Abstract
Multidrug resistant among Acinetobacter baumannii infection is associated with a high mortality rate and limits the therapeutic options. The aim of this study was to assess the safety and efficacy of colistin monotherapy vs. other single antibiotic therapy AND colistin-based combination therapy (with other antibiotics) vs. colistin alone for the treatment of Acinetobacter baumannii infection. Online electronic database were searched for studies evaluating colistin with or without other antibiotics in treatment of patients with drug-resistant Acinetobacter baumannii infection. Totally, twelve studies met the inclusion criteria. For colistin-based combination therapy, six articles including 668 patients were included. Our results showed that the overall clinical response did not differ significantly between colistin-based combination therapy and monotherapy (OR = 1.37, 95% CI = 0.86–2.19, P = 0.18). This insignificance was also detected in ICU mortality, length of stay and nephrotoxicity (P > 0.05). However, the colistin-based combination therapy was shown increasing the microbiological response (OR = 2.14, 95% CI = 1.48–3.07, P < 0.0001). For colistin monotherapy, six studies involving 491 patients were analyzed. The results were in concordance with the findings of the colistin-based combination therapy group. Our results suggest that colistin may be a promising therapy as safe and efficacious as standard antibiotics for the treatment of drug-resistant Acinetobacter baumannii infection.
Acinetobacter baumannii is an aerobic Gram-negative pathogen, which is often associated with nosocomial infections in the immunocompromised patient population1. In 1911, Beijerinck was the first to isolate and describe the organism that would now be recognized as Acinetobacter2. Most clinically significant isolates belong to the species A. baumannii or its close relatives, which together account for the vast majority of infections and hospital outbreaks involving Acinetobacter spp3. Recently, multidrug resistant A. baumannii (MDR-AB), which is resistant to all standard antimicrobial agents, began to expand, resulting in high treatment failure in some areas4. The presence of MDR-AB increases the prevalence of A. baumannii infection, and makes the choice of appropriate antimicrobial treatment difficult.
Colistin and tigecycline remain the only active antibiotics and have become the last resort of treatment for drug-resistant A. baumannii infection5. Previous meta-analysis conducted by Tasina et al. showed that tigecycline was not better than the usually used antimicrobial agents6, which have led to increased reliance on colistin in treating widespread drug-resistant A. baumannii infection. Colistin, a natural substance produced by Bacillus polymyxa subspecies colistinus, is a cationic lipopeptide7. It is rapidly bactericidal against Gram-negative bacteria by interacting with the lipid A moiety of lipopolysaccharide (LPS) to cause rupture of the outer membrane, and leading to cell permeability changes, leakage of the cellular content, and cell death7,8. Colistin sulfate and colistimethate sodium are the two commercially available forms. Recently, colistin has increasingly been used as salvage therapy, either alone or in combination with other antibiotics, for the treatment of severe infections in critically ill patients9. And it has been recommended in American Thoracic Society Guidelines as a therapeutic option for the treatment of VAP (Ventilator-associated pneumonia) caused by drug-resistant gram-negative organisms10. Recent study have also demonstrated that the use of higher doses of colistin, or colistin-based combination therapy (with other antibiotics), may prevent emerging resistance and preserve the activity of polymyxins against A. baumannii.
Though colistin is used in clinical practice, and is considered to be sub-optimal to beta-lactams, some reports have showed that it is very nephrotoxic and resistance to colistin has been reported in a particular area, from which the highest resistance rate was reported in Asia, followed by Europe11. Moreover, colistin heteroresistance and colistin resistance have been described in A. baumannii. Thus, we conducted this meta-analysis to systematically evaluate the safety and efficacy of colistin for the treatment of A. baumannii infection.
Results
Characteristics of studies included
As shown in Fig. 1, out of a total of initially reviewed 168 studies, only 12 studies including 1159 patients were considered eligible for inclusion in this meta-analysis. For colistin monotherapy, 6 studies involving 491 patients, were included. Of them, one was from Thailand12, one from Greece13, one from South Africa14, one from Spain15, one from Korea16 and one from China Taiwan17. For colistin-based combination therapy, 6 articles including 668 patients were contained. Four were from Turkey18,19,20,21, one from Korea22 and one from Italy23. Characteristics of the studies included in this analysis were presented in Table 1.
Figure 1. Flow chart demonstrating studies that were processed for inclusion in the447 meta-analysis.
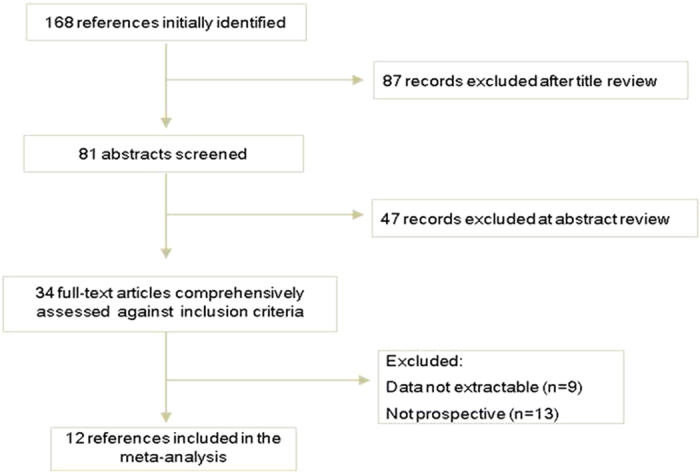
Table 1. Characteristics of the included studies.
| First author | Year | Country | Type of study | Organisms isolated group | Experimental | Control group | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics Sample size | Age | Antibiotics | ||||||||
| Age Sample size | ||||||||||
| Colistin monotherapy | ||||||||||
| Garnacho-Montero J | 2003 | Spain | Prospective cohort | AB | Colistin | 59.3 ± 13.1 | 21 | Imipenem-cilastain | 64.5 ± 11 | 14 |
| Betrosian AP | 2008 | Greece | Prospective cohort | MDRAB | Colistin | 67 ± 9 | 15 | Ampicillin/Sulbactam | 72 ± 5 | 13 |
| Gounden R | 2009 | South Africa | Retrospective cohort | MDRAB | Colistin | 43.5 ± 15.6 | 32 | Tigecycline | 45.6 ± 18.2 | 32 |
| Nakwan N | 2011 | Thailand | Retrospective cohort | EDRAB | Colistin | 38 (28–41) | 8 | Other antibiotics | 29 (28–34) | 7 |
| Chuang YC | 2014 | China Taiwan | Retrospective cohort | MDRAB | Colistin-based | 63.7 ± 19.5 | 119 | Tigecycline-based | 63.8 ± 17.9 | 175 |
| Kwon SH | 2014 | Korea | Retrospective cohort | EDRAB | Colistin | 59.0 ± 19.2 | 39 | Tigecycline | 60.1 ± 12.3 | 16 |
| Colistin combination therapy | ||||||||||
| J ang HJ | 2009 | Korea | Retrospective cohort | MDRAB | Colistin/synergistic antibiotics | 57.0 ± 16.5 | 19 | Colistin | 62.5 ± 17.5 | 22 |
| Simsek F | 2012 | Turkey | Retrospective cohort | AB | Colistin-based | 51.7 ± 18.8 | 21 | Colistin | 51.7 ± 18.8 | 15 |
| Aydemi H | 2013 | Turkey | RCT | CRAB | Colistin/ Rifampicin | 58 ± 23 | 21 | Colistin | 63 ± 17 | 22 |
| Durante-Mangoni E | 2013 | Italy | RCT | EDRAB | Colistin/ Rifampicin | 62 ± 15.1 | 104 | Colistin | 61 ± 15.7 | 105 |
| Batirel A | 2014 | Turkey | Retrospective cohort | EDRAB | Colistin/other antibiotics | 59.1 ± 19.6 | 214 | Colistin | 58.3 ± 20.5 | 36 |
| Kalin G | 2014 | Turkey | Retrospective cohort | MDRAB | Colistin/Sulbactam | 63 (20–89) | 37 | Colistin | 51 (19–96) | 52 |
RCT, randomized controlled trial; AB, Acinetobater baumannii; MDRAB, multi-drug resistant Acinetobater baumannii; EDRAB, extensively drug resistant Acinetobater baumannii; CRAB, carbapenem-resistant Acinetobater baumanni.
Meta-analysis of colistin monotherapy
Clinical outcome
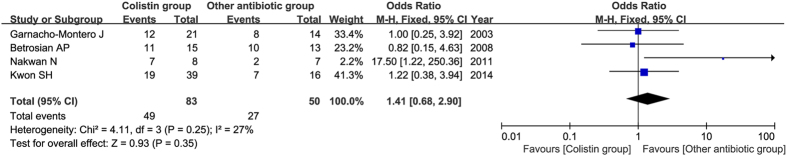
Four studies reported the clinical response, including 133 patients (83 with colistin treatment, and 50 with other antibiotics). No statistical heterogeneity was observed among studies (P = 0.25, I2 = 27%), and the fixed-effects model was employed. Overall, our result found that the effective rate of clinical response in patients with colistin group (experimental group) was a little higher than that in other antibiotic group (control group) (59% versus 54%). However, the overall clinical response did not differ significantly between colistin group and control groups (OR = 1.41, 95% CI = 0.68–2.90, P = 0.35) as shown in Fig. 2.
Figure 2. Clinical response of colistin monotherapy compared with control antibiotics.
Microbiological outcome
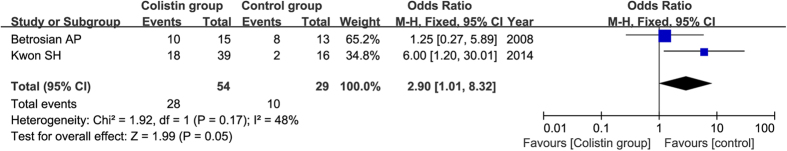
Two studies compared colistin with other antibiotics in terms of microbiological response, including 83 patients (54 with colistin treatment, 29 with other antibiotics). The result from these two studies showed that there was a significant difference between colistin group and control groups (OR = 2.90, 95% CI = 1.01–8.32, P = 0.05) as shown in Fig. 3. No statistical heterogeneity was found among studies (P = 0.17, I2 = 48%).
Figure 3. Forest plot for microbiological response between colistin group and control troup.
Mortality
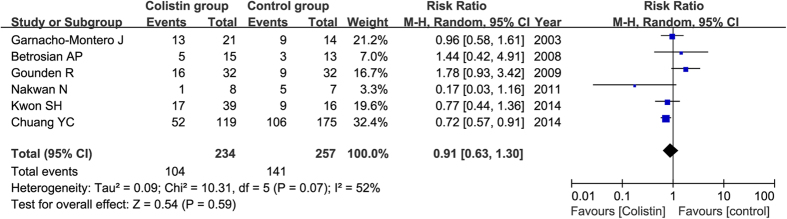
All six studies reported the mortality, including 491 patients (234 with colistin treatment, and 257 with other antibiotics). Although the mortality rate was lower in colistin group than that in control group (43% versus 50%), no significant difference was found between these two groups (RR = 0.91, 95% CI = 0.63–1.30, P = 0.59) as shown in Fig. 4.
Figure 4. Forest plot for risk ratios in terms of mortality of colistin compared with control antibiotics.
Length of stay
All the studies reported the length of stay containing 491 patients. We can’t conduct a statistical analysis due to different means of expression (some articles reported the mean days of length of stay, while others reported the range of days). All of them demonstrated that there was no statistically significant difference in the median length of hospital stay between two groups (P > 0.05).
Safety analysis
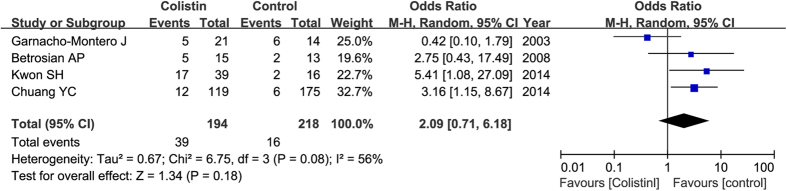
The main adverse effects of colistin treatment are nephrotoxicity and neurotoxicity. Four studies, including 412 patients, reported nephrotoxicity. Overall, it did not differ significantly between colistin group and control group (OR = 2.09, 95% CI = 0.71–6.18, P = 0.18) in a random effect model as shown in Fig. 5. No studies reported the results of neurotoxicity.
Figure 5. Risk of nephrotoxicity with colistin compared with control antibiotics.
Meta-analysis of colistin-based combination therapy
Clinical outcome
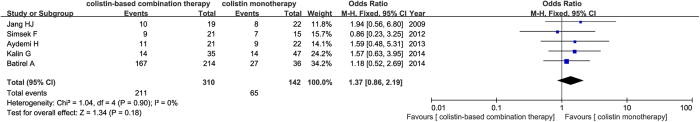
Five studies reported the clinical response, including 412 patients (194 with colistin-based combination therapy, and 218 with colistin monotherapy). The favorable clinical response was higher in patients with colistin-based combination therapy (experimental group) than that with colistin monotherapy (control group) (68.1% versus 45.8%). As shown in Fig. 6, the overall clinical response did not differ significantly between these two groups (OR = 1.37, 95% CI = 0.86–2.19, P = 0.18). No significantly heterogeneity was found between studies (I2 = 0%, P = 0.75).
Figure 6. Clinical response with colistin combination therapy compared with monotherapy.
Microbiological response
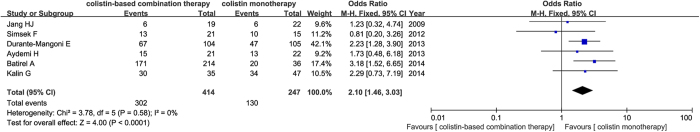
All the six studies reported the microbiological response of colistin-based combination therapy (414 patients) vs. colistin monotherapy (247 patients). Our result showed a significantly association (OR = 2.14, 95% CI = 1.48–3.07, P < 0.0001) in a fixed-effects model as shown in Fig. 7.
Figure 7. Forest plot for microbiological response between colistin combination and monotherapy groups.
Mortality
All six studies reported mortality. As shown in Fig. 8, no significant difference was noted when colistin-based combination therapy was compared with colistin monotherapy with respect to hospital mortality (RR = 0.93, 95% CI = 0.74–1.17, P = 0.54) in a random-effect model.
Figure 8. Risk ratios of mortality between colistin combination and alone groups.
Length of ICU stay
Three studies reported the length of ICU stay. The mean length of hospital stay was 5.05 days (95% CI,-4.35 to 14.45 days, P = 0.29), and it did not differ significantly between the colistin-based combination therapy group and colistin monotherapy group.
Safety analysis
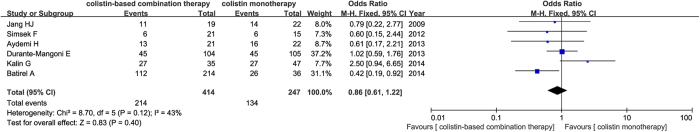
Nephrotoxicity did not differ significantly between colistin-based combination therapy group and colistin monotherapy group (OR = 1.13, 95% CI = 0.74–1.73, P = 0.57).
Sensitivity analyses and publication bias
A single study included in the meta-analysis was deleted each time to reflect the influence of the individual data set to the pooled ORs, and the corresponding pooled ORs were not materially changed. This procedure confirmed the stability of our overall result.
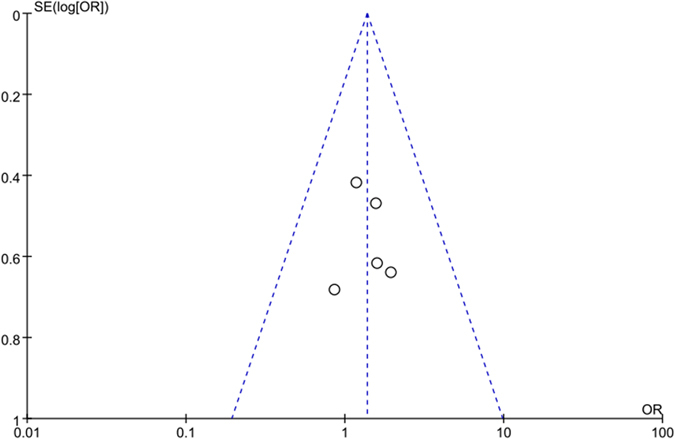
The funnel plot was conducted to assess the publication bias of the literature. The shape of funnel plots did not reveal any evidence of funnel plot asymmetry as shown in Fig. 9.
Figure 9. Funnel plot analysis on the detection of publication bias in the meta-analysis.
Discussion
A. baumannii has emerged as a major cause of nosocomial infections. It has been identified by the Infectious Diseases Society of America as one of the six particularly problematic pathogens24. Due to the shortfall of effective antibiotic development for multidrug resistant A. baumannii, colistin is re-investigated in treating the widespread drug-resistant bacteria and has found renewed interest25.
Colistin has a narrow spectrum of use and is primarily used for A. baumannii infections. It is a multicomponent polypeptide antibiotic, composed mainly of colistin A and colistin B. Its use was limited by its renal toxicity, and it was replaced in the 1970s by antibiotics considered to be less toxic26. Moreover, in 2006 Li et al. first described colistin heteroresistance of A. baumannii, which was defined as the emergence of resistance to colistin by a subpopulation from an otherwise susceptible (MIC ≤ 2 mg/L) population27. More recently, colistin has increasingly been used as salvage therapy in combination with one or more antibacterial drugs for the treatment of severe infections in critically ill patients7,28.
Our study found that clinical response did not change significantly between colistin-based combination therapy against monotherapy AND colistin monotherapy against other antibiotic (P > 0.05). This insignificance was also found in other comparisons such as clinical outcome, mortality, length of stay, or toxicity. However, a significant difference was found between both these two comparison groups in terms of microbiological response. Our study is consistence with a previous meta-analysis conducted by Liu et al. which demonstrated that microbiological response rates favored the colistin group, while these differences were not significant in hospital mortality, lengths of hospital stay or nephrotoxicity in both colistin-based combination regimen vs. colistin alone AND monotherapy regimen vs. other single antibiotic groups29. These results suggest that colistin may be as safe and as efficacious as standard antibiotics for the treatment of A. baumannii infection.
In clinical practice, in order to improve antibacterial activity, colistin is frequently used as combination or single therapy, despite the consequent increase in toxicity. The comparative effectiveness of colistin monotherapy and colistin combination therapy was evaluated30. Most of the microbiological studies examined colistin monotherapy vs. combinations for A. baumannii infections. However, data from the relevant human studies suggest non-inferiority of colistin monotherapy as compared with combination therapy31.
Some studies have investigated the clinical effectiveness of combination therapy and have assessed the issues of the increased toxicity related to combination treatment regimens. Bassetti et al. found that colistin and rifampicin may be an effective and safe combination therapy for severe infections due to MDR-AB32. Although rifampin would be used more for synergy, research has shown that the 30-day mortality was not reduced by addition of rifampicin to colistin. Moreover, the combination of colistin with rifampicin may improve clinical and microbiological outcomes of VAP patients infected with A. baumannii. Cai et al. identified that colistin/rifampicin and colistin/carbapenem were the most studied combinations which showed promising results in vitro, in vivo and in the clinic33. Phee et al. firstly found colistin/ fusidic acid was a novel regimen for the treatment of MDR-AB, the combination was effective at low concentrations, which should be therapeutically achievable whilst limiting toxicity. Lee et al. showed that emergence of colistin-resistant subpopulations was completely suppressed in the colistin-susceptible isolate with all combinations at both inocula and provided important information for optimizing colistin-rifampin combinations against colistin-susceptible and -resistant MDR-AB34. Mutlu Yilmaz et al. identified that in the treatment of infections with a high mortality rate such as pneumonia caused by XDR-AB, combining tigecycline with colistin during the first 48 h and continuing treatment with one of these agents seems a rational approach35. However, Garnacho-Montero et al. demonstrated that clinical outcomes did not differ in patients treated with colistin plus vancomycin from those receiving colistin without vancomycin, and this combination significantly increases the risk of renal failure36.
Other studies have evaluated the effect of colistin monotherapy in severe infections caused by MDR Gram-negative pathogens. Khawcharoenporn et al. found that administration of primary or adjunctive intrathecal or intraventricular colistin therapy was effective for MDR-AB central nervous system infection37. Kang et al. showed that aerosolized colistin may be used as monotherapy for VAP due to A. baumannii infection in pre-term infants38. However, a major concern regarding colistin monotherapy is the potential problem of heteroresistance among Gram-negative bacterial populations exposed to colistin alone39.
Several limitations are presented in our meta-analysis. Firstly, the patient population enrolled and randomized was a very heterogeneous population. The population was inherently complex for the baseline conditions were diversity. Secondly, the patients were not stratified by the additional antibiotics that are known to be effective for A. baumannii, or antibiotics in the control group might have different effect. Thirdly, colistin drug monitoring was not available for drug-drug interactions with these medications may have affected the primary mortality. Lastly, some of the outcomes are presented in different ways, so we can’t make a statistical analysis. Furthermore, the number of included studies for a certain comparison was small.
In conclusion, our results showed that colistin could be a safe and effective alternative therapy for drug resistant A. baumannii infection. Although no differences were found between colistin combination therapy and colistin monotherapy AND between colistin monotherapy and other antibiotics, additional studies are needed to evaluate the effective of colistin in MDR-AB infection.
Materials and Methods
Literature search
We conducted a comprehensive literature search using the electronic database of PubMed, Medline and Embase for relevant articles published between January 2000 and March 2014. We retrieved the relevant articles using the following terms: “colistin or polymyxin E”, “combination therapy”, “monotherapy”, “Acinetobacter baumannii or A. baumannii infection” and “ventilator associated pneumonia or VAP” as well as their combinations. References of retrieved articles were searched with no language restrictions. The search was focused on studies that had been conducted in humans.
Study selection
The inclusion criteria were as follows: 1) the paper should be randomized controlled trials or cohort studies; 2) evaluating the safety and efficacy of colistin monotherapy against other antimicrobial agents, or colistin combination therapy against colistin monotherapy for the treatment of A. baumannii infection; 3) multi-drug resistant A. baumannii was defined as A. baumannii, which showed non-susceptibility to ≥1 agent in ≥3 antimicrobial categories40; and 4) the primary outcome were clinical response and microbiological response (clinical response was defined as complete or partial remission of the signs and symptoms of infection by the end of therapy; microbiological response was defined as negative of culture result at the end of therapy); the secondary outcome were mortality and colistin toxicity/adverse effect (neurotoxicity, nephrotoxicity) (Neurotoxicity was defined as any of the following: seizures, encephalopathy, neuromuscular blockade and apnea; Nephrotoxicity was defined as initiation or as a decline in renal function that prompted renal replacement therapy).
Data extraction
Two investigators independently assessed the quality of the included studies according to the descriptions provided by the authors of the included trials. Any disagreement was subsequently resolved by discussion with a third author. The following information was extracted from each article: first author, year of publication, country, ethnicity, number of patients, type of colistin administered, co-administration of other antibiotics, clinical response, mortality, length of therapy and number of patients with nephrotoxicity.
Statistical analysis
The overall effect was measured by odds ratios (ORs), risk ratios (RRs), and mean difference (MD) with their 95% confidence interval (CI), which were calculated according to the method of Woolf41. The significance of the pooled ratios was determined by the Z test, and a P value less than 0.05 was considered statistically significant. The I2 test was used to assess the proportion of statistical heterogeneity and the Q-statistic test was used to define the degree of heterogeneity. A P-value less than 0.10 for the Q-test and I2 more than 50% was considered significant among the studies. Data were combined using both a fixed-effects model and a random-effects model42,43. The fixed-effects model is used when the effects are assumed to be homogenous, while the random-effects model is used when they are heterogenous. The evidence of publication bias was assessed by visual funnel plot inspection.
To assess whether our results were substantially influenced by the presence of any individual study, we conducted a sensitivity analysis by systematically removing each study and recalculating the significance of the result. Statistical analyses were conducted in Review Manager (version 5.2, The Cochrane Collaboration). All the tests were two-sided.
Additional Information
How to cite this article: Chen, Z. et al. Meta-analysis of colistin for the treatment of Acinetobacter baumannii infection. Sci. Rep. 5, 17091; doi: 10.1038/srep17091 (2015).
Footnotes
Author Contributions Conceived and designed the study by: ZJ.C., Y.C., YG.F., XT.W., YQ.C., QS.Q., F.H. and XG.X.; Performed the experiment by ZJ.C., Y.C., YG.F., XT.W., YQ.C., QS.Q., F.H. and XG.X.; Statistical analyses, discussion and paper writing: ZJ.C., Y.C., YG.F., XT.W., YQ.C., QS.Q., F.H and XG.X.; Revising: ZJ.C., Y.C., and XG.X.
References
- Towner K. Acinetobacter: an old friend, but a new enemy. J Hosp Infect 73, 355–363 (2009). [DOI] [PubMed] [Google Scholar]
- Dijkshoorn L. & Nemec A. The diversity of the genus Acinetobacter. Acinetobacter, Molecular Biology 2, 1–34 (2008). [Google Scholar]
- Bergogne-Berezin E. & Towner K. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clinical microbiology reviews 9, 148 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunenshine R. H. et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 13, 97–103 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N. C. & Wareham D. W. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int J Antimicrob Agents 35, 219–226 (2010). [DOI] [PubMed] [Google Scholar]
- Tasina E., Haidich A. B., Kokkali S. & Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 11, 834–844 (2011). [DOI] [PubMed] [Google Scholar]
- Falagas M. E. & Kasiakou S. K. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 40, 1333–1341 (2005). [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Peptide antibiotics. Lancet 349, 418–422 (1997). [DOI] [PubMed] [Google Scholar]
- Oleksiuk L. M. et al. In Vitro Responses of Acinetobacter baumannii to Two-and Three-Drug Combinations following Exposure to Colistin and Doripenem. Antimicrob Agents Ch 58, 1195–1199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society A. T. Infectious Diseases Society of AmericaAmerican Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 1714, 388–416 (2005). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Ch 50, 2946–2950 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakwan N. et al. Safety in treatment of ventilator‐associated pneumonia due to extensive drug‐resistant Acinetobacter baumannii with aerosolized colistin in neonates: A preliminary report. Pediatr Pulm 46, 60–66 (2011). [DOI] [PubMed] [Google Scholar]
- Betrosian A. P., Frantzeskaki F., Xanthaki A. & Douzinas E. E. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infection 56, 432–436 (2008). [DOI] [PubMed] [Google Scholar]
- Gounden R., Bamford C., van Zyl-Smit R., Cohen K. & Maartens G. Safety and effectiveness of colistin compared with tobramycin for multi-drug resistant Acinetobacter baumannii infections. BMC Infect Dis 9, 26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnacho-Montero J. et al. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis 36, 1111–1118 (2003). [DOI] [PubMed] [Google Scholar]
- Kwon S. H., Ahn H. L., Han O. Y. & La H. O. Efficacy and Safety Profile Comparison of Colistin and Tigecycline on the Extensively Drug Resistant Acinetobacter baumannii. Biol Pharm Bull 37, 340–346 (2014). [DOI] [PubMed] [Google Scholar]
- Chuang Y.-C. et al. Effectiveness of tigecycline-based versus colistin-based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumannii in a critical setting: a matched cohort analysis. BMC Infect Dis 14, 102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydemir H. et al. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol Infect 141, 1214–1222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batirel A. et al. Comparison of colistin–carbapenem, colistin–sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur J Clin Microbiol 33, 1311–1322 (2014). [DOI] [PubMed] [Google Scholar]
- Simsek F. et al. Colistin against colistin-only-susceptible Acinetobacter baumannii-related infections: Monotherapy or combination therapy? Indian J Med Microbiol 30, 448–452 (2012). [DOI] [PubMed] [Google Scholar]
- Kalin G., Alp E., Akin A., Coskun R. & Doganay M. Comparison of colistin and colistin/sulbactam for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Infection 42, 37–42 (2014). [DOI] [PubMed] [Google Scholar]
- Jang H. J. et al. The comparative efficacy of colistin monotherapy and combination therapy based on in vitro antimicrobial synergy in ventilator-associated pneumonia caused by multi-drug resistant Acinetobacter baumannii. Tuberculosis and Respiratory Diseases 67, 212–220 (2009). [Google Scholar]
- Durante-Mangoni E. et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 57, 349–358 (2013). [DOI] [PubMed] [Google Scholar]
- Boucher H. W. et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48, 1–12 (2009). [DOI] [PubMed] [Google Scholar]
- Azzopardi E. A., Ferguson E. L. & Thomas D. W. Colistin past and future: a bibliographic analysis. J Crit Care 28, 219 (2013). [DOI] [PubMed] [Google Scholar]
- Wolinsky E. & Hines J. D. Neurotoxic and nephrotoxic effects of colistin in patients with renal disease. New Engl J Med 266, 759–762 (1962). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Ch 50, 2946–2950 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo N. et al. Combined colistin and rifampicin therapy for carbapenem‐resistant Acinetobacter baumannii infections: clinical outcome and adverse events. Clin Microbiol Infec 11, 682–683 (2005). [DOI] [PubMed] [Google Scholar]
- Liu Q., Li W., Feng Y. & Tao C. Efficacy and Safety of Polymyxins for the Treatment of Acinectobacter baumannii Infection: A Systematic Review and Meta-Analysis. PLoS One 9, e98091 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo N., Ioannidou E. & Falagas M. Colistin monotherapy vs. combination therapy: evidence from microbiological, animal and clinical studies. Clin Microbiol Infec 14, 816–827 (2008). [DOI] [PubMed] [Google Scholar]
- Pogue J. M. & Kaye K. S. Is there really no benefit to combination therapy with colistin? Expert Rev Anti Infect Ther 11, 881–884, 10.1586/14787210.2013.827881 (2013). [DOI] [PubMed] [Google Scholar]
- Bassetti M. et al. Colistin and rifampicin in the treatment of multidrug-resistant Acinetobacter baumannii infections. J Antimicrob Chemother 61, 417–420 (2008). [DOI] [PubMed] [Google Scholar]
- Cai Y., Chai D., Wang R., Liang B. & Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67, 1607–1615 (2012). [DOI] [PubMed] [Google Scholar]
- Lee H. J. et al. Synergistic activity of colistin and rifampin combination against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Ch 57, 3738–3745 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu Yilmaz E. et al. Efficacy of tigecycline/colistin combination in a pneumonia model caused by extensively drug-resistant Acinetobacter baumannii. Int J Antimicrob Agents 40, 332–336 (2012). [DOI] [PubMed] [Google Scholar]
- Garnacho-Montero J. et al. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemotherapy 59, 225–231 (2013). [DOI] [PubMed] [Google Scholar]
- Khawcharoenporn T., Apisarnthanarak A. & Mundy L. M. Intrathecal colistin for drug‐resistant Acinetobacter baumannii central nervous system infection: a case series and systematic review. Clin Microbiol Infec 16, 888–894 (2010). [DOI] [PubMed] [Google Scholar]
- Kang C. H. et al. Colistin inhalation monotherapy for ventilator‐associated pneumonia of Acinetobacter baumannii in prematurity. Pediatr Pulm 49, 381–388 (2013). [DOI] [PubMed] [Google Scholar]
- Hawley J. S., Murray C. K. & Jorgensen J. H. Development of colistin-dependent Acinetobacter baumannii-Acinetobacter calcoaceticus complex. Antimicrob Agents Ch 51, 4529–4530 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos A. P. et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infec 18, 268–281 (2012). [DOI] [PubMed] [Google Scholar]
- Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet 19, 251–253 (1955). [DOI] [PubMed] [Google Scholar]
- Mantel N. & Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22, 719–748 (1959). [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]