, 952375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak G. W. (2014). Opiate pharmacology and relief of pain. Journal of Clinical Oncology, 32(16), 1655– 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami F., Oryan S., Ahmadiani A., Dargahi L. (2012). Morphine preconditioning protects against LPS-induced neuroinflammation and memory deficit. Journal of Molecular Neuroscience, 48(1), 22– 34. [DOI] [PubMed] [Google Scholar]
- Saccani F., Anselmi L., Jaramillo I., Bertoni S., Barocelli E., Sternini C. (2012). Protective role of μ opioid receptor activation in intestinal inflammation induced by mesenteric ischemia/reperfusion in mice. Journal of Neuroscience Research, 90(11), 2146– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M., Imamura K., Nagatsu T. (2006). Role of cytokines in inflammatory process in Parkinson’s disease. Journal of Neural Transmission. Supplement, 70, 373– 81. [DOI] [PubMed] [Google Scholar]
- Spetea M., Asim M. F., Wolber G., Schmidhammer H. (2013). The μ opioid receptor and ligands acting at the μ opioid receptor, as therapeutics and potential therapeutics. Current Pharmaceutical Design, 19(42), 7415– 34. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Schumacker P. T. (2013). Calcium, bioenerkgetics, and neuronal vulnerability in Parkinson’s disease. Journal of Biological Chemistry, 288(15), 10736– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y., Monden M., Shintani M., Kawai A., Shiomi H. (2006). Neuroprotective effects of hibernation-regulating substances against low-temperature-induced cell death in cultured hamster hippocampal neurons. Brain Research, 1108(1), 107– 16. [DOI] [PubMed] [Google Scholar]
- Tatton W. G., Chalmers-Redman R., Brown D., Tatton N. (2003). Apoptosis in Parkinson’s disease: signals for neuronal degradation. Annals of Neurology, 53(3), 61– 70. [DOI] [PubMed] [Google Scholar]
- Tolleson C. M., Fang J. Y. (2013). Advances in the mechanisms of Parkinson’s disease. Discovery Medicine, 15(80), 61– 6. [PubMed] [Google Scholar]
- Wang Y., Xie W. Y., He Y., Wang M., Yang Y. R., Zhang Y., Yin D. M., Jordan-Sciutto K. L., Han J. S., Wang Y. (2006). Role of CDK5 in neuroprotection from serum deprivation by mu-opioid receptor agonist. Experimental Neurology, 202(2), 313– 23. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen Q., Yu L. C. (2008). Morphine: a protective or destructive role in neurons? Neuroscientist, 14(6), 561– 70. [DOI] [PubMed] [Google Scholar]