Abstract
Introduction:
Lower level of estrogen hormone is considered as an important factor for loss of learning and memory in postmenopausal women. Although estrogen replacement therapy is used for compensation, but long-term usage of estrogen is associated with a higher risk of hormone-dependent cancers. Phytoestrogens, due to fewer side effects, have been proposed to prevent menopause-related cognitive decline.
Methods:
24 female Wistar rats weighing 180–220 g were used in this study. The animals were ovariectomized and randomly divided into four groups including, control and two groups which received 8 and 80 mg/kg Vitex agnus castus (VAC) ethanolic extract orally. The last groups were treated with 40 μg/kg of estradiol valerat. Step-through passive avoidance (STPA) test was used for the evaluation of learning and memory. The hippocampal estrogen receptor α (ERα) expression was measured using Real-Time PCR.
Results:
The results demonstrated that VAC extract or estradiol had better performance on step-through passive avoidance test than control group (all P<0.05). Moreover, administration of either estradiol or VAC extract increased the hippocampal mRNA level of ERα and prevented the decrease in uterine weight of ovariectomized rats.
Discussion:
Based on our data, VAC extract improves learning and memory in ovariectomized rats. The positive effect of VAC extract on learning and memory is possibly associated with an increase in ERα gene expression in the hippocampal formation.
Keywords: Vitex agnus-castus, Memory, Ovariectomy, Estrogen receptor alpha
1. Introduction
Menopause is defined by permanent cessation of the primary functions of the human ovaries. Hormonal fluctuations that occur during menopause, may affect on the mood and cognitive processes (Genazzani, Pluchino, Luisi, & Luisi, 2007; Markou, Duka, & Prelevic, 2005). Emotional disorders such as postpartum and postmenopausal depression are the most common forms of mental illness associated with decreased serum levels of ovarian hormones (Toufexis, Myers, & Davis, 2006). Many studies have shown that estrogen deficiency after menopause may impair learning and memory function in women (Pompili, Arnone, & Gasbarri, 2012). Moreover, there is a strong relationship between reduced estrogen levels and incidence of Alzheimer’s disease (AD) (Craig & Murphy, 2009). On the other hand, estrogen replacement therapy is effective for improving or preventing depression and AD, respectively (Amin, Canli, & Epperson, 2005; Marin et al., 2003).In fact, it has been shown that estrogen by reduction in β amyloid accumulation and oxidative damage as well as amplification of cerebral blood flow and neurotransmitter release or action, improves brain functions (McEwen, 1999; Toran-Allerand, Singh, & Setalo, 1999; Xu et al., 1998). Nevertheless, the benefits of estrogen replacement therapy in postmenopausal women are usually overshadowed by serious side effects of estrogen (Warren, 2004). Particularly, the long-term use of estrogen in women may increase the risk of breast and endometrial cancers (Colditz, 1998). Nowadays, there are several propositions for the relief of mood disorders in postmenopausal women, which mainly consists of estrogenic agents, combination therapy and plant-derived natural products (Soares, Prouty, Born, & Steiner, 2005). Phytoestrogens and many other estrogen-like compounds in plants, due to fewer side effects, have been massively proposed to prevent menopause-related cognitive decline (Kennedy & Scholey, 2006).
Phytoestrogens are non-steroidal compounds that can behave as estrogen receptor agonist or antagonist (Makela, Pylkkanen, Santti, & Adlercreutz, 1995). For example, in cell culture, it was observed that these compounds inhibited the growth of estrogen-dependent tumor cells (Santell, Kieu, & Helferich, 2000). In contrast, it has been reported that phytoestrogens may have beneficial effects on cognitive performance in postmenopausal women (Greendale et al., 2012).
Vitex agnus castus (VAC) belongs to the Verbenaceae family and is distributed throughout Mediterranean, Europe, central Asia and the United States (Meier, Berger, Hoberg, Sticher, & Schaffner, 2000).Traditionally, the fruits of this plant have been used as a herbal medicine for treatment of hormone disorders in women so that, the fruit extract is used for the treatment of severe premenstrual syndrome and hot flashes associated with menopause (He et al., 2009). Its phytoconstituent includes flavonoids, terpenoids and glycosides (Chen et al., 2011). It is reported that VAC extract is a proliferation inhibitor and induces apoptosis in prostate epithelial cell lines (Weisskopf et al., 2005). In the current study, we used ovariectomized rats as a model of menopause-related estrogen depletion to evaluate the effect of VAC on learning and memory. Moreover, we investigated the effect of VAC extract on uterine weight and estrogen receptor alpha (ERα) gene expression in the hippocampus of the ovariectomized rats.
2. Methods
2.1. Animals and treatment
24 female Wistar rats weighted 180–220 g were kept under standard laboratory conditions with a temperature of 20 to 22 °C and maintained on a 12 h light/dark cycle with food and water available adlibitum. Rats were operated through dorsal incision under anesthesia with an intraperitoneal injection of ketamine sulfate solution (60 mg/kg) and xylazine (4 mg/kg). Then, ovaries were removed by a cautery device, wound was closed, and 22,000 i.u/kg penicillin was injected for two days after surgery. Vaginal smears were taken from rats to confirm the absence of ovarian cycles for at least five days (Goldman, Murr, & Cooper, 2007).
The animals were randomly divided into four groups (n=6) as following: control (vehicle, saline 1ml/kg); VAC 8 mg/kg; VAC 80 mg/kg and estradiol valerat 40 μg/kg (Bunratsami, Udomuksorn, Kumarnsit, Vongvatcharanon, & Vongvatcharanon, 2015) (Aboureihan Co, Tehran, Iran). All animals were treated daily at 11 A.M. by oral administration (P.O.) of all compounds for three months. The doses of VAC were chosen based on our previous experiments (Honari et al., 2012).
2.2. Extraction of VAC
Vitex agnus castus was purchased from the central herbarium of Isfahan University and was identified by experts of the central herbarium of Isfahan University with registration number 9252, in 2012. The quantities of major components of VAC in Iran have been already reported (Ghannadi et al., 2012). The main compositions of the plant’s seeds are caryophyllene oxide (25%), n-hexadecane (12.5%), α-terpinyl acetate (11.6%) and bicyclogermacrene (8.4%) (Ghannadi et al., 2012). The dried fruit (seeds) of VAC were rinsed with distilled water. After grinding, 500 g of plant was extracted in a Soxhlet apparatus with ethanol. Finally, ethanol was evaporated with a rotary machine and the residual was dried to form a powder. The resultant extract was then reconstituted by solving it in saline before being orally administered to animals.
2.3. Behavioral testing
Step-through passive avoidance (STPA) apparatus was used for the evaluation of learning (Panlab Co, Spain). The apparatus included both light and dark compartments with equal sizes (20 cm×20 cm×30 cm) which were separated by a guillotine door. The floor was made of steel rods with a diameter of 3 mm and 1 cm space. The following experimental protocol was based on the designs described previously (Tahamtan et al., 2013).
Habitation stage: rats were placed in the laboratory for 30 minutes to habitude to the experimental environment. Animals were transferred to the light compartment and following 5 seconds the guillotine door between the two compartments was removed and the animal was allowed moving freely toward the dark compartment for 5 minute. The animal was removed from the study if it did not enter the dark compartment after 300 seconds. Following entry into the dark side, the guillotine door was closed and the rats were returned to the cage after 10 seconds.
Training stage: This stage began 1 h after habituation stage. Each rat was placed in the light compartment following 5 seconds the guillotine door between the two compartments was removed. Following the entrance to the dark compartment, the guillotine door was immediately closed and an electric shock was delivered to the floor bars (0.5 mA, 2s). We repeated the procedure until the latency to enter the dark box was more than 300s. The latency of rat for entering the dark compartment was reported as initial latency (IL).
Retention stage: the retention time was measured 24 hours after the training stage. The animals were returned to the light compartment and the latency for entering the dark compartment was reported as Step-through latency (STL). If the rat did not enter the dark compartment during 300 seconds, it was transferred to its cage and 300 seconds was reported (maximum latency) but if the rat entered to the dark compartment, the retention time of rat in the dark compartment was also recorded as total time in dark chamber (TDC) (Tahamtan et al., 2013).
2.4. Locomotor activity test
Locomotor activity was measured by a glass box with a black plastic floor (35 cm×35 cm×35 cm). Each animal’s activity was monitored by a camera during 30 min. The Ethovision software was used to calculate the total distance traveled and average speed (centimeters per second) for each animal (Rajabi et al., 2012).
2.5. Uterus and brain removal
At the end of behavioral testing, the animals were deeply anesthetized using pentobarbital (80 mg/kg) and then were decapitated by a guillotine. The brains were removed, the hippocampal formations isolated and stored at −80°C until mRNA extraction. The uterine was also removed and weighted in all animals.
2.6. Real-Time PCR
Total mRNA was extracted from hippocampal formation using the RNA extraction kit from Cinnaclon Company (RNX-Solution) (Tehran, Iran). The extracted mRNA quality was identified by measuring absorption at 260/280 nm by a UV spectrophotometer and also using electrophoresis on an ethidium bromide pretreated agarose gel. cDNA was synthesized from extracted mRNA using a cDNA synthesis kit (Parstous, Iran) with oligo (dT) primers. The reverse transcription step was performed using the following steps: 70°C for 10 min (without reverse transcription enzymes), −20°C for 1 min (cooling), addition of reverse transcription enzymes, 42°C for 60 min, following with a final step at 95°C for 10 min. Real-time PCR was performed using a SYBR green master mix (Parstous, Iran), combined with 200 ng of template cDNA with the estrogen receptor alpha (ERα) and Beta-2 microglobulin (B2M), as housekeeping gene, primers (Table 2) in a Bio-Rad CFX96 system (Bio-Rad Company, USA) using the following program: 1 cycle of 95°C for 15 min, 40 cycles of 95°C for 30 s, and 60°C for 30 s and 72°C for 30 s.
Table 2.
The effects of long-term administration of Vitex agnus castus ethanolic extract (VAC8 and 80 mg/kg) and estradiol valerat (40 μg/kg) on passive avoidance task in the ovariectomized rats.
| Control | VAC 80 | VAC 8 | Estradiol valerat | |
|---|---|---|---|---|
| Initial latency | 10.1± 1 | 9.3±1.4 | 9.3±1.3 | 9.7±1 |
| Step through latency (STL) | 28.1±1.6 | 184.3±31.9* | 178.8±15.2* | 220.8±2.2* |
| Time in dark chamber (TDC) | 58.5±4.9 | 31.1±4.5# | 34.5±2.3# | 24.6±1.7# |
| Shock number | 7.5±0.6 | 4±0.7$ | 5.3±0.4 | 2.6±0.3$ |
Significant differences in STL between VAC80, VAC8 and estradiol valerat with control group. All P<0.001.
Significant differences in TDC between VAC80, VAC8 and estrogen with control group. All P<0.001.
Significant differences in shock number between VAC80 and estrogen with control group. All P<0.001. Data expressed as mean±SEM of six animals. Control; group that received saline, VAC80 and VAC8; groups that receive 80 and 8 mg/kg VAC respectively, estrogen; group that received estradiol. All groups were ovariectomized.
Primers were synthesized by the Cinnaclon company (Iran). Real-Time PCR were performed in triplicate and the relative amounts of PCR product were determined by the 2−ΔΔCt formula. The CFX manager software version 1.1.308.111 (Bio-Rad, USA) was used for quantitative analyses of the data and melting curves checking. PCR products were also rechecked by electrophoresis on a 1% gel agarose containing 0.5 mg/ml ethidiumbromide (Hakimizadeh et al., 2014).
2.7. Statistical analysis:
Data are shown as mean±SEM and analyzed by oneway analysis of variance (ANOVA) and Tukey’s test as post-hoc test. In all groups, values were considered statistically significant when P<0.05.
3. Results
3.1. Passive avoidance test
Step-through passive avoidance test was used to assess the memory of animals. ANOVA analysis indicated that oral administration of VAC extract (8 and 80 mg/kg) and estradiol (40 μg/kg) had no significant effect on initial latency (IL) when compared with the control group (Table 2) (ANOVA F3=2.009, P>0.05). The step-through latency (STL) in rats treated with either 8 or 80 mg/kg of VAC extract or estradiol was significantly higher in comparison with control group (F3=23.09, P<0.001). There was no significant difference between the effects of different doses of VAC on STL (P>0.05). The effect of VAC on retention latency was also similar to estradiol (P>0.05) (Table 1).
Table 1.
Primer sequences of evaluated genes.
| Target gene | Primer sequences |
|---|---|
| Estrogen receptor α (ERα) | F: 5′-CAAACCAATGCACCATCGATAA-3′ |
| R: 5′-TTTTCGTATCCCGCCTTTCA-3′ | |
| Beta-2 microglobulin (B2M) | F: 5′-CCTGGCTCACACTGAATTCACAC-3′ |
| R: 5′-AACCGGATCTGGAGTTAAACTGGTC-3′ |
In line with STL, TDC of rats treated with 8 and 80 mg/ kg extract or estradiol was also decreased significantly in comparison with control group (F3=16.23, all P<0.001). However, there was no significant difference between different doses of VAC on TDC (P>0.05). The effect of VAC on TDC was similar to estradiol (F3=16.23, P>0.05) (Table 1). The number of electric shocks needed to reach the criterion (don’t enter in dark chamber for at least 300s) (learning phase) of rats treated with estradiol and VAC (80 mg/kg) decreased significantly compared to control group (F3=13.35, all P<0.001) (Table 1).
3.2. Locomotor activity
One-way ANOVA demonstrated that there was no significant difference in locomotor activity of different groups compared with control rats (data not shown).
3.3. Uterine weight
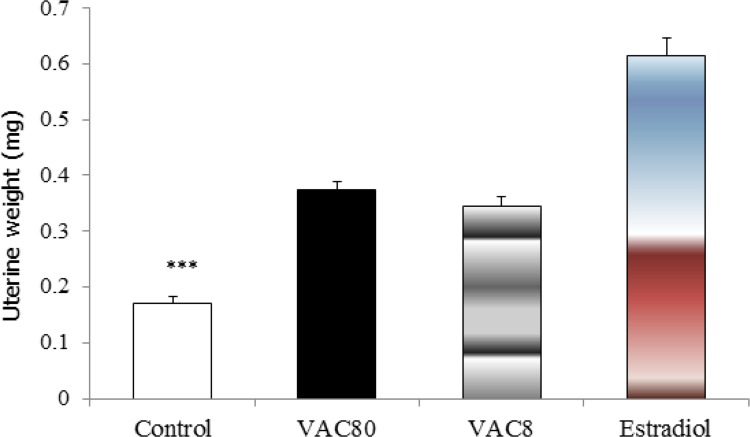
The results revealed a significant increase in the uterine weight in VAC and estradiol treated groups compared to control group (F3=74.97, all P<0.001) (Figure 1).
Figure 1.
Effects of long-term administration of Vitex agnus castus extract (8 and 80 mg/kg) and estradiol on uterine weight in the ovariectomized rats. *** Significant differences among VAC80, VAC8 and estradiol with control group all P<0.001. Data expressed as mean±SEM of six animals. Control; group that received saline (as vehicle), VAC80 and VAC8; groups that received 80 and 8 mg/kg VAC for three months (p.o.) respectively, estrogen; group that received estradiol for three months (p.o.). All groups were ovariectomized.
3.4. mRNA levels of estrogen receptor α in the hippocampal formation
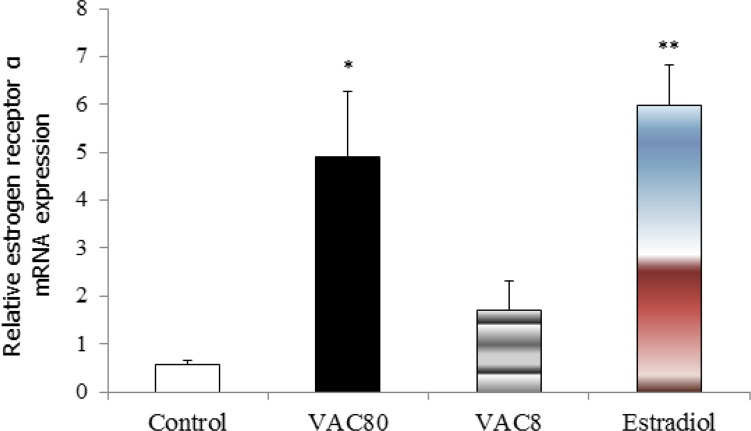
The results showed that mRNA levels of estrogen receptor α in the hippocampus were increased significantly in VAC80 (F3=11.32, P<0.05) and estradiol (P<0.01) groups compared with control group (Figure 2).
Figure 2.
Effects of long-term administration of Vitex agnus castus extract (8 mg/kg and 80 mg/kg) and estradiol on the mRNA levels of α-estrogen receptor in the ovariectomized rats. * Significant differences between Vit 80 with control group P<0.05. ** Significant differences between estradiol with control group P<0.01.
4. Discussion
Our findings indicated that treatment with VAC extract (8 and 80 mg/kg) and estradiol valerat (40 μg/kg) improved learning and memory performance significantly in ovariectomized rats. This improvement of performance in the high dose of VAC was almost equal to estradiol. Furthermore, we observed that VAC extract significantly increased the expression of estrogen receptors α mRNA in hippocampal formation. Our findings also indicated that VAC extract at the dose of 80 mg/kg increased the uterine weight significantly in ovariectomized rats which were similar to estradiol group.
It is well-known that cessation or cyclic fluctuations of ovarian estrogens can influence cognitive functions in woman (Gasbarri et al., 2008). In contrast, hormone or phytoestrogen therapy can minimize cognitive changes in postmenopausal women (Bagger, Tankó, Alexandersen, Qin, & Christiansen, 2005; Duffy, Wiseman, & File, 2003). The role of estrogens in cognitive functions could be explained by the widespread distribution of estrogen receptors in brain regions associated with learning and memory such as hippocampus and basal forebrain (B. S. McEwen & Alves, 1999). Hippocampus is a brain area that plays a critical role in cognitive functions (B. McEwen, 2002) and both ERα and β are expressed in hippo-campus (Register, Shively, & Lewis, 1998). A number of studies indicated ERα mRNA expression in hippocampal CA1 pyramidal cells both during aging and Alzheimer’s disease is lower than normal (Hu et al., 2003; Ishunina, Fischer, & Swaab, 2007). Data from animal studies indicated that estradiol therapy can have beneficial effects on cognition through increasing ERα in the hippocampus (Bohacek & Daniel, 2009; Miller, Jover, Cohen, Zukin, & Etgen, 2005). In line with this, our results also showed that both estradiol and high dose of VAC increased expression of ERα mRNA in the hippocampal formation.
Phytoestrogens are estrogen-like molecules that have a high affinity for estrogen receptors (Kuiper et al., 1998). Despite their very low affinity for binding to the receptor α, some phytoestrogens such as genistein and quercetin have shown a full agonistic effect for ERα and ERβ in vitro studies (Maggiolini et al., 2001). Although the main composition of the plant’s fruit has been already detected and reported (Ghannadi et al., 2012), in the current study, we focused on whole composition of the extract. Conventional wisdom in traditional medicine suggests that the whole part of a herb, due to synergy of its ingredients, is more effective than its single compositions (S. Foster, 2012).
Following three months of treatment with VAC extract and estradiol, we have found a significant increase in ERα mRNA expression in the hippocampus, which was in parallel with the findings of other investigators (J. Liu et al., 2004). It is well known that extract of VAC contains compounds which bind to ERα and ERβ (J. Liu et al., 2004; J. Liu et al., 2001).
Uterus is one of the main target organs for estrogen. Estrogen can increase number of smooth muscle cells, endometrial paranchyme, uterine weight and uterine volume (Hamilton, Arao, & Korach, 2014). So it is expected that drugs with estrogen like activity could also increased uterine weight. Our results, similar to previous reports (Seidlova-Wuttke et al., 2003), showed a significant increase in the uterine weight of animals treated with either estradiol or both low and high doses of the plant extract. This can infer more that the VAC has estrogenic effects.
It is proved that synaptic plasticity plays an important role in hippocampal-dependent learning and memory. On the other hand, in the hippocampal neurons, estradiol-induced synaptogenesis is dependent on ER (F. Liu et al., 2008). Estradiol-induced synaptogenesis is reported by an increase in synaptic number and synaptic molecular markers (Akama & McEwen, 2003; Jelks, Wylie, Floyd, McAllister, & Wise, 2007). Some studies showed that ERα is the predominate receptor for long-term changes on synaptogenesis (T. C. Foster, 2012). So, it may be possible to suggest that estrogen improved memory function by both increase in ER mRNA expression and neuronal plasticity in the hippocampus (C. Li et al., 2004; Spencer et al., 2008). Similarly, it has been reported that phytoestrogens improve learning and memory in ovariectomized rats by increasing synapse density in the hippocampus (H. Li et al., 2008). Thus, on the basis of our results, we can conclude that oral administration of VAC extract may improve learning and memory in the ovariectomized rats and this effect is comparable with estradiol. The effect of VAC extract in the prevention of learning and memory impairment may be associated with an increase in ERα mRNA in the hippocampal formation. However, low dose of VAC (8mg/kg), in spite of preventing learning and memory impairment, cannot change ERα mRNA expression in hippocampus of ovariectomized rats. This led to conclude that other mechanisms, such as antioxidant (Moreno et al., 2015), anti-inflammatory (M. M. Li et al., 2014) properties of VAC may be involved in beneficial effects of this plant on learning and memory. So, the exact mechanism of the VAC extract on cognition needs to be further investigated.
In conclusion, result of this study demonstrated that VAC extract improves learning and memory (possibly by a mechanism associated with an increase in ERα gene expression in the hippocampal formation) in ovariectomized rats.
Acknowledgments
This study was financially supported by the research vice chancellor of Rafsanjan University of Medical Sciences.
References
- Akama K. T., McEwen B. S. (2003). Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. The Journal of Neuroscience, 23(6), 2333– 2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin Z., Canli T., Epperson C. N. (2005). Effect of estrogenserotonin interactions on mood and cognition. Behavioral and Cognitive Neuroscience Reviews, 4(1), 43– 58. [DOI] [PubMed] [Google Scholar]
- Bagger Y. Z., Tankó L. B., Alexandersen P., Qin G., Christiansen C. (2005). Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause, 12(1), 7– 12. [DOI] [PubMed] [Google Scholar]
- Bohacek J., Daniel J. (2009). The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. Journal of Neuroendocrinology, 21(7), 640– 647. [DOI] [PubMed] [Google Scholar]
- Bunratsami S., Udomuksorn W., Kumarnsit E., Vongvatcharanon S., Vongvatcharanon U. (2015). Estrogen replacement improves skeletal muscle performance by increasing parvalbumin levels in ovariectomized rats. Acta Histochemica, 117(2), 163– 175. [DOI] [PubMed] [Google Scholar]
- Chen S. N., Friesen J. B., Webster D., Nikolic D., van Breemen R. B., Wang Z. J., et al. (2011). Phytoconstituents from Vitex agnus-castus fruits. Fitoterapia, 82(4), 528– 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz G. A. (1998). Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. Journal of the National Cancer Institute, 90(11), 814– 823. [DOI] [PubMed] [Google Scholar]
- Craig M. C., Murphy D. G. (2009). Alzheimer’s disease in women. Best Practice & Research Clinical Obstetrics & Gynecology, 23(1), 53– 61. [DOI] [PubMed] [Google Scholar]
- Duffy R., Wiseman H., File S. E. (2003). Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacology Biochemistry and Behavior, 75(3), 721– 729. [DOI] [PubMed] [Google Scholar]
- Foster S. (2012). Tyler’s honest herbal: a sensible guide to the use of herbs and related remedies. Routledge. [Google Scholar]
- Foster T. C. (2012). Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus, 22(4), 656– 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarri A., Pompili A., d’Onofrio A., Cifariello A., Tavares M. C., Tomaz C. (2008). Working memory for emotional facial expressions: role of the estrogen in young women. Psychoneuroendocrinology, 33(7), 964– 972. [DOI] [PubMed] [Google Scholar]
- Genazzani A. R., Pluchino N., Luisi S., Luisi M. (2007). Estrogen, cognition and female ageing. Human Reproduction Update, 13(2), 175– 187. [DOI] [PubMed] [Google Scholar]
- Ghannadi A., Bagherinejad M., Abedi D., Jalali M., Absalan B., Sadeghi N. (2012). Antibacterial activity and composition of essential oils from Pelargonium graveolens L’Her and Vitex agnus-castus L. Iranian Journal of Microbiology, 4(4), 171– 176. [PMC free article] [PubMed] [Google Scholar]
- Goldman J. M., Murr A. S., Cooper R. L. (2007). The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research Part B: Developmental and Reproductive Toxicology, 80(2), 84– 97. [DOI] [PubMed] [Google Scholar]
- Greendale G. A., Huang M. H., Leung K., Crawford S. L., Gold E. B., Wight R., et al. (2012). Dietary phytoestrogen intakes and cognitive function during the menopausal transition: results from the Study of Women’s Health Across the Nation Phytoestrogen Study. Menopause, 19(8), 894– 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimizadeh E., Kazemi Arababadi M., Shamsizadeh A., Allahtavakoli M., Rezvani M. E., Roohbakhsh A. (2014). Morphine reduces expression of TRPV1 receptors in the amygdala but not in the hippocampus of male rats. Iranian Journal of Medical Sciences, 39(3), 261– 267. [PMC free article] [PubMed] [Google Scholar]
- Hamilton K. J., Arao Y., Korach K. S. (2014). Estrogen hormone physiology: reproductive findings from estrogen receptor mutant mice. Reproductive Biology, 14(1), 3– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Chen R., Zhou Y., Geng L., Zhang Z., Chen S., et al. (2009). Treatment for premenstrual syndrome with Vitex agnus castus: A prospective, randomized, multi-center placebo controlled study in China. Maturitas, 63(1), 99– 103. [DOI] [PubMed] [Google Scholar]
- Honari N., Pourabolli I., Hakimizadeh E., Roohbakhsh A., Shamsizadeh A., Vazirinejad R., et al. (2012). [Effect of vitex agnus castus extraction on anxiety-like behaviors in ovariectomized rats (Persian)]. Journal of Babol University of Medical Sciences (Jbums), 14(5), 29– 35. [Google Scholar]
- Hu X. Y., Qin S., Lu Y. P., Ravid R., Swaab D. F., Zhou J. N. (2003). Decreased estrogen receptor-a expression in hippocampal neurons in relation to hyperphosphorylated tau in Alzheimer patients. Acta Neuropathologica, 106(3), 213– 220. [DOI] [PubMed] [Google Scholar]
- Ishunina T. A., Fischer D. F., Swaab D. F. (2007). Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer’s disease. Neurobiology of Aging, 28(11), 1670– 1681. [DOI] [PubMed] [Google Scholar]
- Jelks K. B., Wylie R., Floyd C. L., McAllister A. K., Wise P. (2007). Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-alpha. The Journal of Neuroscience, 27(26), 6903– 6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. O., Scholey A. B. (2006). The psychopharmacology of European herbs with cognition-enhancing properties. Current Pharmaceutical Design, 12(35), 4613– 4623. [DOI] [PubMed] [Google Scholar]
- Kuiper G. G. J. M., Lemmen J. G., Carlsson B., Corton J. C., Safe S. H., van der Saag P. T., et al. (1998). Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology, 139(10), 4252– 4263. [DOI] [PubMed] [Google Scholar]
- Li C., Brake W. G., Romeo R. D., Dunlop J. C., Gordon M., Buzescu R., et al. (2004). Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proceedings of the National Academy of Sciences of the United States of America, 101(7), 2185– 2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li S. L., Gong L., Wang J. L., Li Y. Z., Wu Z. H. (2008). The effects of an herbal medicine Bu-Wang-San on learning and memory of ovariectomized female rat. Journal of Ethnopharmacology, 117(3), 427– 432. [DOI] [PubMed] [Google Scholar]
- Li M. M., Su X. Q., Sun J., Gu Y. F., Huang Z., Zeng K. W., et al. (2014). Anti-inflammatory ursane- and oleanane-type triterpenoids from Vitex negundo var. cannabifolia. Journal of Natural Products, 77(10), 2248– 2254. [DOI] [PubMed] [Google Scholar]
- Liu F., Day M., Muniz L. C., Bitran D., Arias R., Revilla-Sanchez R., et al. (2008). Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nature Neuroscience, 11(3), 334– 343. [DOI] [PubMed] [Google Scholar]
- Liu J., Burdette J., Sun Y., Deng S., Schlecht S., Zheng W., et al. (2004). Isolation of linoleic acid as an estrogenic compound from the fruits of Vitex agnus-castus L. (chaste-berry). Phytomedicine, 11(1), 18– 23. [DOI] [PubMed] [Google Scholar]
- Liu J., Burdette J. E., Xu H., Gu C., van Breemen R. B., Bhat K. P. L., et al. (2001). Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. Journal of Agricultural and Food Chemistry, 49(5), 2472– 2479. [DOI] [PubMed] [Google Scholar]
- Maggiolini M., Bonofiglio D., Marsico S., Panno M. L., Cenni B., Picard D., Andò S. (2001). Estrogen receptor α mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Molecular Pharmacology, 60(3), 595– 602. [PubMed] [Google Scholar]
- Makela S. I., Pylkkanen L. H., Santti R. S., Adlercreutz H. (1995). Dietary soybean may be antiestrogenic in male mice. Journal of Nutrition, 125(3), 437– 445. [DOI] [PubMed] [Google Scholar]
- Marin R., Guerra B., Hernandez-Jimenez J. G., Kang X. L., Fraser J. D., Lopez F. J., Alonso R. (2003). Estradiol prevents amyloid-beta peptide-induced cell death in a cholinergic cell line via modulation of a classical estrogen receptor. Neuroscience, 121(4), 917– 926. [DOI] [PubMed] [Google Scholar]
- Markou A., Duka T., Prelevic G. M. (2005). Estrogens and brain function. Hormones (Athens), 4(1), 9– 17. [DOI] [PubMed] [Google Scholar]
- McEwen B. (2002). Estrogen actions throughout the brain. Recent Progress in Hormone Research, 57(1), 357– 384. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. (1999). Clinical review 108: The molecular and neuroanatomical basis for estrogen effects in the central nervous system. The Journal of Clinical Endocrinology & Metabolism, 84(6), 1790– 1797. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Alves S. E. (1999). Estrogen actions in the central nervous system. Endocrine Reviews, 20(3), 279– 307. [DOI] [PubMed] [Google Scholar]
- Meier B., Berger D., Hoberg E., Sticher O., Schaffner W. (2000). Pharmacological activities of Vitex agnus-castus extracts in vitro. Phytomedicine, 7(5), 373– 381. [DOI] [PubMed] [Google Scholar]
- Miller N. R., Jover T., Cohen H. W., Zukin R. S., Etgen A. M. (2005). Estrogen can act via estrogen receptor α and β to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology, 146(7), 3070– 3079. [DOI] [PubMed] [Google Scholar]
- Moreno F. N., Campos-Shimada L. B., da Costa S. C., Garcia R. F., Cecchini A. L., Natali M. R., et al. (2015). Vitex agnuscastus L. (Verbenaceae) improves the liver lipid metabolism and redox state of ovariectomized rats. Evidence-Based Complementary and Alternative Medicine, 2015, 212378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili A., Arnone B., Gasbarri A. (2012). Estrogens and memory in physiological and neuropathological conditions. Psychoneuroendocrinology, 37(9), 1379– 1396. [DOI] [PubMed] [Google Scholar]
- Rajabi S., Shamsizadeh A., Amini H., Shirazi M., Allahtavakoli M., Abbasnejad M., Sheibani V. (2012). Effect of DSP-4 induced central noradrenergic depletion on tactile learning in rat. Neurological Research, 34(1), 80– 84. [DOI] [PubMed] [Google Scholar]
- Register T. C., Shively C. A., Lewis C. E. (1998). Expression of estrogen receptor [alpha] and [beta] transcripts in female monkey hippocampus and hypothalamus. Brain Research, 788(1–2), 320– 322. [DOI] [PubMed] [Google Scholar]
- Santell R. C., Kieu N., Helferich W. G. (2000). Genistein inhibits growth of estrogen-independent human breast cancer cells in culture but not in athymic mice. The Journal of Nutrition, 130(7), 1665– 1669. [DOI] [PubMed] [Google Scholar]
- Seidlova-Wuttke D., Hesse O., Jarry H., Christoffel V., Spengler B., Becker T., Wuttke W. (2003). Evidence for selective estrogen receptor modulator activity in a black cohosh (Cimicifuga racemosa) extract: comparison with estradiol-17 beta. European Journal of Endocrinology, 149(4), 351– 362. [DOI] [PubMed] [Google Scholar]
- Soares C. N., Prouty J., Born L., Steiner M. (2005). Treatment of menopause-related mood disturbances. CNS Spectrums, 10(6), 489– 497. [DOI] [PubMed] [Google Scholar]
- Spencer J. L., Waters E. M., Romeo R. D., Wood G. E., Milner T. A., McEwen B. S. (2008). Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in Neuroendocrinology, 29(2), 219– 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahamtan M., Allahtavakoli M., Abbasnejad M., Roohbakhsh A., Taghipour Z., Taghavi M., et al. (2013). Exercise preconditioning improves behavioral functions following transient cerebral ischemia induced by 4-vessel occlusion (4-VO) in rats. Archives of Iranian Medicine, 16(12), 697– 704. [PubMed] [Google Scholar]
- Toran-Allerand C. D., Singh M., Setalo G., Jr. (1999). Novel mechanisms of estrogen action in the brain: new players in an old story. Frontiers in Neuroendocrinology, 20(2), 97– 121. [DOI] [PubMed] [Google Scholar]
- Toufexis D. J., Myers K. M., Davis M. (2006). The effect of gonadal hormones and gender on anxiety and emotional learning. Hormones and Behavior, 50(4), 539– 549. [DOI] [PubMed] [Google Scholar]
- Warren M. P. (2004). A comparative review of the risks and benefits of hormone replacement therapy regimens. American Journal of Obstetrics & Gynecology, 190(4), 1141– 1167. [DOI] [PubMed] [Google Scholar]
- Weisskopf M., Schaffner W., Jundt G., Sulser T., Wyler S., Tullberg-Reinert H. (2005). A Vitex agnus-castus extract inhibits cell growth and induces apoptosis in prostate epithelial cell lines. Planta Medica, 71( 10), 910. [DOI] [PubMed] [Google Scholar]
- Xu H., Gouras G. K., Greenfield J. P., Vincent B., Naslund J., Mazzarelli L., et al. (1998). Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nature Medicine, 4(4), 447– 451. [DOI] [PubMed] [Google Scholar]