Abstract
Concurrent diabetes has been linked with an increased risk of death in many cancers, but findings in pancreatic cancer have been inconsistent. We performed a systematic review and meta-analysis to assess the effect of diabetes on survival in patients with pancreatic cancer. Of 4, 463 original articles, 41 were included in the review; 29 studies with 33 risk estimates were included in the meta-analysis. In the overall comparison of patients with pancreatic cancer and diabetes with their nondiabetic counterparts, the former had significantly higher all-cause mortality (pooled HR: 1.13; 95% CI: 1.04–1.22). Subgroup analyses showed that diabetes was associated with poor survival in patients with resectable disease (HR: 1.37; 95% CI: 1.15–1.63) but not in those with unresectable disease (HR: 1.07; 95% CI: 0.89–1.29). The HR (95% CI) was 1.52 (1.20–1.93) for patients with new-onset diabetes (≤2 years of diabetes duration) and 1.22 (0.83–1.80) for those with longstanding diabetes (>2 years). Diabetes was associated with higher mortality overall in patients with pancreatic cancer. The effect of diabetes on overall survival was associated with the stages of tumor and the duration of diabetes.
Diabetes mellitus (DM), or impaired glucose tolerance, is concurrently present in 50–80% of patients with pancreatic cancer (PC), one of the most rapidly fatal malignancies. DM is a known risk factor for PC1,2,3; furthermore, new-onset DM could be an early sign of PC4, resulting from insulin resistance induced by a paraneoplastic syndrome5 or pancreatic β-cell dysfunction6.
Increasing evidence suggests that patients with colorectal, breast, liver, endometrial, and gastric cancers and leukemia7,8 who also have DM are at increased risk of cancer recurrence, cancer-related death, and death from any cause. However, whether and how the concurrent DM may affect clinical outcome in PC has not been determined, and available information on this topic is limited and inconsistent. Some studies found that DM did not have a significant effect on overall survival (OS) duration9,10,11,12,13,14,15,16,17,18,19,20,21,22,23, whereas others found that DM was associated with significantly reduced survival duration24,25,26,27,28,29,30,31,32,33,34. These inconsistent findings may be partially explained by small sample sizes and/or by not adjusting for body mass index (BMI), disease stage, and other possible confounders35. Understanding the prognostic relevance of DM in PC may lead to better clinical management of this devastating disease. We therefore conducted a systematic review and meta-analysis to illustrate the association between preexisting DM and mortality in patients with PC.
Methods
Data sources and searches
We searched PubMed, Embase, and Web of Science (TS) databases from their inception to September 2, 2014, for articles evaluating the association between DM and outcome in PC, including survival, stage at diagnosis, and treatment choice. Our overall search strategy included terms for diabetes (e.g., “diabetes,” “glucose intolerance,” and “hyperglycemia”), pancreatic cancer (e.g., “cancer,” “carcinoma,” “adenocarcinoma,” “pancreatic,” and “pancreas”), and prognosis (e.g., “prognosis,” “survival,” and “mortality”). We also searched the references of included articles. No language or publication type restrictions were imposed (Supplementary Table 1).
Study selection
Our overall search targeted articles describing studies that met the following three criteria: 1) evaluated any prognostic outcome by DM or glycemic status; 2) evaluated a PC patient population; and 3) contained original data analysis. We included studies evaluating type 1 and/or type 2 DM. To avoid overlapping patient populations, we compared data on recruitment years, data source, and geographic location. Publications with duplicate datasets were triaged by keeping the most recent one, the one with the larger study population, or the one with multivariate-adjusted estimates. Articles that met the above three criteria and reported all-cause mortality or OS were included in our systematic review (Fig. 1). To be included in our meta-analysis, articles had to report a risk estimate (e.g., hazard ratio [HR]) relating preexisting DM to subsequent death by using survival analysis regression models, with an estimate of precision, such as standard error (SE) or 95% confidence interval (CI). Articles with missing risk estimates were also included in the meta-analysis if the risk estimates were generated by author contact. Of the 14 authors contacted, 7 responded, 6 provided additional information. We also conducted a systematic review or meta-analyses on long-term, cancer-specific mortality, disease-free survival (DFS), progression-free survival (PFS), postoperative death rate, and choice of cancer treatment in studies with the relevant information including studies that were excluded from the general review and meta-analysis because of overlapping study durations36,37,38.
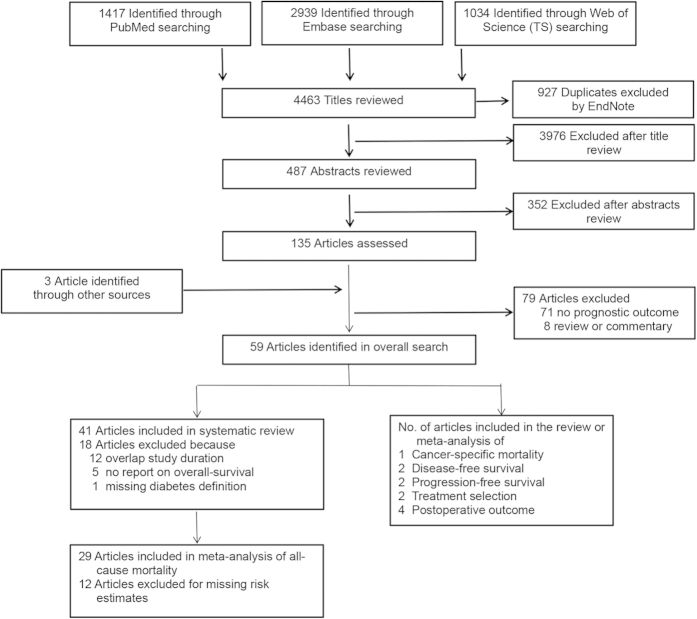
Figure 1. Flowchart of study selection.
Data extraction and quality assessment
Each article was abstracted by one author and reviewed by the second author for accuracy. Any disagreement was resolved by consensus. If several estimates were reported in the same article, we chose the most fully adjusted estimate (i.e., multivariate regression was selected over univariate regression, which was selected over unadjusted Kaplan-Meier analysis). If an article reported multiple estimates by subgroup only, these estimates were entered separately into our relevant meta-analysis dataset.
We also extracted information on key indicators of study quality with use of Meta-analysis of Observational Studies in Epidemiology (MOOSE) standards39 for reporting of meta-analyses of observational studies. From each study, we chose the risk estimates that represented the greatest degree of control for potential confounders. Quality was assessed by using elements of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement40.
Data analyses
The results of the systematic review were summarized qualitatively. The null hypothesis of “no additional mortality risk in cancer patients with preexisting DM” was tested with use of a nonparametric sign test.
For the meta-analysis, P values quoted at less than the specified threshold were assumed to be at the threshold, resulting in a conservative estimate of the significance level. I2 and Cochran Q estimates were performed in a heterogeneity assessment41. A I2 value of >50% or a P value of less than 0.1 represented significant heterogeneity. A DerSimonian-Laird random-effects model (D + L) was used to calculate the pooled HR. Otherwise, an inverse variance fixed-effects model (I-V) was selected. The meta-analysis was performed with use of Stata version 12.0 software (Stata Corp, College Station, TX).
To assess the impact of study quality, we conducted sensitivity analyses. We considered studies to be of higher quality and calculated separate pooled HRs if they were population-based (n = 4) or were adapted from full articles (n = 28), with estimates adjusted for confounders (n = 23) and DM evaluated as the primary exposure variable (n = 10). Publication bias was evaluated by using Begg’s funnel plot. We performed the Duval and Tweedie nonparametric trim and fill procedure42 to further assess potential effects of publication bias. This method considers the possibility of hypothetical missing studies, imputes their HRs, and recalculates a pooled estimate. For all tests, a P value (two-sided) of less than 0.05 was considered statistically significant.
Results
Of the 4, 463 titles identified, 487 abstracts and 135 resulting full articles were reviewed to determine their eligibility (Fig. 1). Three additional articles were identified by searching references9. Of these 138 articles, 59 addressed the effect of DM on PC outcome. Eighteen of the 59 articles were excluded from the review for overlapping study duration, lack of definition for DM, or lack of focus on OS. As a result, 41 articles were included in the systematic review of the association of preexisting DM with long-term, all-cause mortality. Twenty-nine of these 41 articles with 33 risk estimates were included in the meta-analysis (Fig. 1)9,10,11,12,13,14,15,16,18,20,21,22,23,24,25,26,27,28,29,30,31,43,44,45,46,47,48,49,50. Descriptive data for studies included are listed in Table 1 and Supplementary Table 2.
Table 1. Characteristics of 29 studies included in the meta-analysis of the effect of preexisting DM on pancreatic cancer all-cause, long-term mortality.
| Study, year, country | Date of recruitment (range) | Inclusion criteria | Exclusion criteria | Patients with DM No./Total No. (%) | Age at diagnosis (y) | Male No. (%) | Follow-up time (months) | Survival (HR, 95% CI) | Adjustments |
|---|---|---|---|---|---|---|---|---|---|
| Sperti24, 1996, Italy | 1970–1992 | Pathologically confirmed; resected | NA | 62/113 (54.9) | 59.4 (27–81) | 66 (58.4) | NA | 3.02 (1.38–6.60)* | Age, stage, grade |
| Neoptolemos9, 2001, UK | 1994–2000 | Pathologically confirmed; resected | NA | 85/541 (15.7) | 60 (53–67) | 324 (59.9) | 10 (1–25) | 1.93 (0.77–2.17) [margin positive] 1.17 (0.85–2.00) [margin negative] | None |
| Sperti10, 2003, Italy | 1996–2002 | Pathologically confirmed | NA | 20/60 (33.3) | 66.3 (48 –82) | 34 (56.7) | 1–35 | 0.63 (0.32–1.22), P = 0.17† | Age, sex, SUV, tumor stage, tumor grade, treatment, CA 19–9, |
| van de Poll–Franse25, 2007, The Netherlands | 1995–2002 | NA | NA | 245/1211 (20.2) | NA | NA | NA | 1.16 (1.00–1.34), P < 0.05 | Age, sex, stage, treatment |
| Li11, 2007, USA | 1999–2004 | Pathologically confirmed | NA | 88/378 (23.3) | NA | 207 (54.8) | 34 (18–90) | 1.186 (0.901–1.560), P = 0.224 | Age, sex, race, stage |
| Li26, 2009, USA | 2004–2008 | Pathologically confirmed | NA | 221/841 (26.3) | 61.7 (61.0–62.4) | 496 (59.0) | 22.1 (20.2–24.0) | 1.29 (1.02–1.64) | Stage, resection, BMI |
| Chu27, 2010, USA | 2000–2007 | Pathologically confirmed; resected | Other periampullary adenocarcinomas | 93/209 (44.5)‡; new-onset: 55/93 (59.1); longstanding 35/93 (37.6) | 65 (37–86) | 103 (49.3)* | NA | 1.55 (1.02–2.35), P = 0.04; New–onset DM§ 1.75 (1.10–2.78), P = 0.017 Longstanding DM§ 1.30 (0.75–2.25), P = 0.36 | Age, sex, ethnicity, BMI, Charlson, comorbidities, smoking, tumor size, node and margin, perineural and lymphovascular invasion, adjuvant therapy |
| McWilliams12, 2010, USA | 2000–2009 | PAC | Missing height, weight, or disease stage | 472/1529 (30.9) | 66.0 (58.0–74.0)|| | 864 (56.5) | Median 306 d | 1.08 (0.95–1.22), P = 0.229 | Age (continuous variable), sex, BMI |
| Olson13, 2010, USA | 2004–2008 | Over 21 years; pathologically confirmed | NA | 47/475 (9.9) | 63.7 ± 10.8 | 247 (52.0) | NA | 0.97 (0.42–2.26), P = 0.95 [resected]; 0.78 (0.47–1.30), P = 0.34 [unresected] | None |
| Dandona14, 2011, USA | 1995–2009 | Resected | Undocumented BMI, BMI < 18.5 kg/m2 | 116/355 (32.7) | 65.5 ± 10.2 | 192 (54.1) | 32.3 (range, 0.56–51.77) | 0.855 (0.650–1.124) | Age, sex, N–stage, BMI |
| Cannon28, 2011, USA | 2000–2009 | Resected | Margin positive, neoadjuvant chemotherapy | 78/245 (31.8)¶ | 67.0 (58.0–74.0) | 115 (46.9) | Median 4.5 | 1.99 (1.40–2.82), P < 0.001 | Tumor size, node and margin |
| Morizane15, 2011, Japan | 2001–2007 | Pathologically confirmed; metastatic PC with gemcitabine first–line therapy | NA | 171/409 (41.8) | 64 (21–81) | 241 (58.9) | 0.4–41.3 | 0.997 (0.818–1.217) | None |
| Hartwig29, 2011, Germany | 2001–2009 | Resected | Ampullary carcinomas or carcinomas of the distal bile duct | 151/1071 (14.6) | 65.4 (57.9–71.1) | 599 (55.9) | 17 (10–31) | 1.53 (1.20–1.94), P = 0.0005 | Age, CA 19-9, TNM staging, grade, margin status |
| Vickers16, 2012, Canada | 2001–2003 | Pathologically confirmed; unresectable | Prior chemotherapy except for chemoradiation | 175/569 (30.8) | 63.9 (36.1–92.4) | 298 (52.4) | Median 13.9 | 1.21 (0.99–1.47), P = 0.058 | Sex, race, performance status, baseline pain intensity |
| Ben30, 2012, China | 2005–2010 | Histologically confirmed; resected | History of cancers, no FBG test | 107/396 (27.0)# | 63.1 ± 9.1 | (63.5) | 20 (4–62) | 1.385 (1.068–1.796), P = 0.014 | Age, sex, CA19-9, node invasion, stage, neural invasion |
| Inal18, 2012, Turkey | 2005–2011 | ≥18 years; pathologically confirmed; locally advanced or metastatic | NA | 127/406 (31.3) | 63 [Gemcitabine], 57 [Gemcitabine plus Cisplatin] | 273 (67.2) | NA | 0.93 (0.53–1.62) | None |
| Sahin31, 2012, USA | 1996–2011 | Pathologically confirmed; resected | NA | 144/544 (26.5) | NA | 314 (57.7) | NA | 1.29 (1.02–1.64), P = 0.036 | Perineural invasion, margin status, node status, grade |
| Gong43, 2012, USA | 1995–1999 | PAC | NA | 72/510 (14.1) | 66.1 [BMI < 25 kg/m2],63.2 [BMI = 25–30 kg/m2], 62.0 [BMI ≥ 30 kg/m2] | 278 (54.5) | 10.1 y | 0.85 (0.64–1.13) | Age, sex, race, education, BMI, smoking status, stage, tumor grade, tumor site, primary treatment |
| Barbas21, 2012, USA | 1996–2008 | Pathologically confirmed PAC; underwent pancreaticoduodenectomy | Significant missing clinicopathological data | 51/203 (25.1) | <65 (47.8%); 65–74(36.4%); ≥ 75 (15.8%) | 106 (52.2) | NA | 1.2 (0.76–1.90), P = 0.42 | Age, adjuvant therapy, coronary artery disease, histology, neoadjuvant therapy, resection margin, perineural Invasion, lymph node, vascular invasion |
| Hwang20, 2013, USA | 2003–2010 | >40 years; with a diagnostic code for PAC | Had PAC before the start of follow-up in THIN | 745/3147 (23.7)** | 72.3 ± 10 [T2DM], 71.2 ± 11.6 [non-T2DM] | 1524 (48.4) | NA | 1.02 (0.93–1.12), P = 0.620 | Age, sex, resection, history of pancreatitis, Charlson index |
| Zhou44, 2013, China | 2002–2007 | Pathologically confirmed; resected; underwent pancreatectomy | NA | 54/114 (47.4) | 64 (31–79) | 79 (69.3) | 15.0 (0.2–60) | 1.218 (0.765–1.941), P = 0.406 | None |
| Zeiss45, 2013, Germany | 2009–2010 | >18 years; pathologically confirmed; stage III-IV; received palliative first-line gemcitabine-based chemotherapy | Adjuvant chemotherapy | 16/30 (53.3) | 69 (41–82) | 17 (56.7) | 5.8 (0.9–23.5) | 1.49 (0.69–3.23), P = 0.31 | None |
| Mizuno22, 2013, Japan | 1993–2011 | PAC | NA | 256/540 (47.4) | 67 ± 11 | 322 (59.6) | NA | 0.91 (0.74–1.12), P = 0.39 | Symptoms at diagnosis, PS, CA19-9, stage, treatment |
| Lee23, 2013, Korea | 2007–2010 | Pathologically confirmed; received an operation, chemotherapy, or chemoradiotherapy | Received only supportive care, palliative surgery; Referred from other hospitals after receiving treatment or refusing treatment | 57/187 (30.5) | 65 (31–86) | 104 (55.6) | 11.7 (2–59.5) | 0.81 (0.54–1.21), P = 0.312 | Age, sex, PS, stage, tumor site, size, CA19-9, CEA |
| Choi46, 2014, Korea | 2003–2010 | Pathologically confirmed PAC; gemcitabine-based palliative chemotherapy | Double primary advanced malignancies | 182/345(52.8) | 60.1 (20.0–84.7) | 270 (63.5) | 10.3 (9.5–11.1) | 0.774 (0.605–0.991), P = 0.042 | PS, disease extent, weight loss at diagnosis (BMI change ≥ 1), weight loss during chemotherapy (BMI change ≥ 1). |
| Toriola47, 2014, USA | 1993–2001 | Exocrine PC | Missing information on tumor stage or diabetes | 62/504 (12.3) | 64 | 273 (54.2) | NA | 1.52 (1.14–2.04), P < 0.01 (All exocrine pancreas cancer); 1.45 (1.06–2.00), P = 0.02 (Excluding pancreatic cancer cases diagnosed within 3 years of enrolment); 2.31 (1.16–4.58), P = 0.02 (Localized); 1.17 (0.62–2.20), P = 0.65 (Locally advanced); 1.52 (1.04–2.24), P = 0.03 (Metastatic). | Age, sex, BMI, race, smoking, stage |
| Dong48, 2014, China | 2009–2012 | Pathologically confirmed potentially resectable PAC; consecutive patients underwent surgery | Double cancer with life-threatening phenotype; died in within 30 days after surgery | 34/114 (29.8) | 60 (54–67) | 64 (56.1) | NA | 1.820 (1.115–2.972), P = 0.017 | Serum calcium level, histologically poorly-differentiated tumor, existence of vessel invasion |
| Salem49, 2014, USA | 2010–2013 | Albumin-bound paclitaxel plus gemcitabine (Gem/nab) after first-line FOLFIRINOX | NA | NA/44 | 55 | 26 (59.1) | NA | 3.8 (1.0–14.3), P ≤ 0.05 | NA |
| Beg,2014, USA | 1995–2008 | Code for PC | Missing information on DM status or follow up | 1326/4728 (28.0) | 67.2 | 4617 (97.7) | 3.6 (1.3–7.4) | 0.91 (0.849–0.974), P = 0.0065 | Age, tobacco use, disease site, stage, chemotherapy, surgery |
Abbreviations: CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; FBG, fasting blood glucose; GFR, glomerular filtration rate; ICD-9, International Classification of Diseases, 9th revision; NA, not available; PAC, pancreatic adenocarcinoma; PC, pancreatic cancer; SUV, standardized uptake value; THIN, The Health Improvement Network.
*Data from Table 1 of Sperti et al. study.
†Data from Barone et al. study.
‡DM definition: For patients without documented history of DM, FBG was tested for classification.
§New-onset DM: disease duration preceding PDAC diagnosis date of < 24 mo; longstanding DM: disease duration preceding PDAC diagnosis date of ≥24 mo.
||For all patients including those missing DM status and other data.
¶DM definition: A past medical history of, a preoperative fasting glucose greater than 125 mg/dL, two or more outpatient random glucose levels above 199 mg/dL.
#DM definition: A self-reported history of DM or a fasting blood glucose level ≥125 mg/dL (6.9 mmol/L) or postprandial blood glucose level ≥11.1 mmol/L.
**DM definition: With a diagnostic code for T2DM.
Description of studies
Forty-one studies had been conducted in the United States (n = 17), Europe (n = 13), Asia (n = 10), and Canada (n = 1). Sample sizes ranged from 21 to 22, 439 with a median of 367. Across the 41 studies that reported the number of participants with DM, the overall prevalence of DM was 35.7% (range, 9.7%–54.9%). Across the 34 studies that reported participant sex, 64.8% of the study population was male. Reporting of age and follow-up time varied widely across studies.
Survival analyses reported various outcomes, including cumulative one-year mortality rates, OS, DFS, and PFS. The studies used a variety of analytic techniques including 9 studies using Kaplan-Meier survival analysis only, 32 using Cox proportional hazards regression analysis (only 29 had exact HRs and 95% CIs), which were included in the following meta-analysis (Supplementary Table 3). The time origin for survival analysis was generally the time of cancer diagnosis, except in the case of treatment or surgical cohorts, for which the time of origin was the beginning of treatment or the date of tumor resection. Most studies were clinic-based design and 4 studies were population-based cohorts (Supplementary Table 3).
Systematic review of evidence
The best evidence from each study is summarized here. Of 9 studies using Kaplan-Meier survival curves with the log-rank tests, DM was associated with decreased survival in 3 studies33,34,51 and 4 studies19,52,53,54 with and without statistical significance, respectively. DM was associated with nonsignificantly increased survival in 2 studies55,56.
Seven studies provided 9 crude HRs of death for preexisting DM including 3 nonsignificantly decreased risk9,45 and 6 null effect13,15,18,44,57. Of the 25 studies with multivariate HRs of death for DM, 12 reported significantly increased risk24,25,26,27,28,29,30,31,32,47,48,49, 2 reported nonsignificantly increased risk11,16, 2 had significantly decreased risk46,50, and 9 had null effect10,12,14,20,21,22,43,54,58.
Overall, DM was associated with increased risk of death in 25 estimates, decreased risk of death in 4 studies, and null effect in 16 estimates. The nonparametric sign test rejected the null hypothesis of equal mortality in patients with and without preexisting DM (P < 0.001).
Meta-analysis on DM and all-cause mortality
The 29 studies in the meta-analysis reported both risk (HR) and precision (95% CI). The descriptive data, adjustment or restriction variables, and major findings from each study are described in Table 1. The results of the meta-analysis are shown in Fig. 2. Preexisting DM was associated with a 13% increased risk of death from all causes in PC patients (HR, 1.13; 95% CI, 1.04–1.22). The pooled HR (95% CI) was 1.37 (1.15–1.63) from 13 studies conducted in patients with resectable disease, 1.07 (0.89–1.29) from 8 studies in unresectable disease, and 1.01 (0.93–1.10) from 10 studies in those with all-stage diseases. The Begg’s test and Duval and Tweedie trim and fill procedure showed no significant risk of publication bias (Begg’s test P = 0.14; Duvall and Tweedie adjusted HR: 1.10; 95% CI: 1.01–1.20; number of imputed studies = 3) (Supplementary Fig. 1).
Figure 2. Meta-analysis and pooled hazard ratio of long-term, all-cause mortality in 29 studies comparing PC patients with and without preexisting DM.
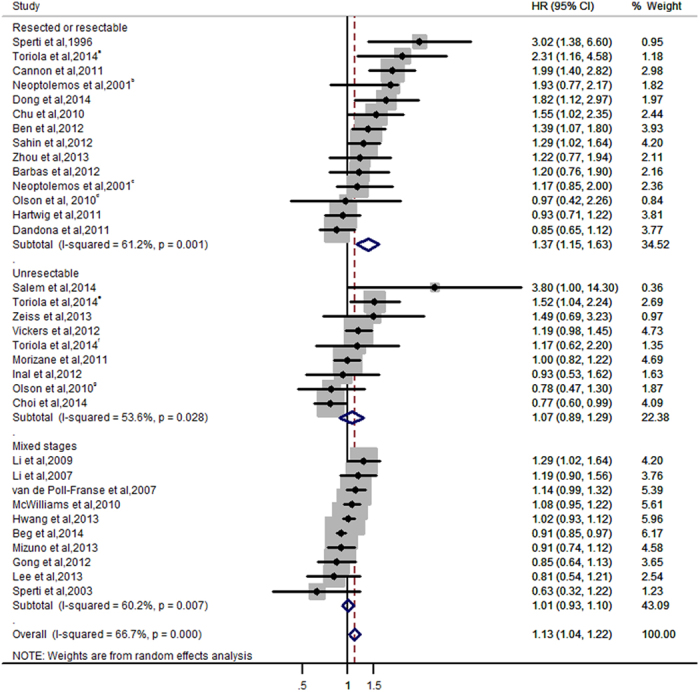
The 29 studies provided 33 estimates. Weights are from random-effects analysis. Data markers are proportional to study sample sizes. CI indicates confidence interval. Squares indicate relative risk in each study. The square size is proportional to the weight of the corresponding study in the meta-analysis; the length of the horizontal lines represents the 95% CI. The unshaded diamond indicates the pooled relative risk and 95% CI.
Sensitivity analyses
Considering the large variations in the covariates included in each study (Table 1), we conducted a sensitivity analysis to confirm robustness (Table 2). Risk estimates from higher-quality studies were similar to the overall estimate. The pooled risk estimate (HR [95% CI]) was 1.21 (1.06–1.39) for studies that took DM as the primary exposure variable. Studies with any adjustments had a pooled HR (95% CI) of 1.13 (1.03–1.24). More specifically, the above pooled HRs (95% CIs) after adjusting for age, BMI, and disease stage were 1.08 (0.98–1.20), 1.17 (0.98–1.39), and 1.09 (0.95–1.26), respectively. The risk estimates did not vary by publication types. The HR (95% CI) was 1.12 (1.03–1.21) for full articles.
Table 2. Pooled hazard ratios of all-cause mortality in pancreatic cancer patients with and without DM.
| Type of estimate | Studies (estimates), No. | Total patients, No. | Patients with DM, No. | Pooled HR (95% CI)* | I2, % | P |
|---|---|---|---|---|---|---|
| Total | 29 (33) | 19818 | 5257 | 1.13 (1.04–1.22) | 66.7 | <0.001 |
| Studies of full articles | 28 (32) | 19774 | 5257 | 1.12 (1.03–1.21) | 66.4 | <0.001 |
| Adjusted for confounders | ||||||
| Any confounders | 23 (25) | 17843 | 4757 | 1.13 (1.03–1.24) | 72.5 | <0.001 |
| Age | 15 (17) | 14601 | 3667 | 1.08 (0.98–1.20) | 66.8 | <0.001 |
| BMI | 6 (8) | 3948 | 1036 | 1.17 (0.98–1.39) | 61.2 | 0.012 |
| Stage | 12 (14) | 6156 | 1523 | 1.09 (0.95–1.26) | 66.7 | <0.001 |
| Patient source | ||||||
| Population-based | 4 (6) | 5372 | 1124 | 1.12 (0.95–1.32) | 59.6 | 0.030 |
| Clinic-based studies | 25 (27) | 14446 | 4133 | 1.13 (1.02–1.25) | 68.6 | <0.001 |
| DM exposure type | ||||||
| Primary exposure | 10 (12) | 11638 | 3110 | 1.21 (1.06–1.39) | 78.6 | <0.001 |
| One of multiple exposures | 19 (21) | 8180 | 2147 | 1.07 (0.96–1.20) | 54.6 | 0.001 |
| Cancer Stage | ||||||
| Resected or resectable | 13 (14) | 4473 | 1037 | 1.37 (1.15–1.63) | 61.2 | 0.001 |
| Unresectable | 8 (9) | 2214 | 718 | 1.07 (0.89–1.29) | 53.6 | 0.028 |
| Mixed stages | 10 (10) | 13131 | 3502 | 1.01 (0.93–1.10) | 60.2 | 0.007 |
Abbreviations: BMI, body mass index; CI, confidence; interval; DM, diabetes mellitus; HR, hazard ratio.
*Estimates calculated with use of a random-effects model.
Analysis of influence revealed that the risk of all-cause mortality among patients with PC and DM remained significant with the omission of each study in turn. Omission of the study by Cannon et al.28 resulted in the lowest pooled estimate (HR: 1.10; 95% CI: 1.02–1.19); omission of the study by Choi et al.46 resulted in the highest pooled estimate (HR: 1.14; 95% CI: 1.05–1.24).
DM and cancer-specific mortality
One study36 provided adjusted HRs of cancer-specific death in patients who had undergone resection. It showed that patients with DM had a significantly higher risk of cancer-specific mortality compared with their non-DM counterparts (HR: 1.37; 95% CI: 1.00–1.89).
Duration of DM and all-cause mortality
Six studies20,27,30,33,37,52 evaluated the association between duration of DM and OS of PC patients. In most studies, two years of diabetes duration was used as the cutoff for defining new-onset and longstanding DM. One study33 demonstrated that both new-onset and longstanding DM were associated with shorter survival by log-rank test. Three studies20,37,52 did not find a correlation between new-onset DM and OS in PC patients. Two studies27,30 indicated that OS duration in PC patients with new-onset DM, but not that in patients with longstanding DM, was significantly shorter than was OS in patients without DM. A meta-analysis of the two studies27,30 with risk estimates revealed new-onset DM as a significant prognostic factor (HR: 1.52; 95% CI: 1.20–1.93). We did not detect any significant heterogeneity (I2 = 0%, Q = 0.48; P = 0.49). However, the same prognostic value was not found in longstanding DM (HR: 1.22; 95% CI: 0.83–1.80) (Supplementary Fig. 2).
DM and DFS, PFS
Two studies28,38 showed that having DM before undergoing tumor resection was independently associated with poor DFS (pooled HR: 1.54; 95% CI: 1.28–1.85) as well as poor OS after adjusting confounders. No significant heterogeneity was detected in the meta-analysis (I2 = 14.3%, Q = 1.17; P = 0.28) (Supplementary Fig. 3). Moreover, two studies17,18 found that in patients with advanced PC who were receiving systemic chemotherapy, PFS did not differ between those with and without DM.
DM and treatment selection, postoperative mortality
No significant difference was found in the percentage of surgery between patients with and without DM, although DM patients were more likely recommended for resection27,34. Patients with DM had a higher likelihood of developing fistulas and acute kidney injury than did those without DM, but overall complication and severity did not differ between them59. The relation between DM and a higher postoperative mortality was not conclusive59,60,61,62.
Discussion
Our study demonstrated that preexisting DM in PC patients, compared with their non-DM counterparts, was associated with increased risk of all-cause mortality. The risk of all-cause mortality was higher in patients with resected or resectable tumors than in those with nonresectable tumors and was higher in patients with new-onset DM than in those with longstanding DM. These observations could not be explained by confounding factors, publication bias, or undue influence by a single study.
To our knowledge, our meta-analysis is the first exclusive study of the association between DM and PC outcome, even though this topic has been investigated by many individual studies. Our results are not in accordance with a previous meta-analysis of long-term all-cause mortality in cancer patients with preexisting DM by Barone et al.8, who found that DM was associated with increased risk of mortality in all cancers (HR: 1.41; 95% CI: 1.28–1.55) but not specifically in PC (HR: 1.09; 95% CI: 0.70–1.69). The discrepancy between the two studies could be partially explained by low power (7%) of the previous study since only four studies in the PC subgroup with a population of 1,681 patients, including 477 DM patients, were analyzed. In the current review and larger-scale meta-analyses, we conducted post hoc power calculations and found that our study had 85% power in demonstrating the association between DM and cancer mortality. Furthermore, we observed that the negative effect of DM on survival occurred primarily in patients with resected or resectable pancreatic tumors (HR, 1.37; 95% CI, 1.15–1.63) but not in patients with late-stage disease (HR, 1.07; 95% CI, 0.89–1.29). These observations support the hypothesis that the previous inconsistent findings between individual studies might be partially explained by the different patient populations involved.
There were several limitations in the literature and thus in our systematic review and meta-analysis. First, studies varied in their inclusion criteria, study population, and adjustment for confounding variables, which may have led to both overestimations and underestimations of risks. Nevertheless, our sensitivity analyses, excluding studies that did not adjust for potential confounders, did not materially change the results. Residual or unknown confounding is still possible after adjusting for most relevant confounding factors. The association may not necessarily be causal as well, particularly in the observational studies63. Given that the start time of survival of each study differs by cancer stage (ie. tumor resection), we also performed a subgroup analysis by cancer stage (resected/resectable, unresectable and mixed stages), which confirmed the association of diabetes with poor survival in patients with resected/resectable tumor.
Second, the status of DM ascertainment varied across studies, and the duration of DM was not directly reported in some studies. Moreover, in most studies, diabetic status was based exclusively on past medical history; thus, there was a chance of misclassification, which may have led to underestimation of the number of patients with DM and of the effect of the disease.
Third, overlap in patient enrollment between some studies may have elevated their weight in the quantitative analysis. For example, the patient cohort used by Cannon et al.28 to derive and test the survival prognostic scoring model included the 209 patients from Chu et al.’s prior study27, and these patients were randomly divided into training and validation sets. In addition, two studies conducted at the same institution, i.e., Li et al.’s study of all-stage patients recruited between 1999 and 2008 and Sahin et al.’s study of patients who had undergone resection and were recruited between 1996 and 2001, may have some overlapping patients. However, we expect that the effects of this overlap on the final results of our analysis to be minimal.
The fourth limitation was that most of the articles did not report the types of anticancer and antidiabetic therapies used or their effects on outcomes. This is important because studies have shown that some therapies (e.g., surgery, adjuvant chemotherapy, and the antidiabetic drug metformin) have a more positive effect than others on cancer outcome64,65.
There are several potential explanations for the observed association between decreased survival time and DM in PC patients. First, DM may enhance tumor progression via the mechanisms of insulin resistance and inflammation, i.e., the same mechanisms that contributed to the higher risk of PC may also be responsible for the poor outcome of the disease. It has been suggested that hormonal or metabolic abnormalities, such as hyperinsulinemia or hyperglycemia, may affect tumor biology at multiple stages, including malignant transformation, growth, and metastasis66.
Second, PC patients with DM may be given less vigorous anticancer regimens because they generally have more contradictions to surgery, chemotherapy, and other treatments, although selection bias associated with types of surgery, chemotherapy, and radiotherapy were not seen in previous studies27. The metabolic abnormalities associated with DM may have an adverse effect on response to cancer treatment67.
Finally, the high mortality rate observed among patients with DM may partially be due to noncancerous factors, such as complications of long-term DM. However, since most PC patients die of the disease within a short period of time, it is unlikely that the complications of long-term DM would make a significant contribution to PC mortality. Only one study measured PC-specific mortality and observed a significant impact of DM36.
Previous studies2,6 have shown that new-onset DM, compared with longstanding DM, was associated with greater risk of PC because new-onset DM was a manifestation of subclinical PC. The main implication of our study is that DM is significantly associated with adverse outcome in PC. Subgroup analyses showed that the effect of diabetes on overall survival was associated with the cancer stages and the duration of diabetes. Our results reveal the need for further prospective studies to confirm DM as a prognostic factor and to assess the possibility of an antidiabetic regimen in the treatment of PC.
Additional Information
How to cite this article: Mao, Y. et al. Effect of Diabetes Mellitus on Survival in Patients with Pancreatic Cancer: A Systematic Review and Meta-analysis. Sci. Rep. 5, 17102; doi: 10.1038/srep17102 (2015).
Supplementary Material
Acknowledgments
This work was supported by the grants from NIH/NCI under award number P30CA016672, National Natural Science Foundation of China (NSFC) 30901765 (Y.M.) and 81272542 (M.T.), China Scholarship Council (Y.M.), the Natural Science Foundation of the First Affiliated Hospital to Soochow University (Y.M.), the Science and Education for Health Foundation of Suzhou for Youth (Y.M.), and the Sheikh Ahmed Center for Pancreatic Cancer Research Funds (D.L.). We thank Lawrence Cohn, Fred Brancati, and Hsin-Chieh Yeh for assistance in post hoc power calculation and Tamara Locke for editing the manuscript. We thank Ali Inal, Chigusa Morizane, Cosimo Sperti, John Neoptolemos, Michael Vickers, Monica Dandona, Olugbenga Olowokure, Shaalan Beg, and Sara Olson for providing additional information from their studies.
Footnotes
Author Contributions Conception and design: Y.M., M.T. and D.L. Collection and assembly of data: Y.M., M.T., X.J., H.X. and K.C. Data analysis and interpretation: Y.M., X.J., H.T. and D.L. All authors read and approved the final manuscript.
References
- Everhart J. & Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 273, 1605–9 (1995). [PubMed] [Google Scholar]
- Huxley R., Ansary-Moghaddam A., Berrington de Gonzalez A., Barzi F. & Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 92, 2076–83 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. & Mao Y. Diabetes as a Risk Factor of Pancreatic Cancer. Pancreapedia: Exocrine Pancreas Knowledge Base 10.3998/panc.2015.2 (2015). [DOI] [Google Scholar]
- Chari S. T. et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 129, 504–11 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permert J. et al. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? American Journal of Surgery 165, 61–67 (1993). [DOI] [PubMed] [Google Scholar]
- Gupta S. et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol 4, 1366–72 quiz 1301 (2006). [DOI] [PubMed] [Google Scholar]
- Barone B. B. et al. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care 33, 931–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone B. B. et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 300, 2754–64 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos J. P. et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Annals of Surgery 234, 758–768 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperti C. et al. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg 7, 953–959 (2003). [DOI] [PubMed] [Google Scholar]
- Li D. et al. Effects of base excision repair gene polymorphisms on pancreatic cancer survival. Int J Cancer 120, 1748–1754 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams R. R. et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer 116, 5054–5062 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson S. H. et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int J Cancer 127, 2412–2419 (2010). [DOI] [PubMed] [Google Scholar]
- Dandona M. et al. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas 40, 931–937 (2011). [DOI] [PubMed] [Google Scholar]
- Morizane C. et al. Construction and validation of a prognostic index for patients with metastatic pancreatic adenocarcinoma. Pancreas 40, 415–421 (2011). [DOI] [PubMed] [Google Scholar]
- Vickers M. M. et al. Comorbidity, age and overall survival in patients with advanced pancreatic cancer—Results from NCIC CTG PA.3: A phase III trial of gemcitabine plus erlotinib or placebo. European Journal of Cancer 48, 1434–1442 (2012). [DOI] [PubMed] [Google Scholar]
- Nakai Y. et al. Clinical Outcomes of Chemotherapy for Diabetic and Nondiabetic Patients With Pancreatic Cancer: Better Prognosis With Statin Use in Diabetic Patients. Pancreas 42, 202–208 (2013). [DOI] [PubMed] [Google Scholar]
- Inal A. et al. Gemcitabine alone versus combination of gemcitabine and cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a retrospective analysis of multicenter study. Neoplasma 59, 297–301 (2012). [DOI] [PubMed] [Google Scholar]
- Ganti A. K. et al. Predictive value of clinical features at initial presentation in pancreatic adenocarcinoma: a series of 308 cases. Med Oncol 19, 233–237 (2002). [DOI] [PubMed] [Google Scholar]
- Hwang A., Narayan V. & Yang Y. X. Type 2 diabetes mellitus and survival in pancreatic adenocarcinoma: A retrospective cohort study. Cancer 119, 404–410 (2013). [DOI] [PubMed] [Google Scholar]
- Barbas A. S. et al. Comparison of Outcomes and the Use of Multimodality Therapy in Young and Elderly People Undergoing Surgical Resection of Pancreatic Cancer. Journal of the American Geriatrics Society 60, 344–350 (2012). [DOI] [PubMed] [Google Scholar]
- Mizuno S. et al. Diabetes is a useful diagnostic clue to improve the prognosis of pancreatic cancer. Pancreatology 13, 285–289 (2013). [DOI] [PubMed] [Google Scholar]
- Lee K. J. et al. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Medical Journal 54, 643–649 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperti C., Pasquali C., Piccoli A. & Pedrazzoli S. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg 83, 625–631 (1996). [DOI] [PubMed] [Google Scholar]
- Van de Poll-Franse L. V. et al. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer 120, 1986–1992 (2007). [DOI] [PubMed] [Google Scholar]
- Li D., Hassan M. M. & Abbruzzese J. L. Obesity and survival among patients with pancreatic cancer: Reply. JAMA—Journal of the American Medical Association 302, 1752–1753 (2009). [DOI] [PubMed] [Google Scholar]
- Chu C. K. et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol 17, 502–513 (2010). [DOI] [PubMed] [Google Scholar]
- Cannon R. M. et al. Multi-institutional analysis of pancreatic adenocarcinoma demonstrating the effect of diabetes status on survival after resection. Hpb 14, 228–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig W. et al. Pancreatic Cancer Surgery in the New Millennium Better Prediction of Outcome. Annals of Surgery 254, 311–319 (2011). [DOI] [PubMed] [Google Scholar]
- Ben Q. et al. Clinical profiles and long-term outcomes of patients with pancreatic ductal adenocarcinoma and diabetes mellitus. Diabetes/Metabolism Research and Reviews 28, 169–176 (2012). [DOI] [PubMed] [Google Scholar]
- Sahin I. H. et al. Association of diabetes and perineural invasion in pancreatic cancer. Cancer Med 1, 357–362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste F. et al. Prognostic factors in exocrine pancreas cancer. 72 patients. Revue de Medecine Interne 13, S62 (1992). [Google Scholar]
- Wakasugi H., Funakoshi A. & Iguchi H. Clinical observations of pancreatic diabetes caused by pancreatic carcinoma, and survival period. Int J Clin Oncol 6, 50–54 (2001). [DOI] [PubMed] [Google Scholar]
- Ragulin-Coyne E. et al. Pancreatic cancer following a diagnosis of new-onset diabetes: A population-based study. Hpb 13, 53–54 (2011). [Google Scholar]
- Mao Y., Tao M., Jia X. & Li D. Diabetes Associated With Short Survival in Pancreatic Cancer. Journal of Clinical Oncology 33, 2120–1 (2015). [DOI] [PubMed] [Google Scholar]
- Busaidy N. L. et al. Survival of resectable pancreatic cancer patients with diabetes. Journal of Clinical Oncology 24, 202s–202s (2006). [Google Scholar]
- Mizuno S. et al. New-onset diabetes mellitus is not associated with the prognosis of pancreatic cancer. Gastroenterology 136, A390 (2009). [Google Scholar]
- Yazbeck C. F. et al. Diabetes mellitus confers a poor prognosis on resectable pancreatic cancer patients. Diabetes 55, A207–A207 (2006). [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–12 (2000). [DOI] [PubMed] [Google Scholar]
- Von Elm E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–7 (2007). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–60 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S. & Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–63 (2000). [DOI] [PubMed] [Google Scholar]
- Gong Z. H., Holly E. A. & Bracci P. M. Obesity and survival in population-based patients with pancreatic cancer in the San Francisco Bay Area. Cancer Causes & Control 23, 1929–1937 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. et al. Upregulation of transgelin is an independent factor predictive of poor prognosis in patients with advanced pancreatic cancer. Cancer Sci 104, 423–430 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiss K. et al. Glucose and lipid metabolism in patients with advanced pancreatic cancer receiving palliative chemotherapy. Anticancer Res 33, 287–292 (2013). [PubMed] [Google Scholar]
- Choi Y. et al. The Impact of Body Mass Index Dynamics on Survival of Patients With Advanced Pancreatic Cancer Receiving Chemotherapy. Journal of Pain and Symptom Management 48, 13–25 (2014). [DOI] [PubMed] [Google Scholar]
- Toriola A. T., Stolzenberg-Solomon R., Dalidowitz L., Linehan D. & Colditz G. Diabetes and pancreatic cancer survival: A prospective cohort-based study. British Journal of Cancer 111, 181–185 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q. et al. Serum calcium level used as a prognostic predictor in patients with resectable pancreatic ductal adenocarcinoma. Clin Res Hepatol Gastroenterol 38, 639–648 (2014). [DOI] [PubMed] [Google Scholar]
- Salem M. E. et al. Albumin-bound paclitaxel plus gemcitabine after first-line FOLFIRINOX therapy in patients with pancreatic cancer. Journal of Clinical Oncology 32, e15252 (2014). [Google Scholar]
- Beg M. S., Dwivedi A. K., Ahmad S. A., Ali S. & Olowokure O. Impact of diabetes mellitus on the outcome of pancreatic cancer. PLoS One 9, e98511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehayem Y. M. et al. Impact of diabetes mellitus on clinical presentation and prognosis of pancreatic cancer. Ann Endocrinol (Paris) 72, 24–29 (2011). [DOI] [PubMed] [Google Scholar]
- Partelli S. et al. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Digestive and Liver Disease 44, 945–951 (2012). [DOI] [PubMed] [Google Scholar]
- Teo M. Y., McDonnell F. & McDermott R. Glycaemic status and survival in locally-advanced pancreatic adenocarcinoma. Pancreatology 12, e18 (2012). [Google Scholar]
- Lee T. Y., Cheon Y. K. & Shim C. S. High hemoglobin A1C level is associated with worse survival in advanced pancreatic cancer patients with diabetes. Gastroenterology 144, S661 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K. et al. Prognostic factors of unresectable pancreatic cancer treated with nafamostat mesilate combined with gemcitabine chemotherapy. Anticancer Research 32, 5121–5126 (2012). [PubMed] [Google Scholar]
- Esbah O. et al. Metformin in diabetic pancreatic cancer patients: Benefit or not-Multicenter experience. Journal of Clinical Oncology 31, e15110 (2013). [Google Scholar]
- Pelucchi C. et al. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas 43, 47–52 (2014). [DOI] [PubMed] [Google Scholar]
- Winter J. M. et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 10, 1199–1210 (2006). [DOI] [PubMed] [Google Scholar]
- Chu C. K. et al. Impact of diabetes mellitus on perioperative outcomes after resection for pancreatic adenocarcinoma. J Am Coll Surg 210, 463–73 (2010). [DOI] [PubMed] [Google Scholar]
- Malleo G. et al. Preoperative diabetes mellitus does not impact on postoperative morbidity after pancreatic resections for ductal adenocarcinoma. Pancreatology 12 (6), 589–590 (2012). [Google Scholar]
- Andren-Sandberg A. & Ihse I. Factors influencing survival after total pancreatectomy in patients with pancreatic cancer. Annals of Surgery 198, 605–10 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkevold K. E. & Kambestad B. Morbidity and mortality after radical and palliative pancreatic cancer surgery. Risk factors influencing the short-term results. Annals of Surgery 217, 356–68 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. A., Gale E. A., Johnson J. A. & Gale E. A. M. Diabetes, insulin use, and cancer risk: are observational studies part of the solution-or part of the problem? Diabetes 59, 1129–31 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowker S. L., Yasui Y., Veugelers P. & Johnson J. A. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia 53, 1631–1637 (2010). [DOI] [PubMed] [Google Scholar]
- Sadeghi N., Abbruzzese J. L., Yeung S. C. J., Hassan M. & Li D. H. Metformin Use Is Associated with Better Survival of Diabetic Patients with Pancreatic Cancer. Clinical Cancer Research 18, 2905–2912 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. Z., Fehsenfeld D. M., Murphy L. O., Permert J. & Adrian T. E. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas 21, 310–20 (2000). [DOI] [PubMed] [Google Scholar]
- Richardson L. C. & Pollack L. A. Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol 2, 48–53 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.